 J Clin Aesthet Dermatol. 2022;15(12):16-18.
J Clin Aesthet Dermatol. 2022;15(12):16-18.
by Noureddine Litaiem, MD; Azza Ghannem, MD; Linda Bel Hadj Kacem, MD; Soumaya Rammeh, MD; and Faten Zeglaoui, MD
All authors are with the Faculty of Medicine of Tunis at University of Tunis El Manar in Tunis, Tunisia. Drs. Litaiem, Ghannem, and Zeglaoui are also with the Department of Dermatology at Charles Nicolle Hospital in Tunis, Tunisia. Drs. Kacem and Rammeh are additionally with the Department of Pathology at Charles Nicolle Hospital in Tunis, Tunisia.
FUNDING. No funding was provided for this study
DISCLOSURES. The authors report no conflicts of interest relevant to the content of this article.
Dear Editor:
Cutaneous pseudolymphoma (CPL) is histologically defined by benign reactive lymphoid proliferations simulating cutaneous lymphomas.1 We report herein two cases of disseminated and delayed CPL due to aluminum-containing allergen immunotherapy indicated for allergic asthma.
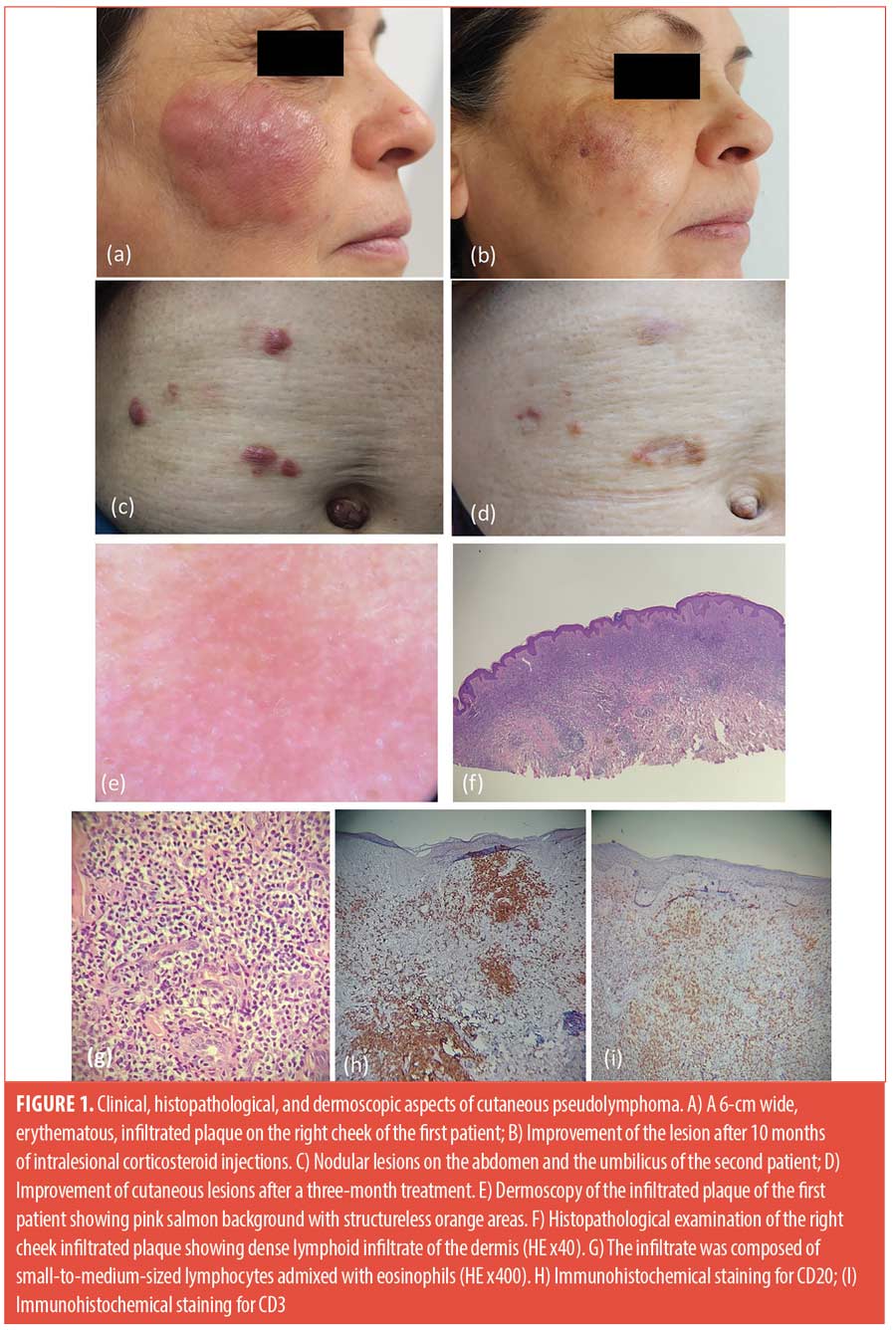
The first patient was a 62-year-old woman who reported the appearance, 20 years earlier, of erythematous nodules on both arms, corresponding to sites of hypersensitizing allergen injections received to treat asthma. The skin biopsy, then performed, was consistent with the diagnosis of cutaneous pseudolymphoma. The lesions resolved spontaneously after discontinuation of hyposensitization vaccines. She presented to our department with pruritic erythematous plaques on the cheeks of one-year duration (Figure 1A). Physical examination revealed bilateral infiltrated plaques on both cheeks of 6cm and 2cm. Dermoscopy showed pink‑orange background color with perifollicular and follicular whitish spots (Figure 1E). Histopathological examination showed dense and nodular lymphoid infiltrate in the dermis