 J Clin Aesthet Dermatol. 2022;15(12):49–51.
J Clin Aesthet Dermatol. 2022;15(12):49–51.
by Ahmet M. Yildirim, MEd; Noelle Lemons, MD; Ahmed Yousaf, MD; and Zachary A. Zinn, MD
All authors are with the Department of Dermatology at West Virginia University in Morgantown, West Virginia.
ABSTRACT: Background. Isotretinoin, the gold standard treatment for nodulocystic acne vulgaris, is contraindicated in patients with a soy allergy. Due to potential cross-reactivity, a history of peanut allergy is listed as a contraindication to isotretinoin use in some countries.
Objective. We sought to further evaluate the safety of isotretinoin use in patients with peanut allergy.
Methods. Using Epic’s SlicerDicer, patients were identified with both an allergy to peanuts and history of isotretinoin use for treatment of acne vulgaris. Clinical manifestation to peanut exposure, peanut-specific skin prick and/or IgE testing, and adverse reactions to isotretinoin use were recorded via chart review and phone interviews.
Results. Ten patients were identified having both a peanut allergy and treatment for acne vulgaris with isotretinoin. All patients tolerated isotretinoin without evidence of allergy.
Conclusion: Isotretinoin use did not result in allergic eruptions in patients with a known peanut allergy, however, more robust clinical studies are needed to confirm the extent of its use in this patient population.
Keywords: Acne vulgaris, isotretinoin, peanut allergy, soy allergy, case series
Oral isotretinoin (13, cis-retinoic acid) is considered the gold standard in treatment for nodulocystic acne vulgaris.1 Likewise, isotretinoin improves health-related quality of life.2 Isotretinoin is contraindicated in patients with a soy allergy, as the capsule contains hydrogenated soybean oil. Due to a potential cross-reactivity between soy and peanuts, package inserts in the United Kingdom specify that isotretinoin is contraindicated in patients with allergies to either soy or peanut.3 Similarly, but to a lesser extent, Australia cautions isotretinoin use in patients with either a soy or peanut allergy.4 Additionally, Epic, an electronic medical record software application holding the medical records of 54% of patients in the United States (2.5% patients worldwide),5 lists history of peanut allergy as a contraindication to isotretinoin. Currently, the literature does not provide evidence as a direct association between peanut allergy and isotretinoin use. We sought to further observe the safety of isotretinoin use in patients with peanut allergy.
Methods
This study was reviewed and approved by West Virginia University Institutional Review Board (approval #2002887995). Using Epic’s SlicerDicer, patients were identified as having both an allergy to peanuts and history of isotretinoin use for the treatment of acne vulgaris over the previous 10 years. Phone interviews were conducted with the identified patients to confirm accuracy of data obtained via Epic. Data collected included clinical manifestation to peanut exposure, peanut allergen skin prick or immunoglobulin E (IgE) testing results, duration and cumulative dose of isotretinoin use, and adverse reactions to isotretinoin use.
