J Clin Aesthet Dermatol. 2023;16(9):20–24.
J Clin Aesthet Dermatol. 2023;16(9):20–24.
by James Q. Del Rosso, DO
Dr. Del Rosso is with Touro University Nevada in Henderson, Nevada. He is also with JDR Dermatology Research in Las Vegas, Nevada, and Advanced Dermatology and Cosmetic Surgery in Maitland, Florida.
FUNDING: Manuscript preparation and editorial support were provided by Dana Lengel, PhD, of AlphaBioCom, a Red Nucleus company, and funded by Sun Pharma.
DISCLOSURES: Dr. Del Rosso has served as a research investigator, consultant, and/or speaker for Allergan, Almirall, Arcutis, Bayer, Bausch Health (Ortho Dermatologics), Biorasi, Bristol Myers Squibb, Cassiopea, Cutera, Dermavant, Dr. Reddy, EPI Health, Evommune, Ferndale Laboratories, Galderma, JEM Health, Journey, Johnson & Johnson, LEO Pharma, L’Oreal, Mayne Pharma, Sebacia, Sol-Gel, Sun Pharma, and Vyne.
ABSTRACT: Objective. Isotretinoin is a widely used and clinically efficacious treatment for severe, recalcitrant, nodular acne vulgaris (AV). Clinicians are generally familiar with safety concerns regarding isotretinoin use, especially teratogenicity. However, there are specific “real-world” challenges with use of conventional formulations based on the original isotretinoin formulation, including poor solubility and food-dependent absorption requiring high fat intake with each dose. This review describes the development and use of new isotretinoin formulations and their potential to improve long-term outcomes in patients with AV.
Methods. PubMed was searched using the terms “acne,” “isotretinoin,” “micronized,” “lidose,” and “efficacy.”
Results. Micronized isotretinoin received US Food and Drug Administration approval in 2019 for the treatment of severe recalcitrant nodular acne in patients aged 12 years or older. This isotretinoin formulation utilizes micronization technology to reduce drug particle size, thereby increasing its dissolution rate and bioavailability, combined with a lipid-based carrier system that enhances gastrointestinal absorption without the need for dietary fat ingestion. Together, these features allow for an approximately two-fold or greater increase in isotretinoin absorption despite a lower administered dose compared with prior formulations, without dependency upon high-fat food intake.
Limitations. Evidence supporting reduction in relapse with micronized isotretinoin is based on studies with the lidose formulation and pharmacokinetic data supporting greater systemic exposure to micronized isotretinoin without food.
Conclusion. The lack of any specific dietary requirements or need for food intake and the enhanced bioavailability of micronized isotretinoin may increase both patient compliance and the rate of prolonged remission of AV after completion of therapy.
Keywords. Acne, isotretinoin, micronized, relapse, absorption, efficacy, safety
Acne vulgaris (AV) is a chronic, multifactorial skin condition affecting at least 50 million people in the US annually.1 It is most prevalent in adolescents and young adults but can develop earlier and may persist into or develop during adulthood.2–4 Acne is associated with adverse effects on quality of life, including symptomatic discomfort, scarring, and emotional and psychosocial distress.5 It is also associated with significant economic costs.1 According to a 2013 national burden of skin disease report, the costs of treatment and lost productivity associated with medical care for acne exceeded $1.2 billion.6 Although combination topical therapy is often the basis of initial treatment for mild-to-moderate acne, systemic treatment is typically required for refractory moderate or severe AV and/or AV that is causing scarring or psychosocial distress, or that has failed to respond to conventional treatments.7,8 Isotretinoin (13-cis-retinoic acid), a vitamin A derivative first approved in the US in 1982 for the treatment of severe recalcitrant nodular AV,9 is a clinically effective therapy that targets all four major pathogenic mechanisms of AV: follicular hyperkeratinization, sebum production, Cutibacterium acnes proliferation, and multiple pathways of inflammation.7
Isotretinoin is recognized as having revolutionized the treatment of severe and refractory AV due to its ability to induce complete or near-complete clearance of acne and sustained periods of remission after completion of therapy.7,10,11 Nonetheless, the use of isotretinoin presents some challenges with “real-world” use. The most significant of these are safety issues, most prominently teratogenicity, but also associations with lipid and musculoskeletal abnormalities.8 However, there are other considerations specific to conventional isotretinoin formulations, including the original brand of isotretinoin (Accutane™, Roche Pharmaceuticals) and several branded-generic formulations approved based on the pharmacokinetic profile of the original isotretinoin formulation. The major limitation of conventional isotretinoin formulations is the dependence on co-ingestion with a high-fat meal for sufficient absorption.8,10 This is of prominent clinical significance, as the long-term clearance rate after completion of isotretinoin therapy correlates directly with the extent of systemic exposure,8 which is dependent on the magnitude of gastrointestinal absorption achieved over a course of therapy.12,13
