J Clin Aesthet Dermatol. 2019;12(11):13–18
 by James Q. Del Rosso, MD; Linda Stein Gold, MD; Jeanett Segal; and Andrea L. Zaenglein, MD
by James Q. Del Rosso, MD; Linda Stein Gold, MD; Jeanett Segal; and Andrea L. Zaenglein, MD
Dr. Del Rosso is with JDR Dermatology Research/Thomas Dermatology in Las Vegas, Nevada. Dr. Stein Gold is with the Department of Dermatology, Clinical Research Division at the Henry Ford Hospital in Detroit, Michigan. Dr. Segal is with Sun Pharmaceutical Industries, Inc. in Princeton, New Jersey. Dr. Zaenglein is with the Departments of Dermatology and Pediatrics, Penn State College of Medicine at the Hershey Medical Center in Hershey, Pennsylvania.
FUNDING: The study was funded by Sun Pharmaceutical Industries, Inc.
DISCLOSURES: Dr. Del Rosso has received grants from and/or served as a consultant/advisor, investigator, and/or speaker for Abbvie, Aclaris, Almirall, Athenex, BioPharmX, Botanix, Cassiopeia, Celgene, Cutanea (Biofrontera), Dermira, Encore, EPI Health, Ferndale, Foamix, Galderma, Genentech, LaRoche Posay, Leo Pharma, Menlo Therapeutics, Novan, Ortho Dermatologics, Pfizer, Promius, Regeneron, Sanofi/Genzyme, Sonoma (Intraderm), Sol Gel, Sun Pharmaceuticals, and Verrica. Dr. Stein Gold has received grants from and/or served as a consultant, investigator and/or a speaker for Foamix, Galderma, Novartis, Ortho Dermatologies, Sol Gel, and Sun Pharmaceuticals. Dr. Segal is an employee of Sun Pharmaceutical Industries, Inc. Dr. Zaenglein has received grants from and/or served as a consultant/advisor and/or investigator for Abbvie, Allergan, Incyte, Innovaderm, Ortho Dermatologics, Pfizer, and Ranbaxy/Sun Pharmaceuticals.
ABSTRACT: Objective. We sought to evaluate long-term relapse rates following lidose-isotretinoin taken without food in patients with severe recalcitrant acne.
Design. In this single-arm, open-label study, 197 patients received twice-daily lidose-isotretinoin without food for up to 20 weeks. Patients with a 75-percent or higher adherence rate with the protocol-designated dosage and end-of-treatment lesion counts were predefined as the per-protocol (PP) population and evaluated in a 104-week post-treatment period (PTP) to determine the proportion of patients requiring retreatment.
Setting. Participants were enrolled from 21 sites across the United States.
Participants. Eligible participants were male or nonpregnant, nonlactating female, aged between 12 to 45 years, weighing 40 to 110 kg, and with no prior exposure to systemic isotretinoin or systemic retinoids. Acne was considered severe enough for treatment if the patient had five or more facial nodule lesions.
Measurements. Patients were observed to determine whether they required retreatment with isotretinoin or any acne therapy during the PTP. Lesion counts and assessments of acne severity, quality of life, and adverse events were completed.
Results. Of the 166 patients in the PP population, seven (4.2%; 95% confidence interval [CI]: 1.7%–8.5%) were retreated with isotretinoin, 25 (15.1%; 95% CI: 10.0%–21.4%) were treated with topical and/or oral nonisotretinoin therapies including over-the-counter therapies or intralesional injections, and 137 (82.5%; 95% CI: 75.8%–88.0%) required no retreatment. Isotretinoin retreatment was most common in male patients aged 14 to 18 years.
Conclusion. Long-term relapse rates for lidose-isotretinoin taken without food for 20 weeks were at the low end of those published for traditional isotretinoin taken with a high-fat/high-calorie meal.
