 J Clin Aesthet Dermatol. 2022;15(3):53–56.
J Clin Aesthet Dermatol. 2022;15(3):53–56.
by Lehavit Akerman, MD; Daniel Mimouni, MD; Adi Nosrati, MD;
Daniel Hilewitz, BSc; and Efrat Solomon-Cohen, MD, MOcch
All authors are with the Sackler Faculty of Medicine at Tel Aviv University in Tel Aviv, Israel. Drs. Mimouni, Nosrati, and Solomon-Cohen are also with the Division of Dermatology at Rabin Medical Center in Petah-Tiqva, Israel.
FUNDING: The authors report no conflicts of interest relevant to the content of this article.
DISCLOSURES: No funding was provided for this article.
ABSTRACT: Background. Postacne facial scars are often associated with significant patient distress. Energy-based devices, including non-ablative lasers, are commonly used for the treatment of postacne scarring. There is relatively limited data regarding the combination of non-ablative lasers with hyaluronic acid injections for postacne scarring.
Objective. We aimed to evaluate the efficacy of a non-ablative 1,540-nm erbium:glass laser combined with a hyaluronic acid injectable for the treatment of postacne scars.
Methods. This was a retrospective analysis of 12 patients who underwent the full treatment protocol. A before and after blinded clinical evaluation was performed independently by two dermatologists and graded on a scale from 0 (indicating a worsening of scarring) to 4 (indicating a 76–100% improvement in scarring). Pain perception, adverse effects, and patient satisfaction were evaluated.
Results. A mean correct blinded before and after evaluation by two dermatologists was 96 percent. Patients demonstrated mild to moderate improvement as assessed by a quartile scale of improvement (25–50%). Mild transient pain was reported by most patients. The satisfaction level of the patients was high (4 out of 5).
Limitations. The limitations of our study include the small cohort, retrospective design, and lack of a histological correlation
Conclusion: Our results suggest that this combination treatment using 1,540-nm fractional erbium:glass laser and hyaluronic acid injections is both safe and effective for patients with postacne facial scars.
Keywords: Erbium:glass laser, hyaluronic acid, acne scars, non-ablative laser, fractional laser, soft tissue fillers
Acne scarring is a common detriment to patients’ self-esteem and quality of life. There are numerous treatments for postacne scarring, but both patients and physicians constantly pursue more safe and effective treatment modalities that provide minimal downtime and a safer side effect profile.1
Current therapeutic modalities for the treatment of postacne scars include, among others: chemical peels, dermabrasion, microneedling, filler injections, surgical procedures, and retinoids.2–7 Energy-based devices (intense pulsed light [IPL], radiofrequency and lasers) are commonly used for this purpose in recent years. The ablative lasers (the 10,600-nm CO2 laser and the 2940-nm Er:YAG laser) have significant adverse effects, including dyspigmentation, erythema, edema, and scarring, as well as significant post-procedure downtime, all which make them less desireable options for patients and physicians.1,3–8 Non-ablative lasers indeed have a better side effect profile and a shorter post-procedure downtime; however, their efficacy in the treatment of postacne scars is heterogenous.2–7
Laser fractionation was developed for both ablative and non-ablative lasers, referring to the formation of islands of spared skin adjacent to the treatment zones. The laser beam is manipulated via a defractive lens, in which multiple microscopic laser beams are delivered into microscopic treatment zones, sparing the intervening areas.8–17 This method of treatment carries minimal epidermal damage, and therefore results in fewer adverse effects and a shorter downtime. Fractionation using the 1,540-nm erbium:glass laser has previously shown to induce collagen production and stimulate dermal remodeling and healing, potentially explaining its effect in skin rejuvenation and in the improvement of postacne scars.8–17
Soft-tissue fillers have been used for a myriad of facial contouring and augmentation purposes, as well as for facial postacne scars, however with limited efficacy.18–22 These soft-tissue fillers include calcium-hydroxylapatite (CaHA), polymethylmethacrylate microspheres in collagen (PMMA-collagen), poly-L-lactic acid injection (PLLA) and hyaluronic acid (HA) gels, among others. There is relatively limited data in the literature regarding the combination of non-ablative lasers with hyaluronic acid injectables for the treatment of postacne scars. We aimed to evaluate the efficacy of a non-ablative 1,540-nm erbium:glass laser combined with a specific highly purified hyaluronic acid injectable for the treatment of postacne scars.