Continued research efforts have focused on the development of improved isotretinoin formulations with the goal of optimizing therapeutic outcomes, especially long-term remission of AV.14–16 In particular, the utilization of a lipid-based carrier system that solubilizes isotretinoin coupled with advanced micronization technology that reduces the physical size and increases uniformity of isotretinoin particles provides an advanced approach that improves gastrointestinal absorption, overall systemic exposure to isotretinoin, and ultimately the rate of prolonged remission of AV.15 This review summarizes the development and use of new isotretinoin formulations in the treatment of AV and their demonstrated potential to improve long-term outcomes in patients.
What are the clinical recommendations for isotretinoin use?
Isotretinoin received US Food and Drug Administration (FDA) approval in 1982 for the treatment of severe recalcitrant nodular AV.9,11 Isotretinoin use is also recommended in practice for selected patients with AV who have responded unsatisfactorily to conventional treatments, or those in whom AV is causing physical scarring and/or psychosocial distress.8 The recommended dosage of conventional isotretinoin is 0.5mg/kg/day given in two divided doses, with dose escalation to 1.0mg/kg/day as tolerated, for a duration of 15 to 20 weeks.8,17 Based on findings from numerous clinical trials and retrospective studies with conventional isotretinoin, achievement of a cumulative isotretinoin exposure range of 120mg/kg to 150mg/kg is recommended to provide the best opportunity to achieve long-term remission.8 Although approaches differ among clinicians in practice, treatment is often continued until AV has cleared and may be extended thereafter if the target cumulative dose of isotretinoin has not yet been achieved.11 In a retrospective analysis including 550 patients who were average or underweight (67%), overweight (18%), or obese (14.9%), higher-weight patients received lower daily doses by weight compared with other patients (P≤0.001), and the duration of treatment was longer in obese vs overweight and average-weight patients (P=0.03). However, there were no statistically significant differences based on weight in AV clearance or recurrence rate after end of treatment.18
The safety profile of isotretinoin is generally favorable, with the most common anticipated side effects being dry skin, dry eyes, and dry lips.13,19 The most prominent safety concern is teratogenicity, and isotretinoin is therefore contraindicated during pregnancy; patients who can become pregnant are required to comply with pregnancy testing and contraception requirements while using isotretinoin.9 A mandated risk-management program (iPLEDGE®) was introduced in 2006 to prevent isotretinoin exposures during pregnancy in patients who can become pregnant and to document the frequency and outcomes of isotretinoin exposure during pregnancy.11 The requirements of the iPLEDGE® program include the simultaneous use of two forms of effective contraception (or a commitment to continuous abstinence with no sexual contact that could result in pregnancy) and mandated monthly pregnancy tests for patients who can become pregnant who are receiving isotretinoin.9 All patients treated in the US with isotretinoin are required to be enrolled in and follow the requirements of the iPLEDGE® program.8
Additional potential adverse effects include lipid abnormalities (especially hypertriglyceridemia),9,20–22 musculoskeletal abnormalities, and elevated liver enzymes.9,23 Routine monitoring of liver function tests and serum lipids is recommended at baseline with periodic retesting during the course of treatment.8,9 Other possible adverse effects, including depression, suicidal ideation, and inflammatory bowel disease, have been associated with isotretinoin; however, these associations are somewhat controversial and have not been definitively substantiated.8,24,25 Nonetheless, prescribing physicians are recommended to monitor patients for indications of inflammatory bowel disease and depressive symptoms and to educate patients about potential risks.8,9
What are the challenges associated with the use of conventional formulations of isotretinoin?