ClinicalTrials.gov Registration. NCT02457520
KEYWORDS: Acne, isotretinoin, lidose-isotretinoin, relapse, retreatment
Isotretinoin is a vitamin A derivative recognized as the standard for the treatment of severe refractory acne.1 A single course of therapy is usually sufficient for complete or nearly complete and prolonged remission.2 Isotretinoin is categorized as a class II drug by the United States Food and Drug Administration and the United States Center for Drug Evaluation and Research, owing to poor solubility, high lipophilicity, and high permeability.3 These properties require that isotretinoin be ingested with a high-fat/high-calorie (HF/HC) meal (50g of fat, 1,000 calories) to achieve optimal gastrointestinal absorption.4,5 Other than lidose-isotretinoin (Absorica®; Sun Pharmaceutical Industries, Inc., Princeton, New Jersey), all currently available isotretinoin formulations are based on bioequivalence to pharmacokinetic data achieved with the original brand of isotretinoin (Accutane®; Roche Laboratories Inc., Nutley, New Jersey), referred to here as traditional isotretinoin.5 Traditional isotretinoin administered under fasted conditions results in plasma isotretinoin levels that are about 60-percent lower than those achieved along with the administration of an HF/HC meal.4 Relapse rates have been correlated directly with decreased total systemic exposure to isotretinoin, meaning that nonadherence or inconsistent adherence with food intake can increase the risk of post-treatment relapse and compromise long-term efficacy.6
The term relapse in acne treatment refers to the appearance of new acne lesions after apparent clearance; however, in some publications, relapse may only include patients who require retreatment after acne therapy has ceased. Published relapse rates for traditional isotretinoin vary widely (5%–65%) depending on sample size, length of follow-up observation, and relapse definition used.7 The cumulative administered dose of traditional isotretinoin, reflective of total systemic exposure, is reported to be an important risk factor for acne relapse, with lower cumulative doses (<120 mg/kg total dose) being more associated with higher relapse rates than higher cumulative doses.7,8
Lidose-isotretinoin is a formulation of isotretinoin presolubilized in a lipid matrix that improves bioavailability through greater gastrointestinal absorption.9,10 This formulation has demonstrated comparable safety and efficacy to that of traditional isotretinoin taken with a HF/HC meal.11 However, the pharmacokinetic profile of lidose-isotretinoin is distinct from those of traditional isotretinoin forms. The bioavailability of lidose-isotretinoin administered following an overnight fast was reduced by 33.2 percent compared to administration following a HF/HC breakfast, whereas the bioavailability of traditional isotretinoin was reduced by 60.4 percent under the same conditions.9 Thus, the gastrointestinal absorption of lidose-isotretinoin is less dependent on the amount and/or type of food intake than that of traditional isotretinoin, enabling patients to achieve similar therapeutic outcomes with lidose-isotretinoin—even when taken in an unfed state—and reducing the inconvenience of consistently having to maintain the correct diet.9 This might improve adherence, especially in adolescents or busy adults treated with isotretinoin who do not necessarily adhere to consistent eating habits.12,13
Relapse rates following the completion of an initial course of lidose-isotretinoin taken without food have not been assessed. Here, we report the proportion of patients who required some form of acne retreatment during the supervised post-treatment period (PTP) of an open-label, Phase IV study in patients with severe recalcitrant nodular acne who were treated with lidose-isotretinoin ingested without food over a fixed duration of 20 weeks. The study endpoints and outcomes during the active treatment period (ATP) of this study have previously been reported.14
Methods
In this open-label, single-arm, multicenter Phase IV study (NCT02457520), lidose-isotretinoin treatment efficacy, safety, and impact on quality of life (QoL) were evaluated in patients with severe recalcitrant nodular acne. In the PTP, the proportion of patients who required retreatment with any oral form of isotretinoin or any other acne medication was assessed. In addition, patient surveillance included inflammatory, noninflammatory, and nodular lesion counts; Investigator’s Global Assessment (IGA) scores; and QoL evaluations. The study was registered on May 29, 2015, and was conducted at 21 sites across the United States; screening began on January 29, 2015, and the study was completed in April 2018.
The study protocol was approved by a central institutional review board and conducted in compliance with the ethical principles originating from the Declaration of Helsinki, Good Clinical Practice guidelines, and local laws and regulations. Before enrollment, written informed consent was obtained from each patient or their parent or guardian if the patient was under the legal age of consent.
Eligibility criteria. Eligible participants were male or nonpregnant, nonlactating females in good general health who were aged 12 to 45 years and weighed 40 to 110 kg. Acne was considered severe enough for treatment if the patient had five or more facial nodule lesions. Participants were not permitted to have any prior exposure to systemic isotretinoin or systemic retinoids. Individuals with a clinically significant unstable medical condition that, in the opinion of the investigator, might have posed a risk to patient safety; with another skin disease or condition that might have interfered with the treatment or evaluation of acne; or with a history of a major psychiatric condition and/or a history of excessive or suspected alcohol or drug abuse were excluded.
In addition, female patients of childbearing potential were required to use two effective forms of contraception simultaneously (following iPLEDGE® requirements10) for one month before, during, and for one month after lidose-isotretinoin treatment unless they committed to continuous abstinence from heterosexual intercourse. Negative serum pregnancy test results as mandated by the iPLEDGE® program were required throughout study participation.