Results
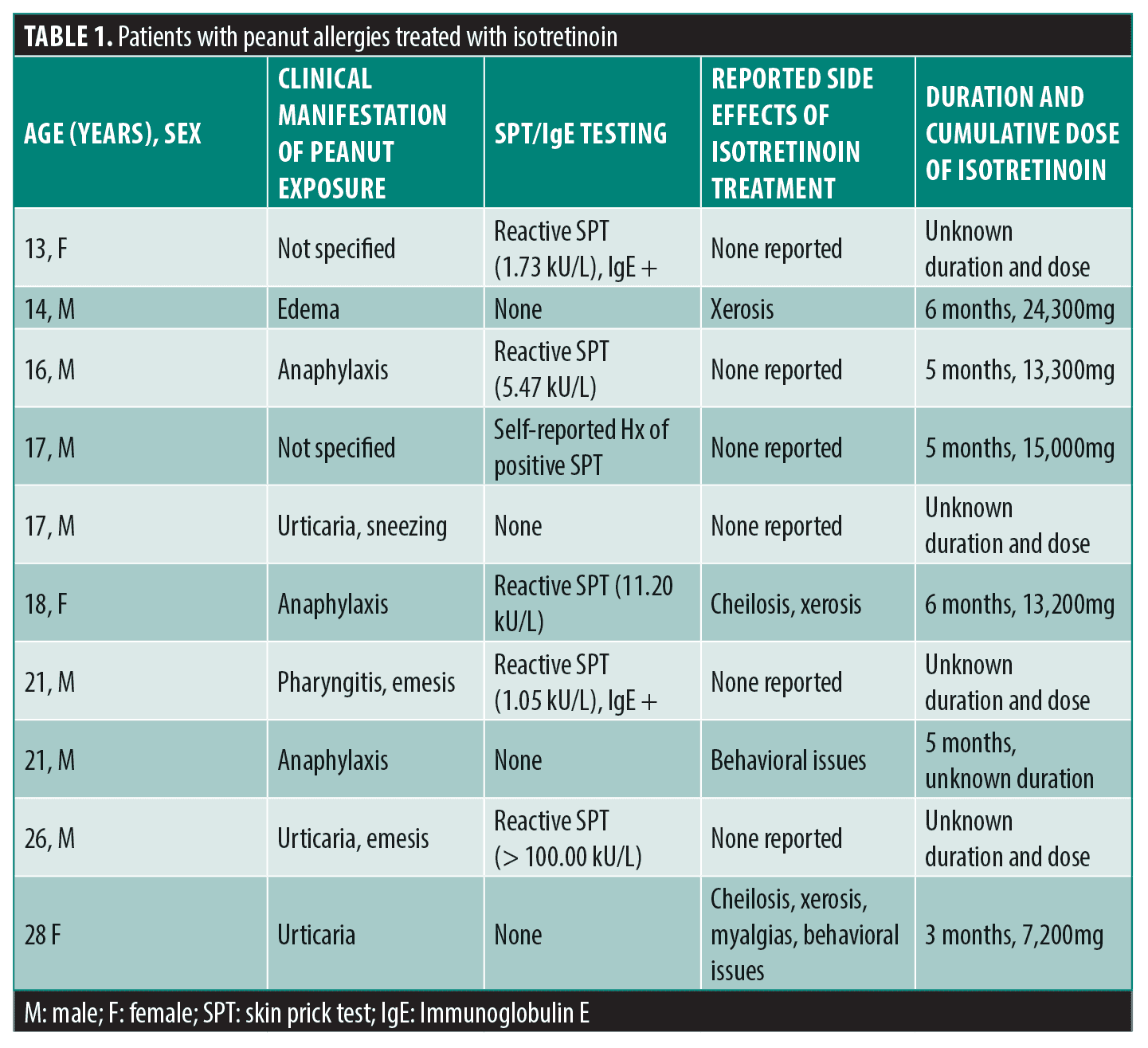
Fifteen patients were identified with peanut allergy and treatment for acne vulgaris with isotretinoin over a 10-year period. Two patients noted that documented history of peanut allergy was inaccurate on a telephone interview and therefore were excluded from analysis. Three additional patients could not be reached via phone interview to verify data obtained from Epic and therefore were excluded from analysis. Ten patients remaining with isotretinoin exposure and documented allergic reaction to peanut (clinical history of allergic reaction +/- confirmatory allergen testing) were included in the analysis (Table 1).
Ten patients (7 males and 3 females) were identified with peanut allergy and treatment for acne vulgaris with isotretinoin over the prior 10-year period. The patient mean age was 19 years (range, 13–28). Reported reactions to peanuts included urticaria, pruritis, edema, emesis, and anaphylaxis. The range of known isotretinoin cumulative dose administered was 7,200 to 24,300mg. The most commonly reported side effects were xerosis, cheilitis, and behavioral issues. All 10 patients tolerated isotretinoin without evidence of allergy (Table 1).
Discussion
In the United States, package inserts for isotretinoin detail no specific mention of peanut allergy,6 and individual case reports from American and European literature have demonstrated use of isotretinoin in patients with peanut allergy without allergic events.7,8 Because allergies to soy and peanuts are due to hypersensitivity to protein antigens, adequately purified oil should not pose an increased risk.9 Even though researchers have previously detailed a cross reactivity to soy and nuts,10 the same researchers also published a case series noting a higher risk in patients with cashew allergy than with peanut allergy and have additionally proposed a protocol for safe use of isotretinoin in nut allergic patients in which all patients who met their criteria for treatment, were treated successfully.11 Similarly, it has also been proposed to administer the initial dose of isotretinoin in a setting with immediate access to appropriate rescue supplies and drugs.12 Though there is no established agreement on the best approach to administer isotretinoin among professionals, our observations, in combination with previous case reports, indicate that isotretinoin is likely well tolerated in patients with known peanut allergies. Strengths of our study include the size of the study. Prior data on this topic is limited to case reports of single patients. Additional strengths include confirmation of clinical allergy with confirmatory testing (skin prick testing [SPT], serum specific IgE testing) in 50 percent of our cohort, with verbal confirmation of history of peanut allergy in the remaining 50 percent.