Due to its high lipophilicity, isotretinoin inherently exhibits poor aqueous solubility, and its bioavailability is greatly dependent on administration with a meal high in fat content.9,16,26 In early pharmacokinetic studies with conventional isotretinoin, the bioavailability of isotretinoin was approximately 1.5 to 2 times greater when administered concomitantly with a meal than when given during a complete fast.26 Based on results from this as well as subsequent pharmacokinetic trials evaluating the bioavailability of conventional isotretinoin when studied with an FDA-recommended high-calorie, high-fat meal consisting of 50g of fat and 800 to 1,000 calories, administration with food, particularly a meal with very high fat content, is recommended for optimal and consistent gastrointestinal absorption of conventional isotretinoin.9,16 When ingested without food, plasma levels of isotretinoin are reduced by approximately 60 percent compared with levels achieved when taken with a high-fat meal,16 which could adversely affect efficacy, especially long-term remission of AV. Unfortunately, patients may not be aware that taking isotretinoin with a high-fat meal is necessary for optimal absorption and efficacy, or they may be unwilling to regularly adhere to taking isotretinoin with such a high intake of dietary fat with each dose. Due in part to the predominant emphasis on pregnancy prevention and other potential adverse effects in patients treated with isotretinoin, relatively limited attention has been paid to educating medical professionals about the importance of taking isotretinoin with high-fat food intake. Even when informed, patients and clinicians may be hesitant to consume or recommend a high-fat diet while using a conventional isotretinoin due to association of isotretinoin use with elevated serum lipid levels, as well as other potential health risks related to high fat intake.
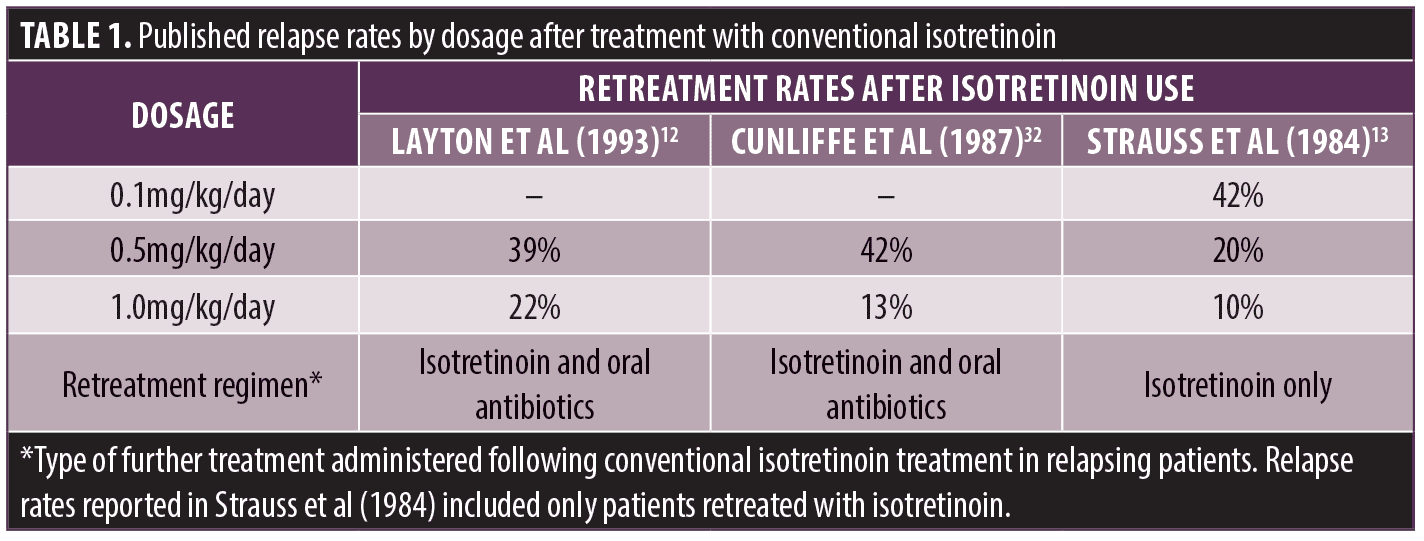
