J Clin Aesthet Dermatol. 2023;16(8):20–26.
J Clin Aesthet Dermatol. 2023;16(8):20–26.
“Skinfluencers” Versus Dermatologists as Creators of the Top Dermatology-related Videos on TikTok
Dear Editor:
Social media platforms allow physicians and laypeople alike to disseminate information quickly and effectively. Over 80 percent of people seek medical information online and 90 percent of Gen Zs often turn to social media for medical advice.1,2 TikTok is the world’s fastest-growing social media platform with over one billion active users. The application allows users to create short videos that range up to one minute in length. Users that generate video content on the platform are known as creators, and they can respond directly to videos, questions, and comments from other users. TikTok uses an artificial intelligence algorithm that provides a personalized feed called the “For You” page. This individualization prompts users to spend more time consuming content on the platform.3 Some users may view more medical advice-related videos than others. Previous studies have analyzed sponsored content among dermatologists on TikTok and one study in 2020 analyzed top dermatology-related posts on TikTok.4,5 In this study, we sought to understand the credentials, aims, and promotions of creators of the top skincare-related TikTok videos in 2022.
The keywords “skincare” and “black skincare” were queried on TikTok in August 2022. The top 50 posts for each search term and the profiles of the creators of these posts were analyzed. Non-English posts were excluded. Data on the topic and purpose of each post were recorded, as well as the creator’s profession, the number of followers, likes, total posts (between May-July 2022), and sponsored posts. Brand names of each promoted product were also recorded. The Federal Trade Commission guidelines were used to define sponsored posts.6
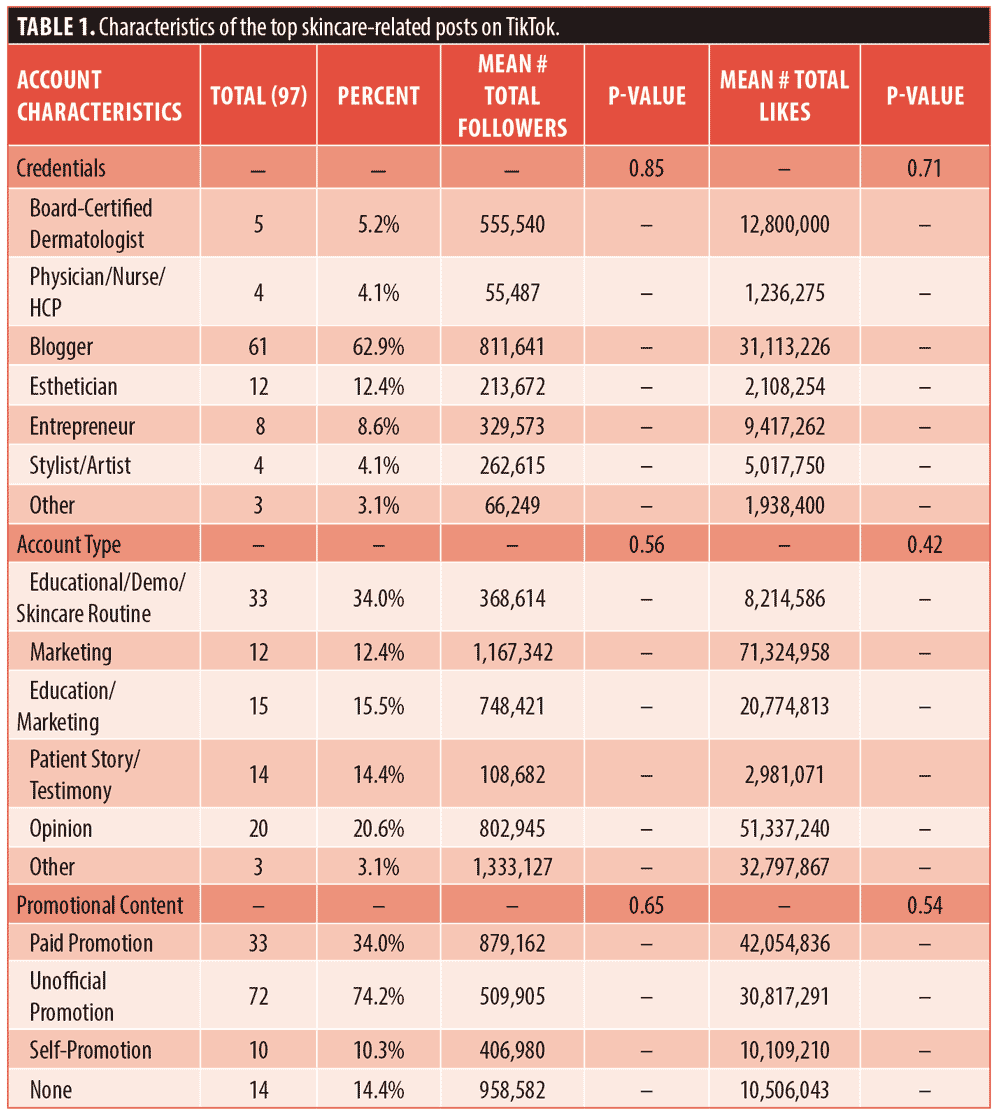
Three posts were excluded as they contained non-English videos, leaving 97 posts in our analysis. Most of these posts were educational (34%) or opinion-based (20.6%), but only 5 out of 97 videos (5.2%) were posted by board-certified dermatologists. Nearly 63 percent of top videos were posted by bloggers. Bloggers’ posts had 2.4 times more likes than those of board-certified dermatologists (31,113,226 vs. 811,641), in addition to having 1.5 times more followers (12,800,000 vs. 555,540) on average (Table 1). Although these differences were not statistically significant, they are sizable and result in varying levels of engagement. and audience size. Furthermore, only 34 percent of creators in our search listed products as paid promotions, while 74.2 percent unofficially promoted products without naming sponsorship.