Our study has limitations. Firstly, the majority of our patient cohort was pediatric (60%) and results may not be generalizable to all patient populations. Even though primary incident cases of acne vulgaris mostly occur in adolescence and reduce in prevalence with age, acne is significantly common in young adults and middle-aged individuals.13–15 Secondly, peanut allergy testing was available for only 5 of 10 patients included in our analysis. Although the remaining patients had documented and orally verified history of adverse reaction to peanut, allergy testing was not performed. This is significant since self-reported (questionnaire-based assessment) pediatric allergen sensitization is considered more reliable when used in combination with blood IgE testing and skin prick testing.16 Therefore, observations may overestimate the safety of isotretinoin use in patients with a history of a peanut allergy. Finally, our study did not capture cashew allergy, and potential concern exists regarding cross-reactivity between cashew and isotretinoin. Despite these limitations, our observations, in agreement with previous case reports, demonstrate that oral isotretinoin for acne vulgaris did not result in allergic eruption in patients with peanut allergies.
Conclusion
All patients with a history of isotretinoin use and an allergy to peanuts tolerated isotretinoin without evidence of allergy. However, more robust clinical studies are needed to confirm the extent of its use in both pediatric and post-adolescent populations.
References
- Vallerand IA, Lewinson RT, Farris MS, et al. Efficacy and adverse events of oral isotretinoin for acne: a systematic review. Br J Dermatol. 2018;178:76–85.
- Chernyshov PV, Tomas-Aragones L, Manolache L, et al. Which acne treatment has the best influence on health-related quality of life? Literature review by the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes. J Eur Acad Dermatol Venereol. 2018;32:1410–1419.
- Isotretinoin capsules. Package leaflet: information for the user. Available at: https://www.medicines.org.uk/emc/files/pil.1114.pdf.
- Isotretinoin capsules. Package leaflet: information for the user. Available at: https://www.guildlink.com.au/gc/ws/ro/pi.cfm?product=roproacc10413.
- Glaze J. Epic Systems draws on literature greats for its next expansion. Wisconsin State Journal 2015.
- Isotretinoin capsules. Package leaflet: information for the user. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/018662s059lbl.pdf.
- Humphrey VS, Phillips SM, James WD. Is oral isotretinoin treatment contraindicated for patients with known peanut allergies? JAAD Case Rep. 2020;6:75.
- Pierret L, Grosber M, Gutermuth J. Is isotretinoin treatment safe in patients with known peanut allergy? J Eur Acad Dermatol Venereol. 2016;30:140–141.
- Awazuhara H, Kawai H, Baba M, et al. Antigenicity of the proteins in soy lecithin and soy oil in soybean allergy. Clin Exp Allergy. 1998;28:1559–1564.
- Alden K, Chowdhury MM, Williams PE, et al. Protocol for investigation of possible soya allergy in patients being considered for treatment with isotretinoin or alitretinoin. Clin Exp Dermatol. 2016;41:326–327.
- Alden K, Chowdhury MMU, Williams PE , et al. Four-year data from use of the nut and soya allergy testing protocol before treatment with isotretinoin and alitretinoin. Clin Exp Dermatol. 2020;45:627–629.
- Fallah H, Rademaker M. Isotretinoin in the management of acne vulgaris: practical prescribing. Int J Dermatol. 2021;60:451–460.
- Cunliffe WJ, Gould DJ. Prevalence of facial acne vulgaris in late adolescence and in adults. Br Med J. 1979;1:1109–1110.
- Lynn DD, Umari T, Dunnick CA, Dellavalle RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584–1590.
- Miao Q, Xiang L, Guan H, et al. Factors related to disagreement between self-reported versus objective measurement of allergen sensitization at a tertiary pediatric center in Beijing, China. BMC Pediatr. 2020;20:259.