Although a single course of conventional isotretinoin therapy can achieve long-term remission in many patients, approximately 23 to 61 percent of patients experience recurrence of acne requiring further topical or systemic treatment,12,27–31 and approximately 20 to 26 percent of patients require a second course of isotretinoin therapy.12,27,29–31 Reported relapse rates are highly variable, due in part to differences in how relapse is defined or identified in specific analyses, the daily and cumulative doses received, and duration of follow-up; however, relapse is less frequent in patients receiving higher doses of isotretinoin.12,13,29,31,32 Interestingly, in early studies of conventional isotretinoin, the initial efficacy by the end of a typical 16- to 20-week course of treatment was similar between low (0.1mg/kg/day), intermediate (0.5mg/kg/day), and high (≥1mg/kg/day) doses.13 However, the rate of relapse following treatment was dose dependent, with higher rates observed in patients receiving low or intermediate doses (20%–42%) compared with those receiving the highest dose (10%–22%; Table 1);12,13,32 the majority of relapses occurred within 18 months of stopping treatment.12 When the cumulative dose was assessed, prolonged remission correlated with a threshold cumulative exposure range of ≥120mg/kg of conventional isotretinoin.12,29 Based on these analyses, a cumulative isotretinoin exposure range of 120 to 150mg/kg is recommended to provide the best opportunity for prolonged remission in patients receiving conventional isotretinoin.8 Achievement of the desired cumulative isotretinoin exposure of ≥120 mg/kg may be particularly challenging for teenagers and young adults, who often exhibit irregular eating habits and may be less likely to adhere to recommendations to ingest isotretinoin with an adequate high-fat meal.33 Ultimately, these factors may adversely affect treatment efficacy and lead to higher rates of relapse in patients receiving conventional isotretinoin for treatment of AV.
How does lidose-isotretinoin improve upon conventional isotretinoin for the effective and convenient management of recalcitrant acne vulgaris?
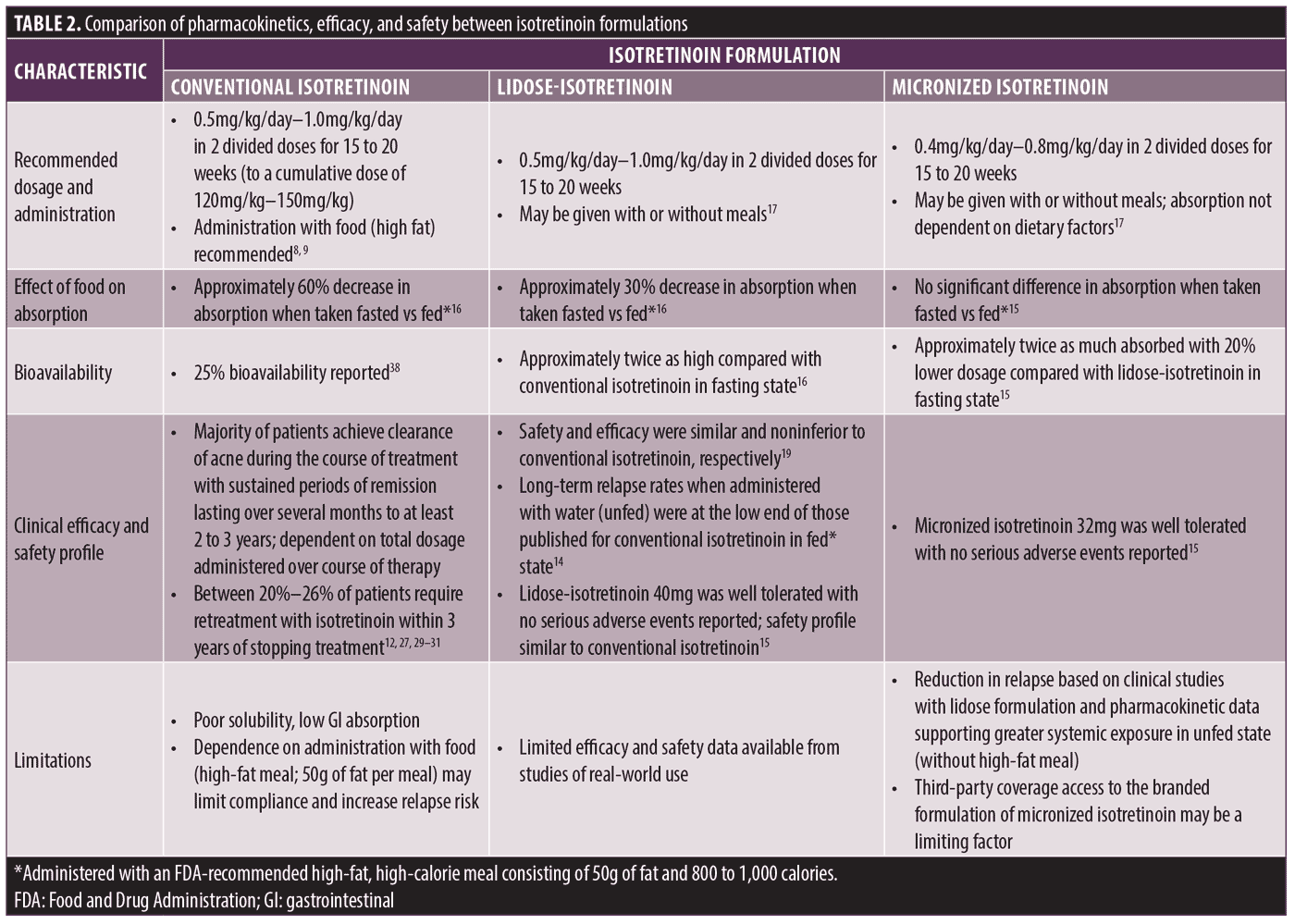