The results of our study coincide with those of a 2020 study by Ngugen, et al. that sought to inform dermatologists to be aware of skincare trends that patients are exposed to online. However, two years later, misinformation and a lack of accredited information on social media are more present than ever. We hope that the results of our study serve to increase patients’ awareness in healthcare decision-making and highlights the need for dermatologists to promote high-quality evidence-based information for skincare on TikTok and other social media platforms. Like it or not, social media is a central place where patients obtain health information, and it is here to stay.
With regard,
Shivali Devjani, MS; Ogechi Ezemma, BA; Sophia Fruechte, BS; Nathalie Ly, BS; Ora Raymond, BA;
Kristen Kelley, BA; Ronda Farah, MD; and Maryanne Senna, MD
Affiliations. Ms. Devjani, Dr. Senna, Ms. Ezemma, and Ms. Kelley are with the Department of Dermatology at Lahey Hospital and Medical Center in Burlington, Massachusetts. Dr. Farah, Ms. Fruechte, Ms. Ly, and Ms. Raymond are with the Department of Dermatology at the University of Minnesota in Minneapolis, Minnesota. Additionally, Dr. Senna is an assistant professor at Harvard Medical School in Boston, Massachusetts.
Funding. No funding was provided for this article.
Disclosures. The authors report no conflicts of interest relevant to the content of this article.
References
- Gantenbein L, Navarini AA, Maul LV, et al. Internet and social media use in dermatology patients: Search behavior and impact on patient-physician relationship. Dermatol Ther. 2020;33(6):e14098.
- Zheng DX, Mulligan KM, Scott JF. TikTok and dermatology: An opportunity for public health engagement. J Am Acad Dermatol. 2021;85(1):e25–e26.
- Bhandari A and Bimo S. (2022). Why’s Everyone on TikTok Now? The Algorithmized Self and the Future of Self-Making on Social Media. Social Media + Society. 8(1).
- Ranpariya V, Chu B, Fathy R, et al. Dermatology without dermatologists? Analyzing Instagram influencers with dermatology-related hashtags. J Am Acad Dermatol. 2020 Dec;83(6):1840–1842.
- Nguyen M, Youssef R, Kwon A, et al. Dermatology on TikTok: Analysis of content and creators. Int J Womens Dermatol. 2021 May 12;7(4):488-489.
- Disclosures 101 for Social Media Influencers. 2021.
Individualized Algorithm of Systemic Treatment in Moderate to Severe Atopic Dermatitis
Dear Editor:
We have reviewed the manuscript “Atopic Dermatitis (AD) Therapeutic Algorithm of JAK-Inhibitors (iJAK) and IL-4/IL-13 Antagonists Utilizing Patient Profiles and Drug Efficacy” published in the Journal of Clinical and Aesthetic Dermatology in the month of February.1
We thank the authors for their contribution, as we were lacking a review of all recently approved iJAK and biologic drugs for AD depending on patients profile, having no algorithm to assist dermatologists in choosing the most appropriate one. However, we would like to add some observations on the review and algorithm that has been proposed.
There is still a considerable lack of data, and plenty of ongoing clinical trials that will keep the scenario changing in the future years. This is, any algorithm will be liable to change in the short term, since some drugs may have not achieved yet approval for the treatment of AD (e.g. lebrikizumab) or comorbidities (e.g. iJAKs for prurigo nodularis). In addition, differences between countries do appear, regarding governmental agencies (FDA, EMA) approvals (e.g. baricitinib is not approved for AD in UE), and thus restrict available treatment options in some countries. Consequently, we hereby propose a modified review (Table 1) and algorithm (Figure 1) of all available treatments for AD, pointing out some details and including all available data as accurately as we were able to summarize.
