 J Clin Aesthet Dermatol. 2023;16(2):29–36.
J Clin Aesthet Dermatol. 2023;16(2):29–36.
by Luc Duteil, PhD; Catherine Queille-Roussel, MD; Hanane ISSA, PhD;
Natalia Sukmansaya MD; Jane Murray, BSc; and Ferial Fanian, MD, PhD;
Drs. Duteil, Queille-Roussel, and Ms. Murray are with the Center of Clinical Pharmacology Applied to Dermatology at L’Archet 2 Hospital in Nice, France. Drs. Fanian, Issa, and Sukmansaya are with FILLMED Laboratories in Paris, France.
ABSTRACT: Background. Skin bio-revitalization improves skin quality globally; it permits the rejuvenation of the skin by increasing hydration and by reconstructing an optimal physiological environment for the skin cells together with a micro-filling effect.
Objective. To assess the comparative efficacy of a non-cross-linked hyaluronic acid (NCHA) preparation (M-HA®10, FILLMED Laboratories, France) on fine lines reduction and on skin hydration, radiance and mechanical properties, after three sessions of multiple intradermal injections, active versus placebo, on the face of subjects presenting aging signs.
Methods. Thirty healthy subjects received filler injections on one side and a control solution (saline) on the contralateral side of the face. Fine lines depth, skin hydration, and mechanical properties were evaluated using instrumental methods. Skin radiance, cheek fold and crow’s feet were scored clinically. In addition, Investigator and subject satisfaction rates were evaluated by the Global Aesthetic Improvement Scale and a subject self-assessment questionnaire.
Results. Ten days after the last multi-injection session, the following significant results were observed compared to the control: a reduction of both crow’s feet wrinkle depth (in the 110 to 1000µm range, -10% for NCHA and +7% for control) and clinical scoring of cheek wrinkles, and increases in skin radiance and hydration (+35%) and also skin firmness (+27%). The Investigator found that NCHA either improved or much improved the aesthetic aspect on 82% of subjects whereas no improvement was found on the saline side. Subjects found that NCHA significantly reduced wrinkles and increased both skin firmness and elasticity.
Conclusion. Intradermal injection of NCHA can improve the quality of facial skin with aging signs by reducing fine wrinkles and improving hydration, firmness and radiance.
Keywords. Non-cross-linked hyaluronic acid, mesotherapy, skin bio-revitalization
Chronological aging and environmental causes (e.g., UV exposure) induce morphologic and mechanical changes that manifest as skin wrinkling, sagging, loss of elasticity and dryness. In particular, decreased synthesis combined with increased degradation of collagen and elastin, reduced proliferative capacity of fibroblasts, and perturbations in the organization of the elastic fiber network leads to alterations in the mechanical properties of the skin resulting in reduced resilience and elasticity. As skin aging has an important social impact, new approaches have been continuously developed to overcome it in recent years. Facial fillers have been widely used as a minimally invasive procedure for skin rejuvenation. Among these products, HA-based fillers are the most popular and are relatively safe.1–3
Skin bio-revitalization improves the skin globally; it permits the rejuvenation of the skin by increasing hydration and by reconstructing an optimal physiological environment for skin cells together with a micro-filling effect. In recent years there has been an increasing emphasis on this type of treatment as an anti-aging strategy. The history of skin bio-revitalization originates from a medical procedure introduced by Pistor in 1958, which consists of intradermal injection of diluted pharmacologic substances, such as nutrients, hormones, vitamins, enzymes and other reagents that are administered directly to the region to be treated.4 In skin rejuvenation, the aim of this treatment is maintenance and/or restoration of healthy and youthful skin texture.5,6 The desired final effect is a firm, bright and moisturized skin which is achieved by injection into the superficial dermis of suitable, perfectly biocompatible, totally absorbable in products.7 Injection of bio-revitalizing products promotes skin rejuvenation by increasing both hydration and fibroblast activation.8,9 Several experimental studies have demonstrated that HA injected into the skin stimulates fibroblasts to express collagen Type 1 (Col-1), matrix metalloprotease-1 (MMP-1), and tissue inhibitor of matrix metalloproteinase-1 (TIMP).10
Non-cross-linked HA is widely used in skin bio-revitalization as it is an endogenous glycosaminoglycan which occurs particularly in the extracellular matrix. It binds and retains water thus improving the tone and elasticity of the skin. HA used in bio-revitalization should not be cross-linked as its main function is to hydrate. Conversely fillers, whose main function is to fill wrinkles, are cross-linked in order to resist tissue hyaluronidase activity.