The first major improvement in isotretinoin formulation was lidose-isotretinoin, in which isotretinoin is pre-solubilized in a lipid matrix for enhanced absorption and a markedly reduced dependence upon ingestion with a meal high in fat content.16 Compared with the original formulation of conventional isotretinoin, lidose-isotretinoin is bioequivalent when taken with a high-fat, high-calorie meal and is absorbed significantly better when taken on an empty stomach (with water).16 In pharmacokinetic studies, when lidose-isotretinoin was administered to fasting patients, plasma isotretinoin levels reached 66.8 percent of those observed when the drug was taken with a high-fat meal, compared with 39.6 percent for conventional isotretinoin taken under the same conditions.16 Therefore, under fasting conditions, patients absorb nearly twice the amount of isotretinoin from lidose-isotretinoin compared with conventional isotretinoin (Table 2). In a Phase III study, the efficacy of lidose-isotretinoin was noninferior to that of standard isotretinoin when both were ingested with a suggested high-fat meal, with a comparable safety profile.19 Furthermore, Phase IV data suggest treatment of patients with severe recalcitrant nodular acne with lidose-isotretinoin taken without food can lead to significant improvements in multiple measures of quality of life.34
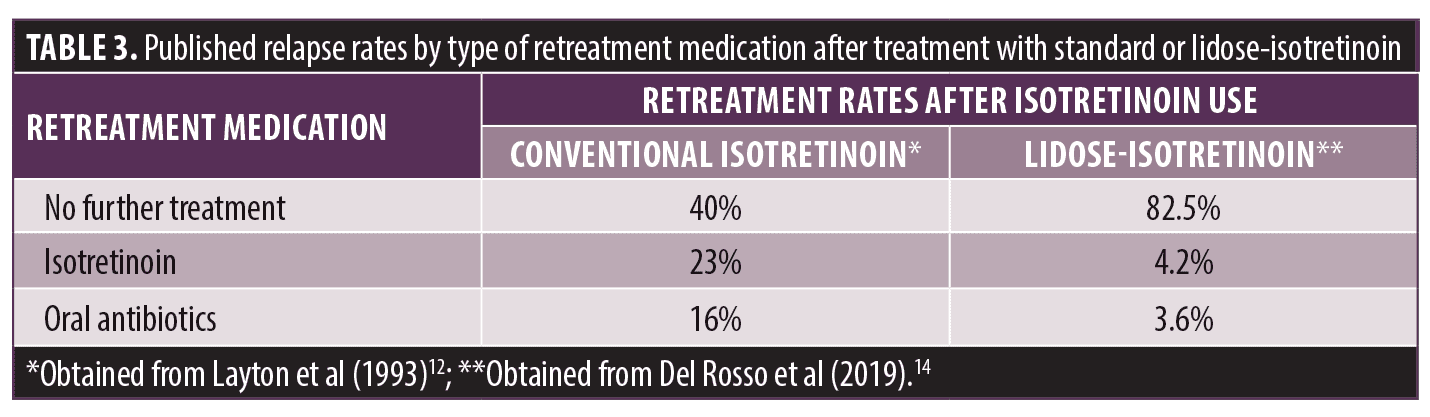
The improvement in the bioavailability of lidose-isotretinoin was shown to translate into low rates of relapse following treatment.14 In a Phase IV study of lidose-isotretinoin given at the recommended weight-based daily dosage of 0.5 to 1.0mg/kg/day divided into two daily doses without food in patients with severe recalcitrant nodular acne, the proportions of patients requiring any type of further treatment over a 104-week posttreatment period were low, supporting an apparent markedly lower relapse rate when compared with the range of published relapse rates in patients who received conventional isotretinoin ingested with a high-fat, high-calorie meal (Table 3).14 Thus, the increased bioavailability of lidose-isotretinoin administered without food and its reduced dependence on ingestion with a high fat content meal demonstrated the potential for improved long-term remission after completion of treatment along with improvements in quality of life in patients with AV.