with small size lymphocytes and eosinophils (Figure 1F and 1G). Immunohistochemical study revealed CD20-positive cells admixed with CD3-positive cells (Figure 1H, 1I). The diagnosis of recurrent cutaneous pseudolymphoma was established based on clinicopathological correlation. The patient received monthly intralesional corticosteroid injections, using a ¼ dilution of betamethasone dipropionate 5mg/mL with a 0.9% saline solution. Both clinical and dermoscopic remission was obtained (Figure 1B).
The second patient was a 55-year-old female patient with a past medical history of allergic rhinitis and allergic asthma treated with hypersensitizing airborne allergen vaccines. Twenty-two years later, she presented with four firm and itchy nodules on the abdomen and the umbilic (Figure 1C). Histopathological examination showed similar dense dermal infiltration of small to medium‑sized lymphocytes supporting the diagnosis of cutaneous pseudolymphoma. The patient was started on corticosteroid intralesional injections with good response (Figure 1D).
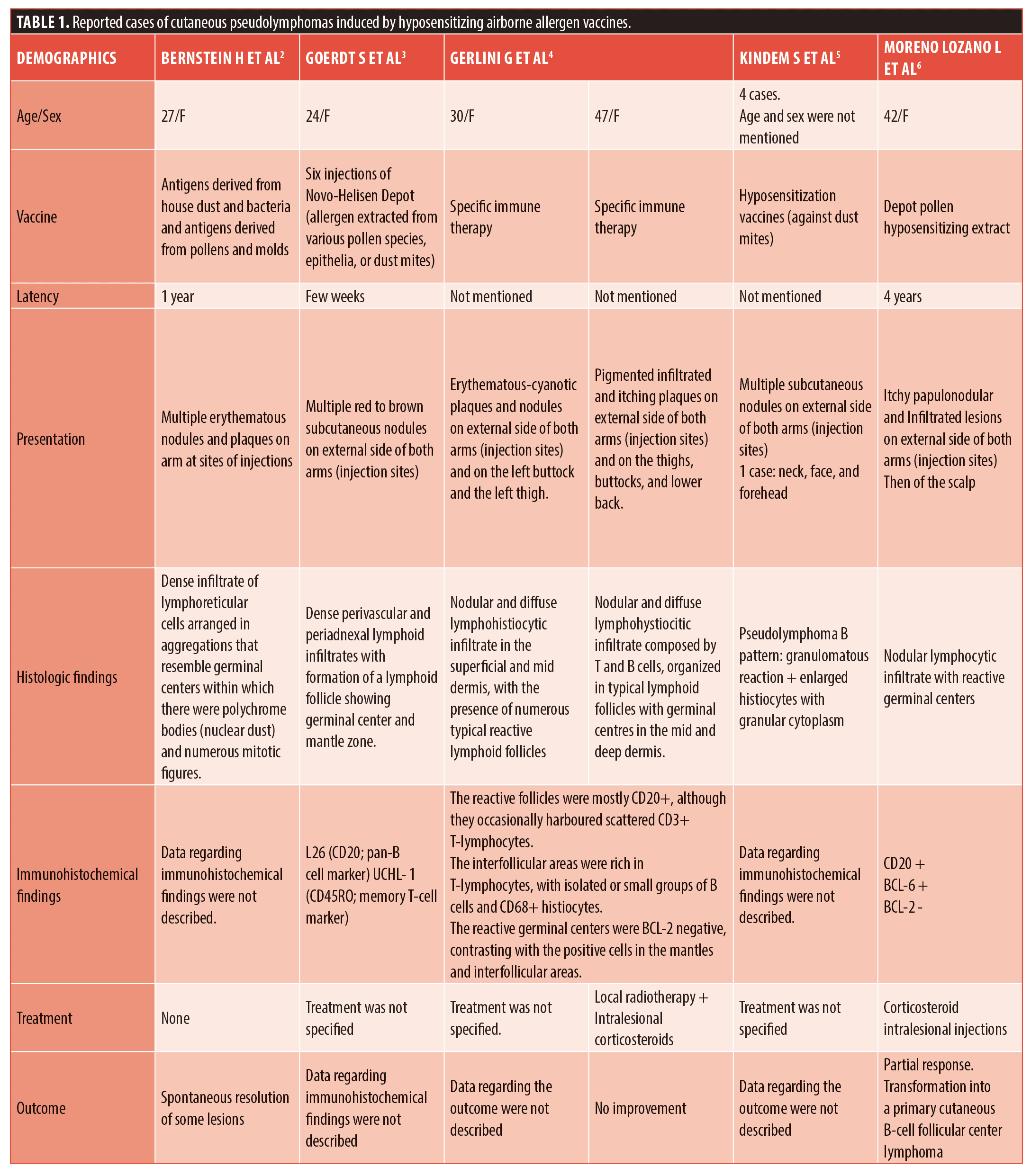
Multiple causes of CPL have been identified including infections, drugs, and foreign agents.1 However, only a few cases of CPL associated with allergen injections for hyposensitization have been described (Table 1).2–6 CPL is reported to appear after a delay of 1 to 4 years (Table 1). One possible hypothesis is that continuous chronic antigenic stimulation can induce activation of antigen-specific B cell clones and lead to disseminated lesions.4 In our patient, CPL developed several years after the culprit allergen immunotherapy. Furthermore, the occurrence of CPL lesions, far from the injection sites, has been previously reported in only two cases.4,5