Methods
Patients. This was a retrospective cohort study. We included patients 18 years or older who underwent treatment in our clinic using the 1,540-nm fractional erbium:glass laser in combination with a hyaluronic acid injectable due to postacne facial scars.
The exclusion criteria included prior treatment with an ablative laser up to one year prior to the study treatment, prior treatment with any laser up to three months prior to the study treatment, application of any topical treatment up to 14 days prior to the study treatment, pregnancy, and/or a significant systemic illness.
Standardized high-resolution digital photography of the treatment area was performed for each patient at baseline, before each treatment, and at the three-month follow-up visit. All patients gave their written informed consent for the treatment in adherence with acceptable ethical guidelines.
Treatment. The protocol consisted of treatment with a non-ablative 1,540-nm erbium:glass laser (ClearSkin Pro, Alma Lasers, GmbH, Nuremberg, Germany) every four consecutive weeks, with a total of four treatment sessions. The first and third treatment sessions were immediately followed by administering a highly purified hyaluronic acid injectable into the postacne facial scars (Profhilo®, IBSA Farmaceutici Italia Srl, Lodi, Italy, Europe). This is a stabilized hybrid HA complex produced using a patented thermal treatment technology. It consists of combining 32mg of high molecular weight HA (between 1100 and 1400 KDa) and 32mg of low molecular weight HA (between 80 and 100 KDa) in a 2-mL syringe.
The treatment area was disinfected prior to each treatment. Protective eyewear was used during treatments. Laser settings included a spot size of 11*11 mm with a fractionation of 49 pixels, fluence of 2,500 to 3,000mJ/pulse (51–61mJ per pixel). Two stacked pulses were emitted at a rate of 1Hz for 2 to 4 passes per treatment session (2,500mJ/pulse was used for the initial treatment, and increased to 3,000mJ/pulse according to the patient’s response). Each treatment session took approximately 20 to 30 minutes. Settings were pre-adjusted according to the tolerability of the patient.
Outcome measures. Patients were followed for 1 to 3 months after the last treatment session. A before/after blinded clinical evaluation was performed independently by two dermatologists, and thereafter graded on a scale from 0 (indicating a worsening of scarring) to 4 (indicating a 76–100% improvement in scarring).
Pain perception was assessed by the patient using a 0 to 10 visual analog scale (VAS), where 0=none and 10=severe. Adverse effects were estimated by the treating physician, as follows: erythema, edema, blistering, flaking, dryness, dyspigmentation and/or pruritus, using a 0 to 3 assessment scale during each treatment and follow-up visits (0= none, 1 = mild, 2 = moderate, 3 = severe). Any additional adverse events were recorded. Patient satisfaction was assessed at the posttreatment follow-up visit at Month 3, graded on a scale of 1 to 5 (1= not satisfied, 5 = very satisfied).
Results
Twelve patients (11 female, 1 male) were included. Ages ranged from 32 to 57 years (mean 44 ± 5.2). Fitzpatrick Skin Types were I to III. All patients had mild to moderate postacne scars at baseline and were seeking to aesthetically improve their appearance. All 12 patients completed both treatment and follow-up period.
The blinded before/after analysis by two dermatologists (D.M, A.N) yielded a correct identification of 100 percent and 92 percent respectively (mean 96%).
Improvement in overall scar appearance occurred gradually over the course of treatment sessions. Following completion of treatment, all patients demonstrated a mild to moderate (25–50%) improvement as assessed by the quartile scale: mean of 1.63 points of improvement in overall scar appearance by evaluator 1 (SD=0.5), and a mean of 2.13 points in overall scar appearance by evaluator 2 (SD=0.65) (mean 1.88 points, SD=0.57). Interobserver agreement for overall improvement was 80 percent. Figures 1 and 2 portray representative cases. Patient-reported satisfaction ranged from 3 to 5 (mean 4 ± 1.06) at the three-month follow up visit. All adverse effects were mild and well-tolerated by patients and included mild transient erythema, mild edema, and mild to moderate pain (mean VAS 3.8 ± 1.5). No vesiculation or scaling were noted. No adverse effects were noted at the follow-up visits. No downtime was reported following treatment by any of the patients.
