 by Douglas C. Wu, MD, PhD; Isabella Guiha, BSc; and Mitchel P. Goldman, MD
by Douglas C. Wu, MD, PhD; Isabella Guiha, BSc; and Mitchel P. Goldman, MD
Drs. Wu and Goldman and Ms. Guiha are with Cosmetic Laser Dermatology, San Diego, California.
J Clin Aesthet Dermatol. 2017;10(8):31–36
Funding: This study was sponsored and funded by LEO Pharma.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Keywords: Ingenol mebutate, actinic keratosis, local skin response, chest, photoaging, photodamage
Abstract: Background. Ingenol mebutate gel 0.05% once daily for two consecutive days provides high clearance rates for actinic keratosis on a 25cm2 area of the chest. However, it may cause intense local skin responses.
Objective. The objective of this study was to determine whether a lower dose of ingenol mebutate gel 0.015% could clear actinic keratosis on the chest with acceptable tolerability and a possible beneficial effect on photodamage and cosmesis.
Design. This was a noncomparative, open-label study (NCT02446223).
Participants. In total, 21 subjects were enrolled, and 20 completed the study.
Measurements. Ingenol mebutate gel 0.015% was applied once daily for three consecutive days to a contiguous area of the chest less than 100cm2 containing four or more actinic keratoses.
Results. The actinic keratosis lesion count decreased by an average of 76 percent compared with baseline. Signs of photoaging were reduced significantly at the end of the study, with greater than 60 percent of the subjects reporting moderate or complete satisfaction with skin improvement. Local skin responses generally resolved within two weeks of treatment. There were no adverse reactions.
Limitations. This was a noncomparative, open-label study that evaluated a relatively small number of subjects.
Conclusion. Ingenol mebutate gel 0.015% applied to a less than 100cm2 area of the chest once daily for three consecutive days reduced the actinic keratosis count and significantly improved signs of photoaging with high patient satisfaction and good tolerability.
Introduction
Actinic keratoses (AKs) are common epidermal lesions on sun-exposed skin from chronic, cumulative ultraviolet (UV) radiation.[1,2] AK may progress to squamous cell carcinoma (SCC), although some experts consider AK to be SCC in situ.[2,3] The risk for progression provides the primary rationale for treatment.[2] An additional inducement for treatment is the cosmetic liability of these lesions.[4] Cosmesis following ablation of AK is important, especially for patients who are concerned about skin appearance, due to cosmetic outcome differences across the various AK treatment options.[5] Moreover, patient experience and satisfaction with cosmetic results may influence future decisions to seek treatment for AK.
For isolated AKs, lesion-directed cryosurgery is commonly used. Field treatment, however, has the advantage of ablating not only visible lesions but also subclinical lesions, and it is appropriate when multiple lesions affect contiguous areas of sun-damaged skin.[6] Ingenol mebutate as a field therapy for AK is approved by the United States Food and Drug Administration (FDA) for use on contiguous 25cm2 areas on the face or scalp and the trunk or extremities.7 The dosage is a once-daily application of the 0.015% formulation for three consecutive days for the face or scalp and once-daily use of the 0.05% formulation for two consecutive days for the trunk or extremities.[7,8]
The chest, as an area on the trunk, can be especially challenging to treat due to skin differences at this site9 that can affect healing and also cosmetic results. In the pivotal studies of ingenol mebutate, AKs on the chest were not identified separately from AKs on other nonface/nonscalp areas when the data were analyzed, nor were cosmetic outcomes considered; thus, no conclusions can be drawn from these studies regarding a specific effect of ingenol mebutate on the chest.[8]
In women, the issue of cosmesis often arises when discussing treatment of AK on the chest due to the occurrence of AK on the décolletage in association with extensive photodamage.[10] This is most common among women with Fitzpatrick Skin Types I and II.[10] Older women often express interest in noninvasive cosmetic procedures for photoaged décolletage,[11] and if they are undergoing field therapy for AK, they may ask about a treatment that can concurrently provide cosmetic improvement.[11] Although the 0.05% formulation of ingenol mebutate is the indicated formulation for use on the chest, this dose can be associated with poor tolerability. Pellacani et al[12] observed greater local skin responses (LSR) on the chest than at other trunk and extremity sites following application of ingenol mebutate gel 0.05% to a 25cm2 area for two consecutive days. In our practice, we also have observed poor tolerability of ingenol mebutate gel 0.05% on the chest, particularly among women.