Dosage and visit schedule. During the 20-week ATP, patients received lidose-isotretinoin capsules twice daily without food (i.e., more than one hour before the next meal or two or more hours after the ingestion of food or any beverages other than water). An initial titration dosage of 0.5mg/kg/day for four weeks was followed by a dosage of 1.0mg/kg/day for 16 weeks (120–150mg/kg cumulative target dose). Each dosage was divided into two daily doses. Patients were seen at two-week intervals for the first month of treatment and at four-week intervals thereafter.
After the Week-20 visit, patients discontinued lidose-isotretinoin treatment and entered the 104-week PTP. The first visit of the PTP took place at Week 24, followed by visits at Weeks 32, 46, 72, 98, and 124 conducted to evaluate relapse rates and continue the evaluation of efficacy and safety and patient QoL. Unscheduled visits were completed if patients contacted the study site about an acne flare. End-of-treatment assessments describe results as of the date patients discontinued the ATP, while end-of-study assessments describe results as of the date patients discontinued the PTP.
Efficacy evaluations. Patient relapse rates were assessed and the type and date of treatment used were reported at each visit during the PTP. Relapse was defined as the point at which a patient required any form of acne treatment, including any oral or topical therapies. The primary efficacy endpoints for the PTP were the proportion of patients who required retreatment with any formulation of oral isotretinoin during the PTP and the time at which retreatment was required.
Secondary efficacy endpoints included the proportion of patients who required treatment with prescription oral and/or topical nonisotretinoin antiacne medication, topical retinoid and nonretinoid medication, and/or over-the-counter medication during the PTP and the time to treatment. Patients who were retreated with a second course of isotretinoin had an end-of-study assessment and were withdrawn from the study. Patients who used nonisotretinoin therapies remained in the study and their medication use and acne severity were recorded. Patients could use more than one retreatment medication during the PTP.
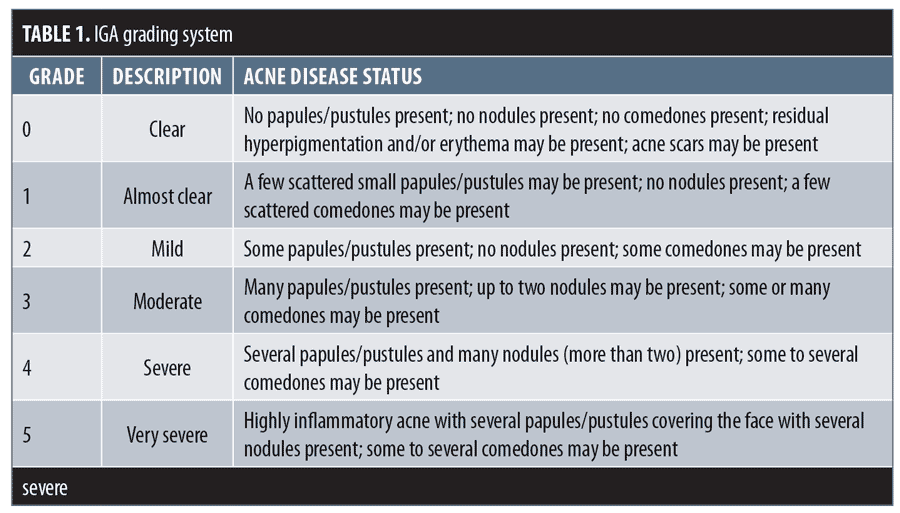
Efficacy assessments in both periods of the study included lesion counts, IGA, and a validated acne-specific QoL questionnaire (Acne-QoL). These assessments were performed at baseline and every visit thereafter. The IGA rated overall lesion severity, independent of baseline score, on a six-point scale (0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe, and 5=very severe) (Table 1).
Safety evaluations. The frequency and severity of adverse events (AEs) in all enrolled patients were evaluated until patients discontinued or completed the study.
Statistical analysis. A target sample size of 200 was selected to provide adequate estimates of probable events in the population. Estimates of the endpoints of interest were calculated with 95% two-sided confidence intervals (CIs) wherever possible. The intention-to-treat (ITT) population was defined as patients who received at least one dose of the study medication. In the PTP, efficacy analyses were assessed using the per-protocol (PP) population, defined as all patients who completed a Week-20 acne lesion count, with 75-percent or greater adherence with the dosing regimen. Patients in the PP population were stratified by sex (male or female) and baseline age (<14, 14–18, or >18 years) at Week 20 for analysis during the PTP. Safety analyses were conducted in the safety population, defined as all patients enrolled. Efficacy and safety data were summarized by descriptive statistics.