First of all, prior to receiving any of these new advanced therapies, most patients require to be treated for their moderate or severe AD with any of the following: systemic steroids, systemic immunosuppressants (e.g. cyclosporine A) or methotrexate. Systemic steroids provide a rapid but unsustained improvement, while methotrexate’s use, though it may be beneficial, is off-label. Cyclosporine A is an approved and effective therapy, yet it should not be maintained over a year’s time. It is mandatory to know that both systemic steroids (Category B) and cyclosporine (Category C) are not prohibited during pregnancy, and it is necessary to always balance their benefits over the risk of their use. Meanwhile, methotrexate is strictly prohibited (Category X). As a result, only some of these systemic therapies should also be regarded in a treatment algorithm prior to the use of any advanced therapy, in any kind of patient (e.g. in Spain, it is compulsory to first treat a patient with cyclosporine to get access to another advanced therapy).
Secondly, it is well known that AD is often associated with asthma, allergic rhinoconjunctivitis and nasal polyposis, eosinophilic esophagitis or phenotypically can also appear as prurigo nodularis. For the treatment of all of these, dupilumab has been proved to be effective (Table 1), as well as in AD1. Thus, all mentioned AD related comorbidities, if present, should be taken into account when making a decision, being dupilumab so far the leading option. While tralokinumab failed to demonstrate efficacy for asthma in clinical trials, other biologic drugs have not been studied for these indications, so their utility for these indications cannot be denied. Likewise, patients with concomitant inflammatory diseases such as rheumatoid or psoriatic arthritis, ankylosing spondylitis, ulcerative colitis or alopecia areata can benefit from treatment with specific iJAKs, as some of them are already licensed for some of these indications.3–5 It is true in the case of an adult without any cardiovascular risk factor, severe renal or hepatic impairment, history of malignancy or cytopenia, all available treatments could be an option; and AD patients are mostly young, without any accompanying diseases and unmedicated. As said before, comorbidities should be kept in mind.
Additionally, in the case where all treatment options could be used, the dermatologist’s criteria and the patient’s characteristics and needs will lead to the choice of the drug. For example, in the case when a rapid response or pruritus relief would be needed, an iJAK could be a preferred option;3–5 while to avoid analytical monitoring or in the presence of any risk factors, anti-IL4/IL13 or anti-IL13 drugs would be the leading option.2,6 In our opinion, needle phobia, though it may be taken into account, might not be relevant enough to be included in a treatment algorithm.
Lastly, the proposed algorithm obviously excludes the use of iJAKs during pregnancy. Nevertheless, these can be used safely prior to conception, as long as a washout period of one week for baricitinib and four weeks for upadacitinib and abrocitinib is observed.3–5 Additionally, pregnancy in women under 18 years old is an scenario that is also plausible and must be beheld. Dupilumab, though it has no assigned category for pregnancy, has proved to be safe in some reported cases, while the same profile of security for tralokinumab during pregnancy could be assumed.2,6
We would also like to note that it might be early and risky to talk about efficacy when comparing different clinical trials, as it is well known that there are always methodological differences between them, regarding e.g. length and follow-up during studies, responding criteria or characteristics of the patients included. Indeed, only two head-to-head clinical trials have been carried out so far: JARE DARE (abrocitinib vs dupilumab) and HEADS UP (upadacitinib vs dupilumab). Therefore, we have little comparative data of efficacy regarding these new treatments, and a lack of efficacy and safety data for most of them (except for dupilumab) in real clinical practice.
As a result, we hereby propose a modified summary and algorithm (Table 1 and Figure 1) to assist dermatologists in the choice of an AD treatment, taking into account every aspect highlighted by the authors of the annotated manuscript, as well as our remarks discussed above.
With regard,
Miguel Recio-Monescillo, MD; Araceli Sánchez Gilo, PhD; and Rubén García-Castro, PhD
Affiliations. Drs. García-Castro and Recio-Monescillo are with the Dermatology Department at Hospital Universitario Fundación Jiménez Díaz in Madrid, Spain. Dr. Sánchez Gilo is with the Dermatology Department at Hospital Universitario Rey Juan Carlos in Móstoles, Madrid, Spain.
Funding. No funding was provided for this article.
Disclosures. The authors report no conflicts of interest relevant to the content of this article.
References
- Haque A, Ahmed F, Amanullah A. Atopic Dermatitis Therapeutic Algorithm of JAK-Inhibitors and IL-4/IL-13 Antagonists Utilizing Patient Profiles and Drug Efficacy. J Clin Aesthet Dermatol. 2023;16(2):11-13.
- Sanofi-Aventis. 2017. Dupixent 300 mg solution for injection in pre-filled syringe: Dupixent INN-Dupilumab. Deutschland.
- AbbVie. 2022. Rinvoq 15/30/45 mg extended release pills: Rinvoq INN-Upadicitinib. United States of America.
- Pfizer. 2022. Cibinqo 50/100/200 mg film-coated pills: Cibinqo INN-Abrocitinib. Belgium.
- Eli Lilly. 2021. Olumiant 2/4 mg film-coated pills: Olumiant INN-Baricitinib. Netherlands.
- LEO Pharma. 2022. Adtralza 150 mg solution for injection in pre-filled siringe: Adtralza INN-Tra