To address these issues in AK treatment of the chest, with special consideration for the cosmetic concerns of women, we studied the effects of applying the facial formulation of ingenol mebutate gel 0.015% to a larger area of the chest, less than100 cm2. In addition to assessing efficacy and safety, we assessed the cosmetic effects, changes in signs of photodamage, and patient satisfaction with skin appearance.
Methods
Study design. This was a single-center, single-arm, open-label study (ClinicalTrials.gov Identifier NCT02446223). The clinical study protocol, any amendments to the clinical study protocol, and informed consent forms were approved by the appropriate Institutional Review Board.Subjects. The study enrolled adults at least 18 years old with four or more clinically typical AKs within one contiguous area of the chest less than 100cm2. Subjects were asked to refrain from the use of topical products containing alpha-hydroxy acids, retinoic acid, retinol, salicylic acid, and vitamins C or D (or their derivatives) in the treatment area for seven days prior to and during the entire study period. Women of child-bearing potential were required to use an accepted form of birth control. Subjects were excluded if hypertrophic or hyperkeratotic AK lesions were present in the prospective treatment area or if any skin condition was present or intervention was required that might interfere with evaluation of the treatment area. Other exclusion criteria were pregnancy or infant nursing, planning a pregnancy during the study period, or known hypersensitivity to, prior allergic reaction to, or previous chest treatment with ingenol mebutate gel. Subjects were not enrolled if they had any dermatologic condition that might interfere with the diagnosis or evaluation of study parameters or if they had received ablative laser treatments on the chest within six months of entry or other topical therapies for AK within three months of entry, or had received intense pulsed light treatments within 30 days of entry. Use of self-tanners or excessive exposure to sunlight or artificial UV radiation within seven days of entry was prohibited. All subjects provided written informed consent.
Interventions. The schedule of treatment included investigator-guided, in-office application of ingenol mebutate gel 0.015% at the first visit on Day 0 (baseline) and subjects’ self-administration of ingenol mebutate gel on Days 1 and 2. Six follow-up visits were scheduled on Days 1, 3, 10, 17, 24, and 59.
Study assessments. The primary efficacy end point was complete clearance of all clinically visible AKs at Day 59 as compared with the pretreatment lesion count. A secondary efficacy end point was stratification of subjects into groups based on percent clearance as follows: partial clearance (?75%), mild clearance (?10% and <75%), minimal to no clearance (<10%), worsened (observable increase in AK), and unable to be assessed. The cosmetic effect of ingenol mebutate gel was measured by investigator-evaluated changes in photodamage across six categories (e.g., dyschromia, erythema/telangiectasias, keratoses, texture, and rhytides), which were assessed with the modified Alexiades-Armenakas comprehensive grading scale, an 8-point grading scale (0–4, with half-numerals between 1 and 4, where higher numbers indicate greater severity).[13] Subjects self-assessed satisfaction with skin appearance by grading improvement in signs of skin aging (e.g., rhytides, dyschromia, telangiectasias, keratosis, erythema, and overall satisfaction) with a 7-point scale (0–6), where a score of 3 was neutral, and higher numbers indicated higher satisfaction. Subjects also completed the 14-item Treatment Satisfaction Questionnaire for Medication (TSQM, version 1.4).[14] Pretreatment photography was obtained at baseline and at each follow-up visit, and safety was assessed by individual LSR (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ ulceration), evaluated with a photographic guide and graded on a scale of 0 to 4, with higher numbers indicating greater severity.[8,15] In addition, adverse events (AE) were recorded at each follow-up visit.