Can micronization technology both improve isotretinoin absorption and decrease food dependency?
The next technological improvement in isotretinoin formulation was the micronization of isotretinoin coupled with a lipid-based carrier system. Micronization technology reduces the physical drug particles to a more uniform micrometer size,35 which allows for a more consistent distribution of isotretinoin along the mucosa of the intestinal tract. This reduced particle size of micronized isotretinoin increases the surface area of mucosal exposure per unit mass of isotretinoin, thereby increasing its dissolution rate, absorption, and bioavailability36 and allowing for a 20-percent decrease in the administered dose as compared with lidose-isotretinoin.15
Micronized isotretinoin received FDA approval in 2019 for the treatment of severe recalcitrant nodular acne in nonpregnant patients aged 12 years of age or older based on a pharmacokinetic study comparing the properties of micronized isotretinoin 32mg and lidose-isotretinoin 40mg.15,17,37 When ingested with a high-fat, high-calorie meal, micronized isotretinoin was bioequivalent to lidose-isotretinoin. However, under fasting conditions, patients absorbed approximately twice the amount of isotretinoin from micronized isotretinoin, even with a 20-percent lower administered dosage, than from lidose-isotretinoin (Table 2).15 Both lidose-isotretinoin and micronized isotretinoin were well tolerated with no serious adverse events reported. Collectively, these data suggest that the markedly enhanced bioavailability of micronized isotretinoin may enable this formulation to provide optimal treatment efficacy when taken on an empty stomach, with a 20-percent lower administered dosage, compared with lidose-isotretinoin or conventional isotretinoin, without concerns regarding concomitant administration with a high-fat, high-calorie meal.