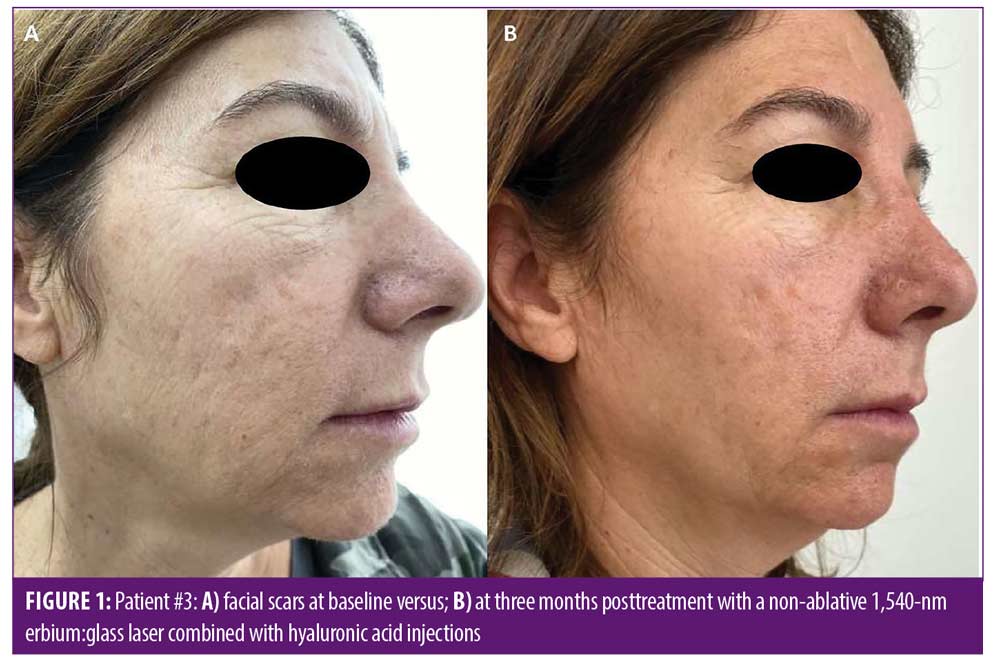
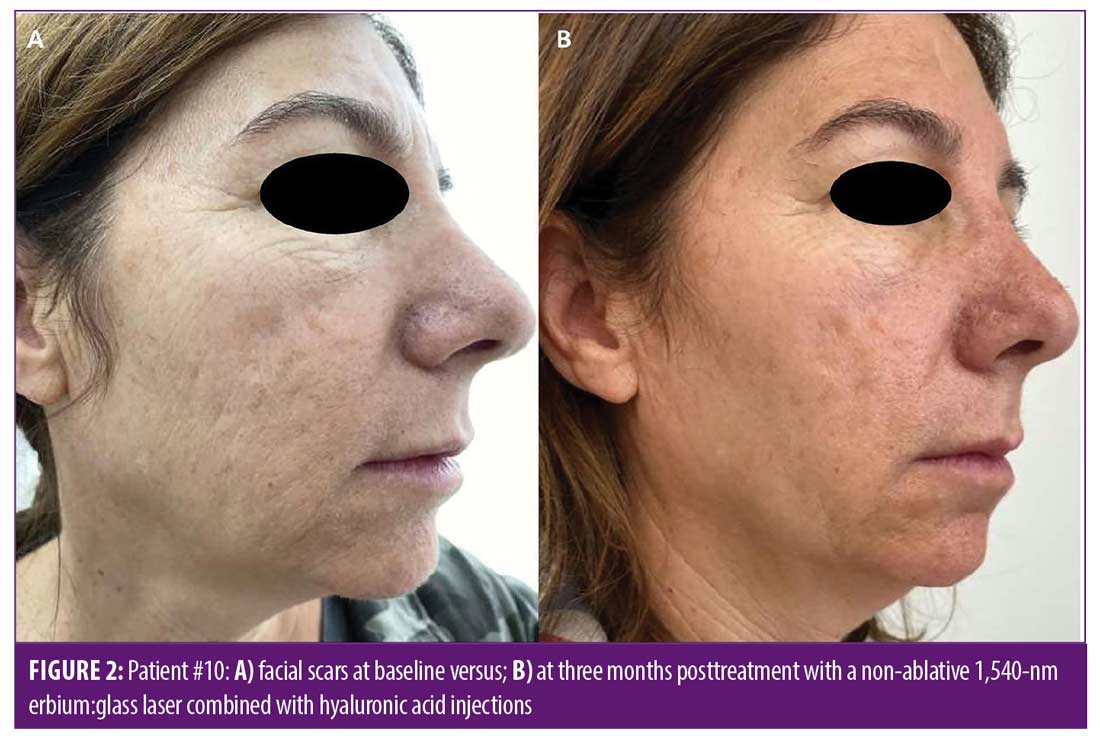
Discussion
Postacne scars are aesthetically challenging and impose a significant burden on the self-esteem of affected patients. Various treatment modalities are of current use for the treatment of postacne scars, with growing trends towards the non-ablative lasers, in combination with other modalities, such as filler injections, as previously mentioned.1–6, 18–22 Fractional non-ablative lasers are considered safer modalities compared to the ablative ones as they allow less pain, safer side effect profile and a shorter downtime, as demonstrated in previous studues.8–17
We presented a novel combination treatment protocol, which overall demonstrated an average mild to moderate improvement in postacne facial scars appearance, with high patient satisfaction three months after treatment and a good safety profile.