Statistical analysis. All statistical tests were two-sided and interpreted at a five-percent significance level. Descriptive statistics (i.e., mean, standard deviation [SD]) were provided for all continuous variables, and frequencies were provided for all categorical variables. Single-factor analysis of variance (ANOVA) tests were used to track changes for individual variables across all follow-up visits, while comparisons between two individual visits were performed using two-sample t-tests assuming equal variance. P-values less than 0.05 were considered clinically significant.
Results
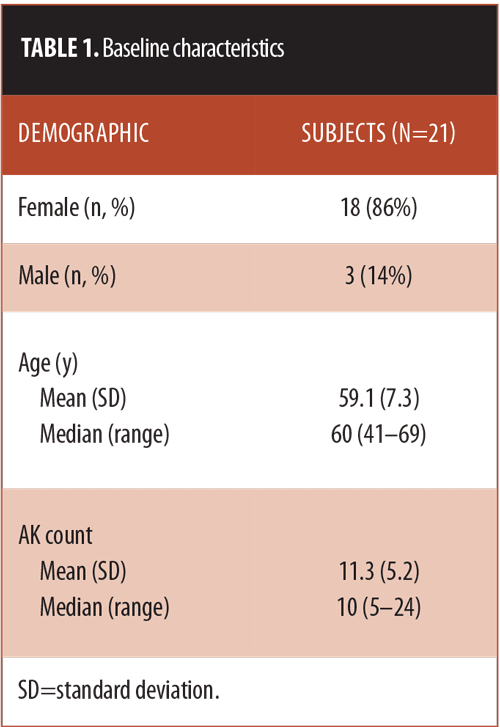 Study population. A total of 21 subjects (18 women; 3 men) with a mean (SD) AK count of 11.3 (5.2) were enrolled and received treatment (Table 1).
Study population. A total of 21 subjects (18 women; 3 men) with a mean (SD) AK count of 11.3 (5.2) were enrolled and received treatment (Table 1).
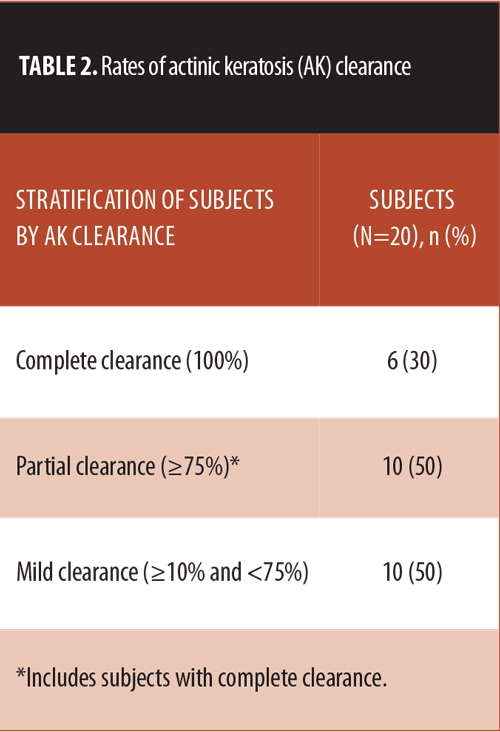 Efficacy. AK clearance was assessed at Day 59 in the 20 subjects who completed the study (1 patient was lost to follow-up) (Table 2). For less than complete clearance of AK, counts at Day 59 were compared with counts at baseline. Treatment with ingenol mebutate gel 0.015% reduced the mean (SD) AK count by 76 percent, from 11.3 (5.2) at Day 0 to 2.7 (2.68) at Day 59 (P<0.001) (Figure 1).