How can micronized isotretinoin achieve improved long-term outcomes in patients?
The use of micronized isotretinoin may provide numerous benefits in clinical practice due to its improved bioavailability and lack of dietary requirements. The association between the extent of systemic exposure to isotretinoin and risk of recurrence after completion of a course of isotretinoin therapy has been established, with fewer relapses observed in patients who received a cumulative dose of conventional isotretinoin of 120 to 150mg/kg,8,12,29 suggesting that there may be a minimal threshold of systemic exposure necessary to produce prolonged therapeutic effects after stopping treatment.15 Patients receiving conventional isotretinoin, including those ingesting a cumulative dose of ≥120mg/kg, may fall short of the desired cumulative exposure to isotretinoin absorbed from conventional formulations if isotretinoin is not taken with an adequate high-fat meal. Thus, reducing the food dependency of isotretinoin absorption is likely to benefit long-term treatment efficacy in patients, as evidenced by the low proportion of patients requiring retreatment after use of lidose-isotretinoin taken with only water compared with that historically observed with conventional isotretinoin taken with a high-fat meal.14 Therefore, the enhanced absorption and decreased food dependency of micronized isotretinoin suggest that this formulation may further improve upon the long-term efficacy observed with lidose-isotretinoin and further facilitate prolonged remission in patients with AV.
Conclusion
Compared to conventional isotretinoin, the micronized formulation provides two principal technological and functional improvements: 1) Increased dissolution rate and gastrointestinal absorption through the use of micronization technology, thereby achieving similar serum concentrations of isotretinoin with a 20-percent lower dosage when administered with a high-fat, high-calorie meal; and 2) Enhanced isotretinoin solubility with reduced dependency upon high-fat meal ingestion through the use of a lipid-based carrier system. Because micronized isotretinoin increases isotretinoin absorption with a lower dose and no food dependency relative to other formulations, patients may more readily achieve the threshold systemic exposure necessary to facilitate prolonged remission after completion of therapy, thereby optimizing long-term outcomes of treatment for AV. Furthermore, the decreased food dependency with this formulation may increase treatment adherence, particularly in teenagers and young adults, who are the most common recipients of acne treatment and who often exhibit irregular eating habits. Ultimately, micronized isotretinoin may be a better option to help patients remain clear without relapse. The micronized formulation of isotretinoin also provides patients and clinicians the greatest increase in flexibility for the effective and convenient management of severe and refractory AV, especially by eliminating the dietary requirements needed to optimize use of conventional isotretinoin.
Acknowledgments
Manuscript preparation and editorial support were provided by Dana Lengel, PhD, of AlphaBioCom, a Red Nucleus company, and funded by Sun Pharma.
References
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500.
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–59.
- Goldberg JL, Dabade TS, Davis SA, et al. Changing age of acne vulgaris visits: another sign of earlier puberty? Pediatr Dermatol. 2011;28(6):645–648.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10(1):5754.
- Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(suppl 1):3–12.
- Lim HW, Collins SAB, Resneck JS Jr., et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958–972.e2.
- Savage LJ, Layton AM. Treating acne vulgaris: systemic, local and combination therapy. Expert Rev Clin Pharmacol. 2010;3(4):563–580.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33.
- Accutane™ (isotretinoin capsules). Prescribing Information. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Del Rosso JQ. Face to face with oral isotretinoin: a closer look at the spectrum of therapeutic outcomes and why some patients need repeated courses. J Clin Aesthet Dermatol. 2012;5(11):17–24.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3–S21.
- Layton AM, Knaggs H, Taylor J, et al. Isotretinoin for acne vulgaris–10 years later: a safe and successful treatment. Br J Dermatol. 1993;129(3):292–296.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10(3):490–496.
- Del Rosso JQ, Stein Gold L, Segal J, et al. An open-label, phase IV study evaluating lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12(11):13–18.