The main differential diagnosis is primary cutaneous B cell lymphoma (PCBCL). The possible occurrence of vaccine-induced PCBCL and the risk of progression of vaccine-induced CPL into PCBCL, make the diagnosis even more challenging.6 Histologically, it is difficult to differentiate PCBCL from CPL. Dense mixed infiltrate arising from the papillary dermis with prominent germinal centers and prominent vasculature are characteristic of pseudolymphomas.2 The detection of B-cell clonality favors the diagnosis of PCBCL, but clonal B-cell proliferation was also described in nodular CPL1. Thus, the diagnosis is based on careful clinical, histopathological, and immunohistochemical correlation. In our patients, histopathological findings and benign clinical course were consistent with the diagnosis of CPL. Intralesional corticosteroid injections showed good efficacy and safety.
References
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma—A review on the spectrum and a proposal for a new classification. J Cutan Pathol. 2020;47(1):76–97.
- Bernstein H, Shupack J, Ackerman AB. Cutaneous Pseudolymphoma Resulting From Antigen Injections. Arch Dermatol. 1974; 110:756–757.
- Goerdt S, Spieker T, Wölffer LU, et al. Multiple cutaneous B-cell pseudolymphomas after allergen injections. J Am Acad Dermatol. 1996;34(6):1072–1074.
- Gerlini G, Mori M, Massi D, et al. Specific immune therapy-related cutaneous B-cell pseudolymphoma with following dissemination. J Eur Acad Dermatol Venereol. 2003;17:208–212.
- Kindem S, Llombart B, Llorca D, et al. Subcutaneous nodules in patients with hyposensitization to aluminium-containing vaccination. J Am Acad Dermatol. 2012;66(4):AB82.
- Moreno Lozano L, Sánchez Caminero M, García Arpa M, et al. Cutaneous B-cell lymphoma at the injection site of airborne allergen immunotherapy followed by cutaneous metastasis. J Investig Allergy Clin Immunol. 2021;32(4).