Efficacy. AK clearance was assessed at Day 59 in the 20 subjects who completed the study (1 patient was lost to follow-up) (Table 2). For less than complete clearance of AK, counts at Day 59 were compared with counts at baseline. Treatment with ingenol mebutate gel 0.015% reduced the mean (SD) AK count by 76 percent, from 11.3 (5.2) at Day 0 to 2.7 (2.68) at Day 59 (P<0.001) (Figure 1).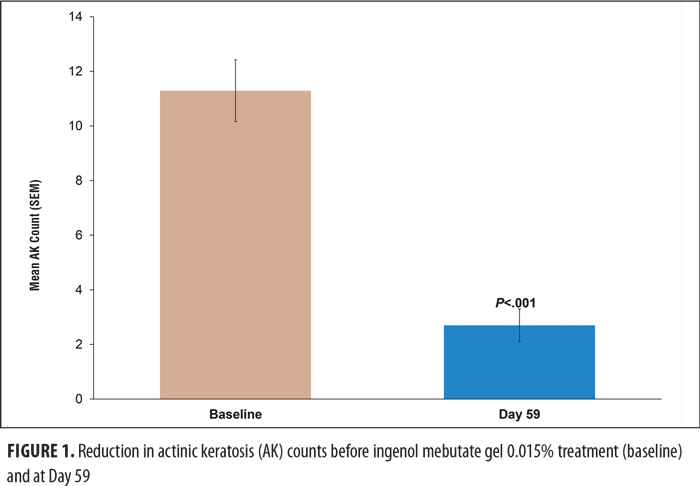
Quantitative analysis of photoaging. The six individual aspects of photoaging described above as well as seborrheic keratoses were assessed by the investigator with the modified Alexiades-Armenakas comprehensive grading scale at baseline (Day 0) and after application of ingenol mebutate on Days 24 and 59 (Figure 2). Grades for all six signs of photodamage were significantly lower after ingenol mebutate gel treatment at Day 59 as compared with baseline. The largest point decrease after treatment (1.7) was observed for “texture,” from a mean grade of 3.0 at baseline to 1.3 at Day 59.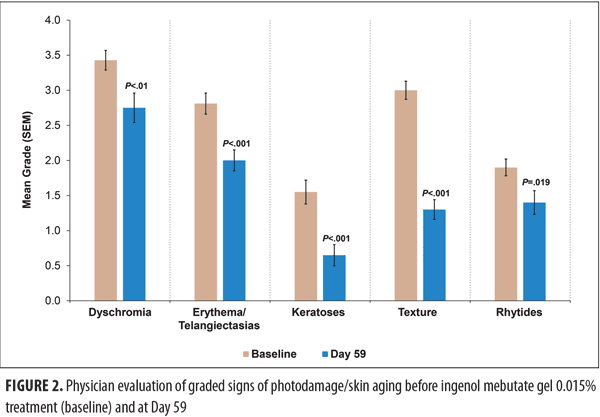
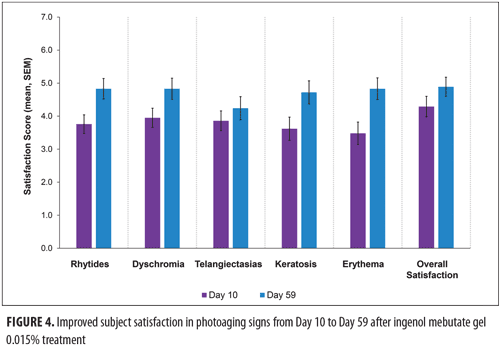
Subject satisfaction with skin improvement. Subjects completed a survey that assessed their overall satisfaction with improvement in photoaging as well as their satisfaction with improvement in five individual characteristics of photoaged skin on Days 10, 17, 24, and 59. At Day 59, the most frequently cited satisfaction score was “6, completely satisfied” (Figure 