Results
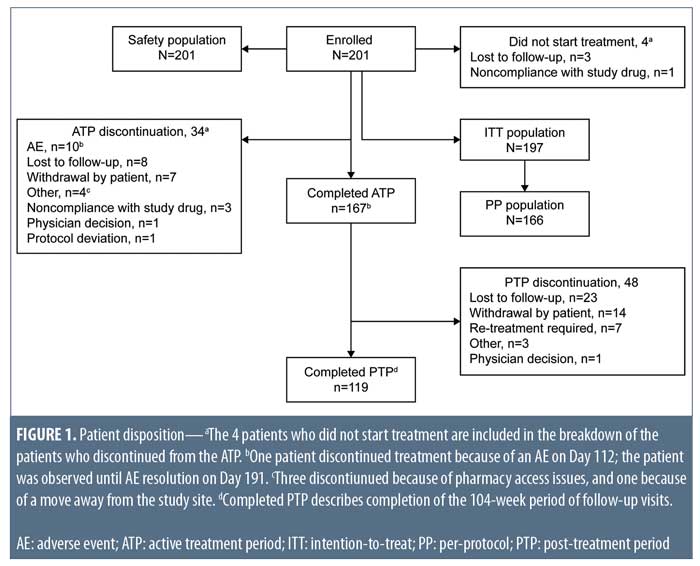
Demographics and baseline characteristics. Of the 232 patients screened, 201 were enrolled in the study and included in the safety population, with 197 forming the ITT population (Figure 1). Conversely, the PP population consisted of 166 patients.
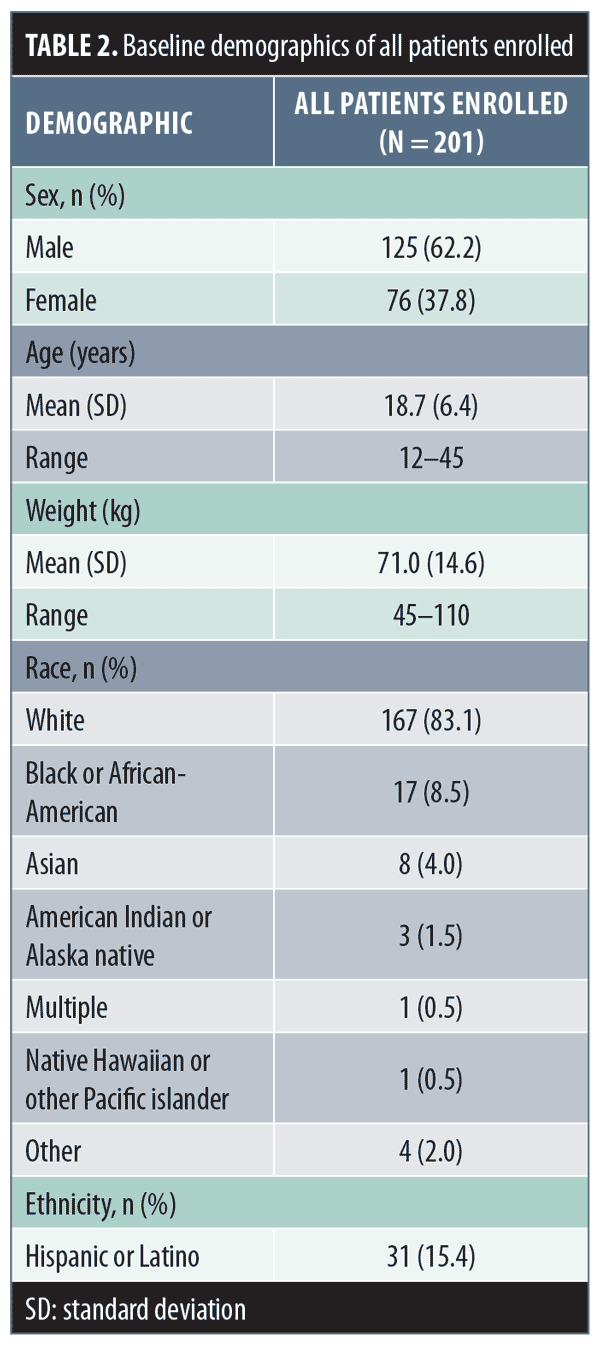
Of the 201 enrolled patients, 125 (62.2%) were male, 167 (83.1%) were white, and 170 (84.6%) were non-Hispanic/Latino, with a mean (standard deviation [SD]; range) age of 18.7 (6.4; 12–45) years and weight of 71.0kg (14.6; 45–110)
(Table 2).