- Madan S, Kumar S, Segal J. Comparative pharmacokinetic profiles of a novel low-dose micronized-isotretinoin 32 mg formulation and lidose-isotretinoin 40 mg in fed and fasted conditions: two open-label, randomized, crossover studies in healthy adult participants. Acta Derm Venereol. 2020;100(4):adv00049.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, 4-treatment, crossover study. J Am Acad Dermatol. 2013;69(5):762–767.
- ABSORICA® (isotretinoin) capsules/ABSORICA LD™ (isotretinoin) capsules. Prescribing Information. Cranbury, NJ: Sun Pharmaceutical Industries, Inc.; 2020.
- Tallmadge M, Patel A, Liegl MN, et al. Isotretinoin use for acne in obese and overweight young people: a retrospective study. J Am Acad Dermatol. 2022 Aug;87(2):461–464.
- Webster GF, Leyden JJ, Gross JA. Results of a phase III, double-blind, randomized, parallel-group, non-inferiority study evaluating the safety and efficacy of isotretinoin-lidose in patients with severe recalcitrant nodular acne. J Drugs Dermatol. 2014;13(6):665–670.
- Alajaji A, Alrawaf FA, Alosayli SI, et al. Laboratory abnormalities in acne patients treated with oral isotretinoin: a retrospective epidemiological study. Cureus. 2021;13(10):e19031.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7(1):171–174.
- Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016–1022.
- Karaosmanoglu N, Mulkoglu C. Analysis of musculoskeletal side effects of oral Isotretinoin treatment: a cross-sectional study. BMC Musculoskelet Disord. 2020;21(1):631.
- Crockett SD, Gulati A, Sandler RS, Kappelman MD. A causal association between isotretinoin and inflammatory bowel disease has yet to be established. Am J Gastroenterol. 2009;104(10):2387–2393.
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76(6):1068–1076.e9.
- Colburn WA, Gibson DM, Wiens RE, et al. Food increases the bioavailability of isotretinoin. J Clin Pharmacol. 1983;23(11–12):534–539.
- Azoulay L, Oraichi D, Berard A. Isotretinoin therapy and the incidence of acne relapse: a nested case-control study. Br J Dermatol. 2007;157(6):1240–1248.
- Demirci Saadet E. Investigation of relapse rate and factors affecting relapse after oral isotretinoin treatment in patients with acne vulgaris. Dermatol Ther. 2021;34(6):e15109.
- Haryati I, Jacinto SS. Profile of acne patients in the Philippines requiring a second course of oral isotretinoin. Int J Dermatol. 2005;44(12):999–1001.
- White GM, Chen W, Yao J, et al. Recurrence rates after the first course of isotretinoin. Arch Dermatol. 1998;134(3):376–378.
- Stainforth JM, Layton AM, Taylor JP, et al. Isotretinoin for the treatment of acne vulgaris: which factors may predict the need for more than one course? Br J Dermatol. 1993;129(3):297–301.
- Cunliffe WJ, Norris JF. Isotretinoin–an explanation for its long-term benefit. Dermatologica. 1987;175(suppl 1):133–137.
- Deshmukh-Taskar PR, Radcliffe JD, Liu Y, et al. Do breakfast skipping and breakfast type affect energy intake, nutrient intake, nutrient adequacy, and diet quality in young adults? NHANES 1999-2002. J Am Coll Nutr. 2010;29(4):407–418.
- Zaenglein AL, Segal J, Darby C, et al. Lidose-isotretinoin administered without food improves quality of life in patients with severe recalcitrant nodular acne: an open-label, single-arm, phase IV study. J Clin Aesthet Dermatol. 2020;13(9):15–20.
- Joshi JT. A review on micronization techniques. J Pharm Sci Technol. 2011;3(7):651–681.
- Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:195727.
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research. ABSORICA LD (isotretinoin capsules) NDA 211913 multidisciplinary review and evaluation, August 17, 2018. Retrieved from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211913Orig1s000MultidisciplineR.pdf.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1(3):162–169.