3); less than 60 percent of the subjects indicated that they were “moderately satisfied” or “completely satisfied” with improvements in signs of photoaging. The mean and standard error of the mean (SEM) scores for subject satisfaction increased from Day 10 to Day 59 for the six characteristics of photoaging to a score intermediate between mildly and moderately satisfied (Figure 4).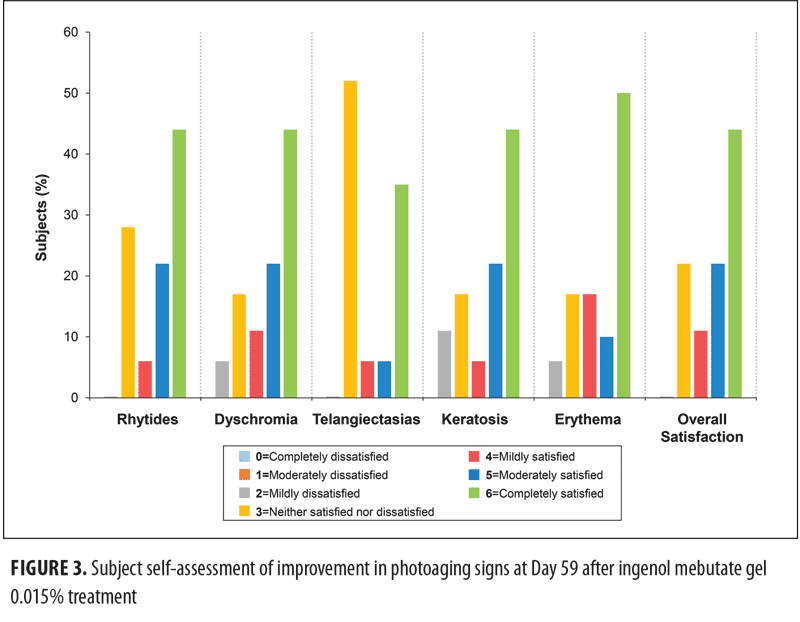
All subjects completed survey questions in the effectiveness, convenience, and overall satisfaction domains of the 14-item TSQM at one day after the last application of ingenol mebutate gel (Day 3) and at the end of the study (Day 59). All TSQM domain scores were less than 70 on Day 59; the convenience scores (91 at Days 3 and 59) were the highest of the four domains and likely reflect satisfaction with the three-day treatment regimen (Figure 5). Only subjects who indicated that they experienced any side effects of the medication (n=15 and n=8 on Days 3 and 59, respectively) answered questions in the side effects domain.
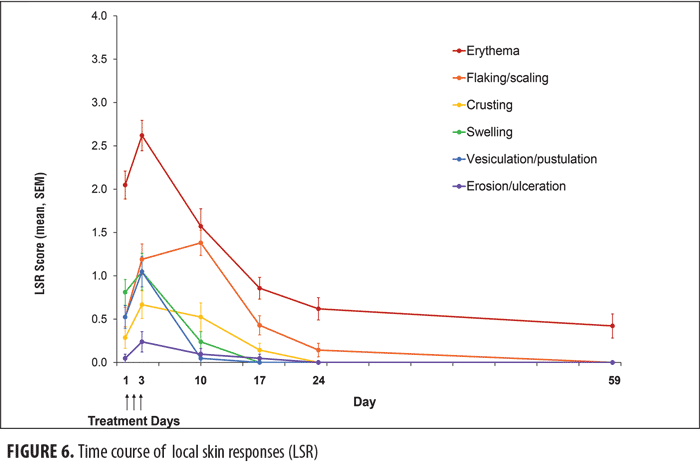
Safety. LSR developed within one day of treatment, generally peaked one day after the last application, and declined over the next 14 days (Figure 6). Scores for erythema were the highest across the six parameters. The mean composite LSR score (sum of the 6 individual reactions) at one day after the last application was 6.8. No adverse events were reported.