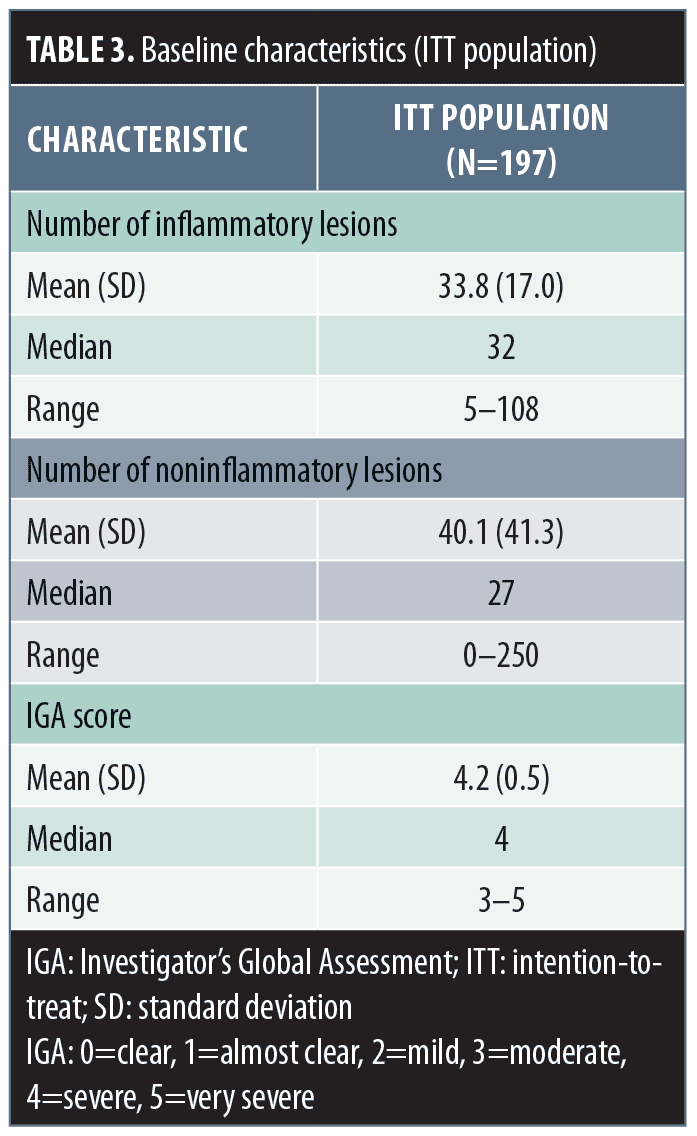
For the 197 patients in the ITT population, the baseline mean (SD) inflammatory lesion count was 33.8 (17.0) and noninflammatory lesion count was 40.1 (41.3) (Table 3). Additionally, the mean (SD; range) baseline IGA score was 4.2 (0.5; 3–5) (Table 3).
Patient disposition. In total, 167 patients completed the ATP and 119 completed the PTP (Figure 1). Thirty-four of the 201 enrolled patients (16.9%) prematurely discontinued before completing the ATP, most commonly because of AEs (10 patients), loss to follow-up (eight patients), and patient withdrawal (seven patients). Forty-eight patients discontinued from the PTP primarily owing to loss to follow-up (23 patients), patient withdrawal (14 patients), and retreatment with isotretinoin (seven patients).
Relapse rates. At end of treatment in the 20-week ATP, 59 of 166 subjects were rated as clear (IGA=0), 70 of 166 were rated as almost clear (IGA=1), and 17 of 166 were rated as mild (IGA=2).14 Of these 166 patients (PP population) followed in the PTP, 137 (82.5%; 95% CI: 75.8%–88.0%) required no retreatment during the 104-week PTP (Figure 2). Retreatment rates with isotretinoin were low (4.2%; 95% CI: 1.7%–8.5%). Because of the small number of patients requiring retreatment, data were insufficient to estimate a median time to retreatment.