The non-ablative 1,540-nm fractional erbium:glass laser probe utilized in our study can deliver a fluence of up to 3,000 mJ/pulse, which results in significant coagulation and increased efficacy.2–4,17 Additionally, treatment duration is relatively short, causes no epidermal injury, and therefore allows minimal to no downtime, all which make this treatment attractive for both patients and physicians. Much like other non-ablative modalities, repeated multiple sessions are required.2–4
Hyaluronic acid injectables have been used for biomedical applications, including for osteoarthritis treatment, tissue augmentation, and ocular surgery, and as scaffold for tissue engineering.18,23 In particular, it has been well established that HA is associated with tissue repair, being involved in cell proliferation and migration, partly due to its hydrophilic and highly osmotic features.18,22–25 In addition, it has been shown to propagate the inflammatory response through the induction of macrophages and chemokine response, thus contributing to the healing process.23–28 The use of a highly purified hyaluronic acid injectable, has been shown to have an effect in wound healing in in-vitro studies, promoting fibroblast and keratinocyte proliferation and migration.25–28 We believe that this contributes to the synergistic effect of the HA injectable and the non-ablative laser used in our presented protocol.
Limitations. Our study holds several limitations, including a small cohort, a retrospective nature, and lack of a control group. A histological correlation to support our hypothesis is lacking, and remains to be investigated in the future. However, the clinical response and improvement observed in all of our patients is noticeable, the high patient satisfaction and lack of side effects, and no reported downtime, yields promise for further research.
Conclusion
In this study, we demonstrated beneficial outcomes using a new high fluence 1,540-nm fractional erbium:glass laser probe in combination with a highly purified hyaluronic acid injectable, for the treatment of facial postacne scars. Further utilization of this treatment protocol in future larger cohorts is warranted.
References
- Zaleski-Larsen LA, Fabi SG, McGraw T, et al. Acne Scar Treatment: A Multimodality Approach Tailored to Scar Type. Dermatol Surg. 2016 May;42 Suppl 2:S139-49.
- Politi Y, Levi A, Lapidoth M. et al. Integrated Cooling-Vacuum-Assisted Non-Fractional 1,540 nm Erbium:Glass Laser is Effective in Treating Acne Scars. J Drugs Dermatol. 2016 Nov 1;15(11):1359-1363.
- Politi Y, Levi A, Enk CD, et al. Integrated cooling-vacuum-assisted 1,540-nm erbium:glass laser is effective in treating mild-to-moderate acne vulgaris. Lasers Med Sci. 2015 Dec;30(9): 2389–2393.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008 May;58(5):719-37; quiz 738-40.
- Akaishi S, Koike S, Dohi T, et al. Nd:YAG Laser Treatment of Keloids and Hypertrophic Scars. Eplasty. 2012;12:e1.
- Pathak A, Mohan R, Rohrich RJ. Chemical Peels: Role of Chemical Peels in Facial Rejuvenation Today. Plast Reconstr Surg. 2020 Jan;145(1):58e-66e.
- Brightman LA, Brauer JA, Anolik R, et al. Ablative and fractional ablative lasers. Dermatol Clin. 2009 Oct;27(4):479-89, vi-vii.