Discussion
Ingenol mebutate gel 0.05% applied once daily for two consecutive days is indicated for the treatment of AK on a 25cm2 contiguous area of the chest. However, this formulation may cause intense LSR at this site. Therefore, we conducted a study to evaluate the efficacy, safety, and effect on photodamaged skin of ingenol mebutate gel, 0.015%—the concentration approved for use on the face and scalp—applied once daily for three consecutive days. Subjects who presented with four or more AK lesions applied the ingenol mebutate on an expanded contiguous area of the chest, up to 100cm2. At Day 59, the lesion count was reduced by an average of 76 percent as compared with baseline. This lesion reduction was comparable to the 75-percent median reduction in AK lesion count at Day 57 found in the pivotal Phase 3 studies, in which the trunk and extremities were treated with ingenol mebutate gel 0.05% applied once daily for two consecutive days.8 Only a small subset of the subjects in the pivotal studies, however, treated AK of the chest specifically.[7]
The calculated mean composite LSR score (6.8) of subjects in the present study appeared to be lower than that of subjects in a Phase 3b study who used the 0.05% formulation to treat AK on the chest (mean composite score, approximately 10–14).[12] However, the results are not directly comparable, because subjects in the pivotal studies and the Phase 3b study treated an area of 25cm2 and not up to 100cm2, as in our study. Nevertheless, the results suggest that treatment of AK on the chest with ingenol mebutate gel 0.015% once daily for three consecutive days delivers clinically meaningful clearance of AK and may be more tolerable than ingenol mebutate gel 0.05% once daily for two consecutive days.
LSR following application of the 0.05% ingenol mebutate gel formulation are considered too intense for use of this dose on the face. Gaide and Cattin16 reported the case of a 72-year-old woman who inadvertently applied ingenol mebutate gel 0.05% to her cheeks instead of her chest. Very shortly thereafter, she experienced pronounced erythema of the skin over the cheekbones, superficial erosions, and conjunctivitis. Thirty days later, in addition to clearance of the AK, there was excellent cosmesis and smooth skin, with a rejuvenated look, and clearance of numerous solar lentigines. The authors who observed these results suggested a potential role for ingenol mebutate for skin rejuvenation.[16]
One relevant difference between chest skin and facial skin is the reduced number of pilosebaceous units: 30 times fewer units for the chest versus the face.[17] Pilosebaceous units are the progenitors of new epithelium after skin damage, including damage from topical agents used for field treatment of AK.17 Areas of skin that contain many pilosebaceous units tend to re-epithelialize more rapidly than areas with fewer units.[17] Another difference is the fact that the epidermis and dermis are thinner on the chest, and this also may impede healing after topical AK treatment. Finally, chest skin has a more variable distribution of subcutaneous fat compared with facial skin, which can result in an increased risk of scarring and pigmentary changes.[9,10]
Photoaging of the chest, as at other anatomic sites, is caused by a combination of damage from UV radiation and the intrinsic aging process.[18,19] Photodamaged chest skin is characterized by laxity, lines and wrinkles, hyperpigmentation, erythema, tactile roughness, atrophy, and telangiectasias.[10] Erythema and hyperpigmentation are more common on the superior décolletage, which is subject to more intense, cumulative UV exposure, whereas lines, wrinkles, and laxity folds develop more frequently on the inferior décolletage.
Topical ingenol mebutate may provide a beneficial effect on photodamaged skin. Erlendsson et al20 studied the preventive effect of ingenol mebutate 0.015% against progressive cutaneous photodamage from UV exposure over 20 weeks in hairless mice. The investigators assessed keratosis grade, epidermal hypertrophy, dysplasia, and dermal actinic damage. Monthly topical application of ingenol mebutate resulted in a significantly lower composite UV-damage score as compared with UV radiation alone, and it prevented progression of cutaneous photodamage in this murine model. An effect on photoaging in humans was reported by Braun and Gerber,[21] who observed a clearing of mottled pigmentation and a reduction in tactile roughness six weeks after treating AK on the scalp of an 82-year-old man with ingenol mebutate 0.015% once daily for three consecutive days. The authors commented that ingenol mebutate “may exert a noteworthy cosmetic effect on signs of skin aging” and proposed its use as a novel therapeutic option for photoaged skin. In our study, we observed a significant reduction from baseline on Day 59 in all six assessed signs of photoaging. In addition, patients’ assessment of the skin manifestations of photoaging and patient satisfaction with treatment improved across all of these parameters from Day 10 to Day 59. TSQM scores for medication effectiveness and overall satisfaction also increased from Day 3 to 59. This finding is consistent with improvements in signs of photoaging reported for a variety of field-based, topical, and procedural therapies for AK.[22]
Limitations. This was a noncomparative, open-label study that evaluated a relatively small number of subjects, thus precluding any direct comparison with the results of the pivotal clinical trials.