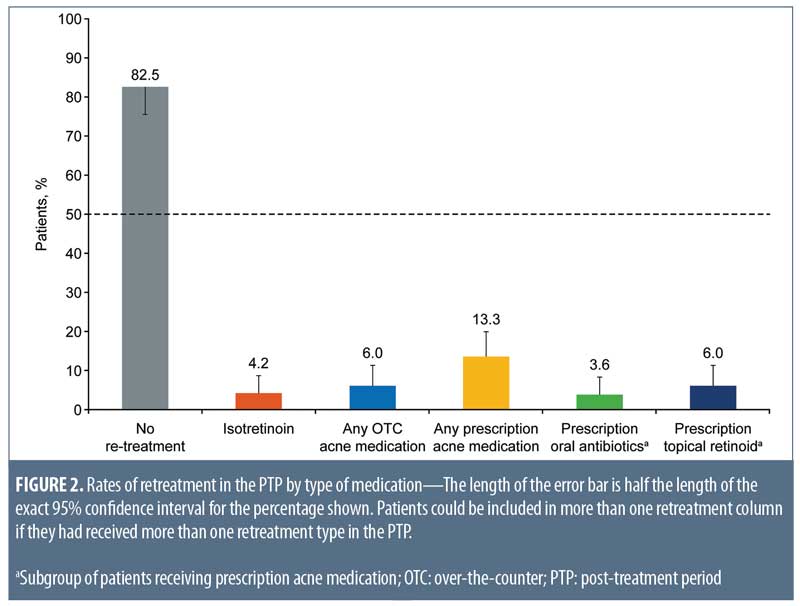
In total, 25 of 166 patients (15.1%; 95% CI: 10.0%–21.4%) were treated with topical and/or oral nonisotretinoin therapies including over-the-counter therapies or intralesional injections. Of the patients requiring nonisotretinoin treatment, prescription medication was more common than over-the-counter medication (13.3%; 95% CI: 8.5%–19.4% and 6.0%; 95% CI: 2.9%–10.8%, respectively). Commonly prescribed acne treatment options were topical retinoids (6.0%; 95% CI: 2.9%–10.8%) and oral antibiotics (3.6%; 95% CI: 1.3%–7.7%) (Figure 2).
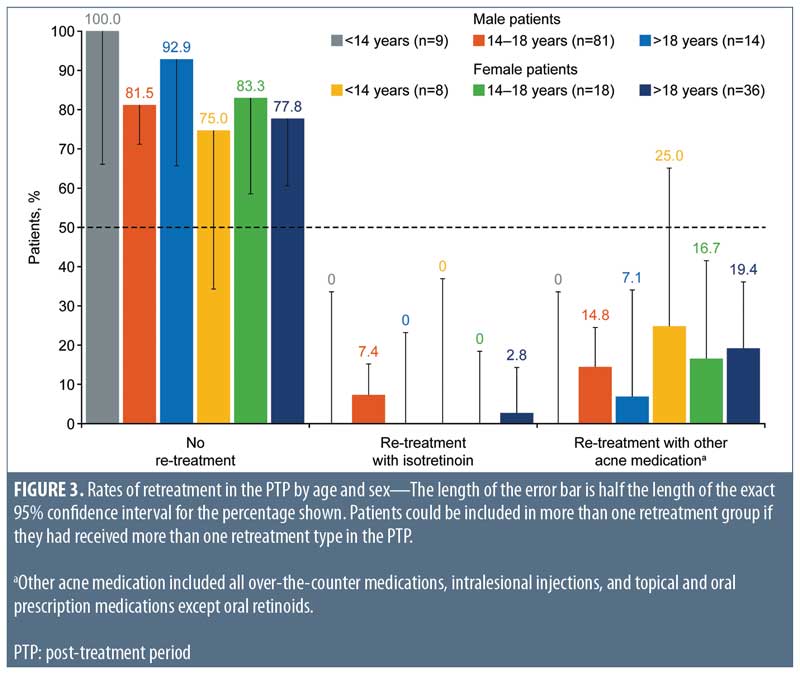
A higher proportion of male patients (n=6; 5.8%; 95% CI: 2.2%–12.1%) than female patients (n=1; 1.6%; 95% CI: <0.1%–8.7%) required retreatment with isotretinoin. These male patients were all aged 14 to 18 years, while the female patient was aged 21 years (Figure 3). More female patients (19.4%; 95% CI: 10.4%–31.4%) than male patients were treated with intralesional injections and over-the-counter and prescription nonisotretinoin therapies (12.5%; 95% CI: 6.8%–20.4%). Among male patients treated with nonisotretinoin therapies, none were aged younger than 14 years, 12 (14.8%; 95% CI: 7.9%–24.5%) were aged 14 to 18 years, and one (7.1%; 95% CI: 0.2%–33.9%) was aged older than 18 years. Nonisotretinoin treatment was required for two female patients (25.0%; 95% CI: 3.2%–65.1%) aged younger than 14 years, three female patients (16.7%; 95% CI: 3.6%–41.4%) aged 14 to 18 years, and seven female patients (19.4%; 95% CI: 8.2%–36.0%) aged older than 18 years.