- Lipozen?i? J, Mokos ZB. Will nonablative rejuvenation replace ablative lasers? Facts and controversies. Clin Dermatol. 2013 Nov-Dec;31(6):718-24.
- Tidwell WJ, Owen CE, Kulp-Shorten C, et al. Fractionated Er:YAG laser versus fully ablative Er:YAG laser for scar revision: Results of a split scar, double blinded, prospective trial. Lasers Surg Med. 2016;48(9):837-843.
- Al-Dhalimi M, Jaber A. Treatment of atrophic facial acne scars with fractional Er:Yag laser. J Cosmet Laser Ther. 2015;17(4):184–188.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010 Mar;36(3):299-306.
- H, Al Anwer M. Fractional versus ablative erbium:yttrium-aluminum-garnet laser resurfacing for facial rejuvenation: an objective evaluation. J Am Acad Dermatol. 2013 Jan;68(1):103–112.
- Borges J, Araújo L, Cuzzi T, et al. Fractional Laser Resurfacing Treats Photoaging by Promoting Neocollagenesis and Cutaneous Edema. J Clin Aesthet Dermatol. 2020 Jan;13(1):22–27.
- Carniol PJ, Hamilton MM, Carniol ET. Current Status of Fractional Laser Resurfacing. JAMA Facial Plast Surg. 2015 Sep-Oct;17(5):360-6.
- de Sica RC, Rodrigues CJ, Maria DA, et al. Study of 1550nm Erbium Glass Laser Fractional non-ablative treatment of photoaging: Comparative clinical effects, histopathology, electron microscopy and immunohistochemistry. J Cosmet Laser Ther. 2016 May 25:1-36.
- de Angelis F, Kolesnikova L, Renato F, et al. Fractional nonablative 1,540-nm laser treatment of striae distensae in Fitzpatrick skin types II to IV: clinical and histological results. Aesthet Surg J. 2011 May;31(4):411-9
- Solomon-Cohen, E, Lapidoth, M, Mimouni, D, et al. 1,540-nm fractional erbium: Glass laser is a safe and effective modality for nonablative facial rejuvenation. J Cosmet Dermatol. 2021;00:1–5.
- Mckee D, Remington K, Swift A, et al. Effective Rejuvenation with Hyaluronic Acid Fillers: Current Advanced Concepts. Plast Reconstr Surg. 2019 Jun;143(6):1277e-1289e.
- Forbat E, Ali FR, Al-Niaimi F. The role of fillers in the management of acne scars. Clin Exp Dermatol. 2017 Jun;42(4):374-380.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014 Jul;71(1):77-83.
- Koren A, Isman G, Cohen S, et al. Efficacy of a combination of diluted calcium hydroxylapatite-based filler and an energy-based device for the treatment of facial atrophic acne scars. Clin Exp Dermatol. 2019 Jul;44(5):e171-e176.
- Dierickx C, Larsson MK, Blomster S. Effectiveness and Safety of Acne Scar Treatment with Nonanimal Stabilized Hyaluronic Acid Gel. Dermatol Surg. 2018 Nov;44 Suppl 1:S10-S18.
- Oh JH, Kim YK, Jung JY, et al. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci. 2011; 62(3):192
- Sparavigna A, Tenconi B. Efficacy and tolerance of an injectable medical device containing stable hybrid cooperative complexes of high- and low-molecular-weight hyaluronic acid: a monocentric 16 weeks open-label evaluation. Clin Cosmet Investig Dermatol. 2016;9:297-305.
- Tagami H. Quantitative measurements of water concentration of the stratum corneum in vivo by highfrequency current. Acta Derm Venereol Suppl (Stockh). 1994;185:29-33.
- La Gatta A, Schiraldi C, Papa A, et al. Comparative analysis of commercial dermal fillers based on crosslinked hyaluronan: Physical characterization and in vitro enzymatic degradation. Polym Degrad Stab. 2011; 96(4):630
- Bellas E, Seiberg M, Garlick J, et al. In vitro 3D fullthickness skin-equivalent tissue model using silk and collagen biomaterials. Macromol Biosci. 2012; 12(12):1627
- Laurino C, Palmieri B, Coacci A. Efficacy, Safety, and Tolerance of a New Injection Technique for High- and Low-Molecular-Weight Hyaluronic Acid Hybrid Complexes. Eplasty. 2015 Oct 8;15:e46.