ConclusionsTreatment of AK on an expanded area of the chest (?100cm2) with ingenol mebutate gel 0.015% reduced the AK lesion count by an average of 76 percent compared with baseline. Signs of photoaging were reduced significantly from baseline by the end of the study, and fewer than 60 percent of subjects indicated that they were “moderately” or “completely” satisfied with improvements in signs of photoaging. Ingenol mebutate gel 0.015% was well tolerated, with resolution of LSR generally within two weeks of completing treatment.
Acknowledgments
Writing assistance was provided by William Fiedelman, from p-value communications. LEO Pharma Inc. provided the funding for this assistance.
References
- Criscione VD, Weinstock MA, Naylor MF, et al. Department of Veterans Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523–2530.
- Feldman SR, Fleischer AB. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201–207.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9–22.
- Touma D, Yaar M, Whitehead S, et al. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol. 2004;140:33–40.
- Uhlenhake EE. Optimal treatment of actinic keratoses. Clin Interven Aging. 2013;8:29–35.
- Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? Part 1: Overview and investigational topical agents. Cutis. 2012;89:241–250.
- Picato® (ingenol mebutate) gel 0.015%, 0.05% [package insert]. Madison, NJ: LEO Pharma Inc.; September 2016.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010–1019.
- Fabi SG. Fillers for the décolletage area. Pract Dermatol. 2015;Sep:29–30, 40.
- Peterson JD, Kilmer SL. Three-dimensional rejuvenation of the décolletage. Dermatol Surg. 2016;42(Suppl 2):S101–S107.
- Schlessinger J, Green B, Edison BL, et al. A firming neck cream containing N-acetyl glucosamine significantly improves signs of aging on the challenging neck and décolletage. J Drugs Dermatol. 2016;15:47–52.
- Pellacani G, Peris K, Guillen C, et al. A randomized trial comparing simultaneous vs sequential field treatment of actinic keratosis with ingenol mebutate on two separate areas of the head and body. J Eur Acad Dermatol Venereol. 2015;29:2192–2198.
- Alexiades-Armenakas M. Rhytides, laxity, and photoaging treated with a combination of radiofrequency, diode laser, and pulsed light and assessed with a comprehensive grading scale. J Drugs Dermatol. 2006;5:731–738.
- Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the Treatment Satisfaction Questionnaire for Medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(suppl 1):S9–S24.
- Rosen R, Marmur E, Anderson L, et al. A new, objective, quantitative scale for measuring local skin responses following topical actinic keratosis therapy with ingenol mebutate. Dermatol Ther. 2014;4:207–219.
- Gaide O, Cattin V. Ingenol mebutate 500 ?g on the cheekbones with concomitant conjunctivitis. Dermatology. 2016;232(Suppl 1):4–6.
- Niamtu J III. Skin resurfacing. In: Niamtu J IIl. Cosmetic Facial Surgery. New York: Elsevier Health Sciences; 2011:517–603.
- Trojahn C, Dobos G, Lichterfeld A, et al. Characterizing facial skin ageing in humans: disentangling extrinsic from intrinsic biological phenomena. Biomed Res Int. 2015;318586:1–9.
- McDaniel DH, Hamzavi IH, Zeichner JA, et al. Total defense + repair: a novel concept in solar protection and skin rejuvenation. J Drugs Dermatol. 2015;14(suppl 7):S3–S11.
- Erlendsson AM, Thaysen-Petersen D, Bay C, et al. Repeated treatments with ingenol mebutate prevents progression of UV-induced photodamage in hairless mice. PLoS One. 2016;11:e0162597.
- Braun SA, Gerber PA. Cosmetic effects of ingenol mebutate gel in the treatment of field-cancerized photodamaged skin. Dermatol Surg. 2015;41:1328–1329.22. Lanoue J, Do T, Goldenberg G. Therapies for actinic keratosis with a focus on cosmetic outcomes. Cutis. 2015;96:165–172, 193.