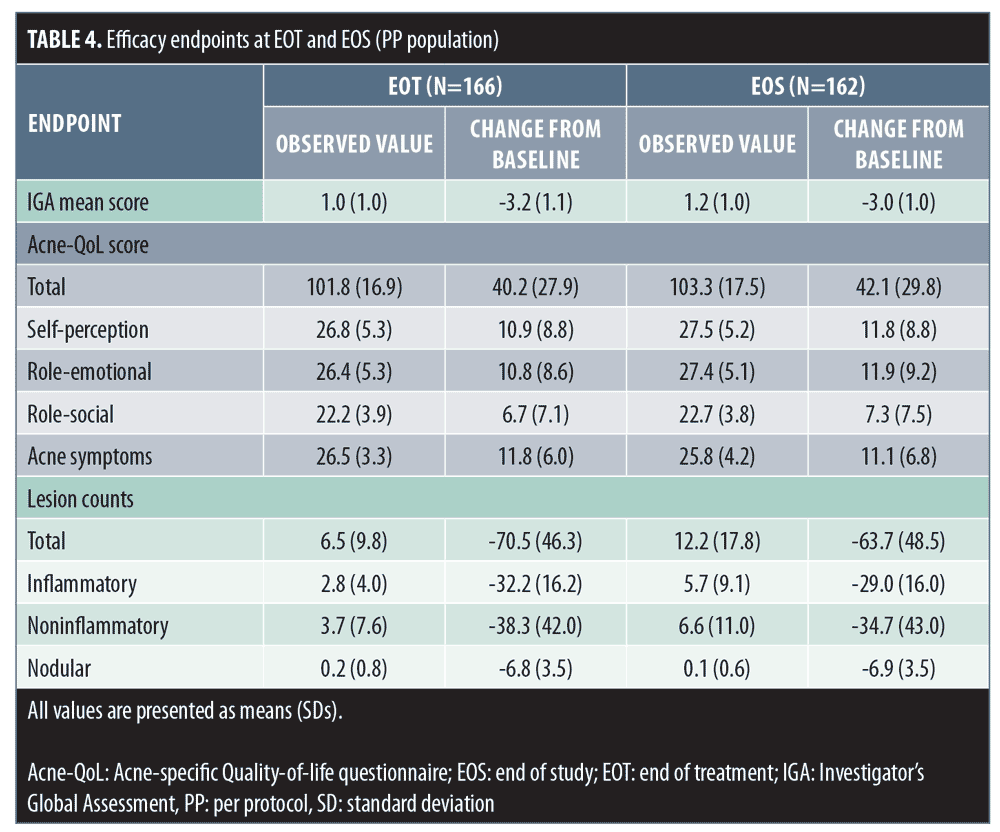
Changes in lesion counts, IGA score, and patient QoL. Statistically significant improvements from baseline in terms of mean lesion count, IGA score, and patient QoL observed at end of treatment were maintained during the PTP (Table 4).
Safety. No new AEs were reported during the PTP. The most common treatment-related AEs during the ATP were dry skin (10.9%), dry lips (11.4%), and cheilitis (9.0%).14
Discussion
Therapies aiming to improve upon traditional options for the treatment of severe recalcitrant acne must exhibit long-term post-treatment efficacy to sufficiently establish their advantage over traditional isotretinoin. In a 104-week PTP following 20 weeks of treatment with twice-daily lidose-isotretinoin without food in patients with severe recalcitrant nodular acne, only 4.2% (95% CI: 1.7%–8.5%) of patients required retreatment with isotretinoin and 17.5% (95% CI: 12.0%–24.1%) required treatment with any acne medication. In the absence of a large-scale comparative randomized study, these results indicate low post-treatment relapse rates following lidose-isotretinoin treatment as compared with traditional isotretinoin, without ingestion with an HF/HC meal.7
Reported relapse rates are affected by a diverse array of factors that can vary widely between study protocols, making comparisons between studies difficult. Research assessing relapse rates following traditional isotretinoin treatment with sample sizes or lengths of follow-up similar to the present study tended to report higher relapse rates.6
A prospective open study with a mean follow-up period of 104 weeks reported a 52-percent relapse rate with traditional isotretinoin.15 However, this study only observed 52 patients and defined relapse as an increase in lesion counts following the completion of traditional isotretinoin treatment. Further, it did not include any data on the need for retreatment.
Chivot and Midoun16 reported that 21 percent of 172 patients required retreatment with traditional isotretinoin following an initial course of isotretinoin over a mean observation period of 19 months. They also found that young age and acne severity were factors that influenced the observed relapse rates. Not all studies have found these associations.17 Data reported here indicate that age and sex might influence retreatment rates. The retreatment rate with any formulation of isotretinoin in this study (4.2%; 95% CI: 1.7%–8.5%) was a fraction of the rate reported by Chivot and Midoun, and retreatment was most commonly required in male patients aged 14 to 18 years.
In this study, the trial design prevented investigators from continuing initial lidose-isotretinoin therapy beyond 20 weeks without the subjects being considered as retreated. Some clinicians suggest extending isotretinoin treatment off-label for an additional four weeks beyond the timepoint when complete acne clearance (IGA = 0) is achieved. Whether this approach may further reduce post-treatment relapse rates or not is not known.
A limitation of this study is that the trial design did not include a direct comparison with traditional isotretinoin. However, given that long-term treatment success is highly dependent on cumulative total drug exposure, the increased bioavailability of lidose-isotretinoin as compared with traditional isotretinoin under fasted conditions may offer advantages in terms of increasing treatment success.5 It should be noted that pharmacokinetic profile differences between lidose-isotretinoin and traditional isotretinoin mean that they are not rated as bioequivalent; therefore, neither can be interchanged with the other if one is specified as necessary by the prescribing clinician.
Conclusion
The present data indicate that relapse rates over a 104-week PTP following 20 weeks of treatment with lidose-isotretinoin in the unfed state are at the low end of the range of published relapse rates for traditional isotretinoin taken with a HF/HC meal. By offering an efficacious alternative that does not include dietary requirements and by providing what appears to be greater long-term efficacy than traditional isotretinoin, lidose-isotretinoin is a rational treatment option for patients with severe recalcitrant acne.
Acknowledgments
The authors thank the patients who took part in this study. Editorial support was provided by Tamsin Brown, MSc, of JK Associates, Inc., part of the Fishawack Group of Companies, and was funded by Sun Pharmaceutical Industries, Inc. Statistical support was provided by Charles Darby of Sun Pharma Advanced Research Company, Ltd. and Novella Clinical and was funded by Sun Pharmaceutical Industries, Inc.
References
- Ingram J, Grindlay D, Williams H. Management of acne vulgaris: an evidence-based update. Clin Exp Dermatol. 2010;35(4):351–354.
- Merritt B, Burkhart C, Morrell D. Use of isotretinoin for acne vulgaris. Pediatr Ann. 2009;38(6): 311–320.
- United States Center for Drug Evaluation and Research. Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system: guidance for industry. Available at: https://www.fda.gov/media/70963/download. Accessed June 30, 2019.
- Colburn W, Gibson D, Wiens R, Hanigan J. Food increases the bioavailability of isotretinoin. J Clin Pharmacol. 1983;23(11–12):534–539.
- Leyden J, Del Rosso J, Baum E, McGuigan K. The use of isotretinoin in the treatment of acne vulgaris. J Clin Aesthet Dermatol. 2014;7(2 Suppl):S4–S22.
- Del Rosso J. Face to face with oral isotretinoin: a closer look at the spectrum of therapeutic outcomes and why some patients need repeated courses. J Clin Aesthet Dermatol. 2012;5(11): 17–24.
- Azoulay L, Oraichi D, Berard A. Isotretinoin therapy and the incidence of acne relapse: a nested case-control study. Br J Dermatol. 2007;157(6): 1240–1248.
- Haryati I, Jacinto S. Profile of acne patients in the Philippines requiring a second course of oral isotretinoin. Int J Dermatol. 2005;44(12): 999–1001.
- Webster G, Leyden J, Gross J. Comparative pharmacokinetic profiles of a novel isotretinoin treatment (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, 4-treatment, crossover study. J Am Acad Dermatol. 2013;69(5):762–767.
- ABSORICA® (isotretinoin) capsules. Prescribing information. Gurugram, India: Ranbaxy Laboratories Inc.; 2015.
- Webster G, Leyden J, Gross J. Results of a phase III, double-blind, randomized, parallel-group, non-inferiority study evaluating the safety and efficacy of isotretinoin-Lidose in patients with severe recalcitrant nodular acne. J Drugs Dermatol. 2014;13(6):665–670.
- Rampersaud G, Pereira M, Girard B, et al. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc. 2005;105(5): 743–760.
- Deshmukh-Taskar P, Nicklas T, Radcliffe J, et al. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic Examination Survey (NHANES): 1999–2006. Public Health Nutr. 2012;16(11):2073–2082.
- Zaenglein A, Segal J, Darby C, Del Rosso J. Lidose-isotretioin capsules administered without food improve quality of life in patients with severe recalcitrant nodular acne: an open-label, single-arm, phase 4 study. Submitted to J Am Acad Dermatol. 2019.
- Quereux G, Volteau C, N’Guyen J, Dreno B. Prospective study of risk factors of relapse after treatment of acne with oral isotretinoin. Dermatology. 2006;212(2):168–176.
- Chivot M, Midoun H. Isotretinoin and acne—a study of relapses. Dermatologica. 1990;180(4):240–243.
- Layton A, Knaggs H, Taylor J, Cunliffe W. Isotretinoin for acne vulgaris—10 years later: a safe and successful treatment. Br J Dermatol. 1993;129(3):292–296.