Hyaluronic acid is a natural polysaccharide which is largely biocompatible and has a short half-life. It is a ubiquitous component of the extra cellular matrix and is one of the main components of the connective-tissue matrix. The dermis contains 50% of body HA. Key in the role of skin hydration and elasticity, HA forms the elastoviscous fluid matrix in which collagen and elastin fibers are embedded. The hydrophilic nature of HA attracts and maintains water within the extracellular space, thus affecting dermal volume and compressibility. The molecular mass of HA in the skin is 1kDa, occurring at concentrations of 15µg/g in the epidermis and 740µg/g in the dermis. Sutherland11 demonstrated that one gram of HA can bind up to six liters of water. A unique characteristic of HA fillers is isovolumetric degradation. As individual molecules of HA degrade, those remaining are able to bind more water and the overall volume of the gel remains unchanged. In other words, the concentration of the gel decreases during reabsorption, but volume of the gel remains steady until the last molecules of HA are degraded. Clinically, this translates as an implant that maintains more than 95 percent of its initial space-filling volume until the last of the material is completely reabsorbed.
A study was conducted by Röck et al12 to investigate whether human skin fibroblasts are affected by exogenous NCHA with respect to proliferation, migration and extracellular matrix composition. The results showed that proliferation was significantly stimulated by HA whereas migration was unaffected. Importantly, exogenous HA was incorporated into fine HA filaments of the pericellular fibroblast matrix. According to these results, published in 2010, this was the first evidence that a human-use, cosmetic HA formulation stimulates fibroblast proliferation.
In order to elucidate the cellular and molecular processes underlying mesotherapy, Jäger et al13 analyzed the effect of five distinct formulas on pivotal parameters involved in skin aging (i.e., collagen expression, cell proliferation and morphological changes) using in vitro normal human skin fibroblast (HSF) cultures. The authors concluded that the distinct formulations currently used in mesotherapy induced strikingly divergent molecular and cellular processes in HSF in vitro. They suggested that more detailed studies were warranted to elucidate whether and how the cellular and molecular processes induced by the five formulas tested in HSF in vitro, are involved in facial skin rejuvenation in vivo, and whether these processes are similarly efficient, independent of the age of the recipients.
The main objective of the present study was to assess the efficacy of a NCHA preparation (M-HA®10, FILLMED Laboratories, France) on crow’s feet wrinkle fine lines reduction after three sessions of multiple intradermal injections. The secondary objectives were to assess the efficacy of M-HA®10 on skin quality parameters such as hydration, radiance and elasticity as well as the cheek fold and crow’s feet clinical scores. In addition, Investigator and subject GAIS, and subject SAQ were completed.
Methods
The study was performed in accordance with the Good Clinical Practices and the principles of the Declaration of Helsinki. The procedures used in this study were approved by the Ethics Committee Ouest III of Poitiers, France (N° 2019-A01473-54). Before any study procedure, the subjects received the necessary written and verbal information including an informed consent form. Eligibility was determined by physical examination and confirmation of all inclusion/non-inclusion criteria.
Subjects. Healthy subjects of both sexes aged at least 19 years or older with Skin Type I to IV according to the Fitzpatrick scale14 were recruited for the study. Subjects had to present aging signs on the face: Grade 3 or 4 on the Bazin crow’s feet severity scale15 and Grade 2, 3 or 4 on the Bazin cheek fold severity scale.15 They had to avoid exposure to ultraviolet (UV) radiation (tanning beds, phototherapy, and sunlight) on the face for at least one month before the screening visit and for the whole duration of the study. Subjects with a history of aesthetic facial injections (HA within the previous year, botulinum toxin or mesotherapy within the previous six months and long-term temporary injectable implants (semi-permanent implants) within the previous two years) were not included in the study. In addition, subjects having had laser sessions for skin rejuvenation or laser resurfacing during the 12 previous months or a surgical facelift during the two years before the study were not included. Subjects also agreed to not apply cosmetic, medical or aesthetic treatments on the face for the whole study duration.
Treatments and study design. The Investigational product was a sterile, biodegradable, viscoelastic, colorless, transparent, isotonic and homogenized injectable gel implant (M-HA®10, FILLMED laboratories, France). M-HA®10 is composed of 10mg/mL of non-cross-linked, non-animal sodium hyaluronate, obtained by bio-fermentation. Physiological saline (NaCl 0.9 %, injectable solution, Lavoisier, France) was used as control product.
This was a randomized, double-blind, placebo-controlled, split-face study and consisted of three injection/assessment sessions at three-week intervals. Assessments were performed before each injection session at baseline (Day 1), on Day 22 and on Day 43. A last assessment visit (Day 53) was performed ten days after the third session of injections.
At each injection session, the tested products were injected at 0.05mL per point for a total amount of 3mL by split face. The 3mL syringes equipped with 32G x 4mm needle were used to inject the tested products and also placebo (normal saline). Injections were made at an angle of 10° to 15° to the skin surface. The plane of injection was the deep dermis and each injection was spaced from 1 to 1.5cm apart (see Figure 1). The treatment allocation to the left/right side of the face was determined by a randomization list. The randomization list was generated using the R software version 4.0.1. This list randomized the thirty subjects to receive study injection vs placebo on left versus right. The list was balanced by block of 10 subjects. In order to keep the double blinding and as the syringes of study product and placebo were not quite identical, the injector covered the subjects’ eyes during the injection, so the subjects were not aware of the nature of the product on either side of the face. In parallel, the “evaluator” was different from the “injector” investigator. The “injector” investigator was not involved in the clinical assessments which were performed by the main investigator (evaluator).
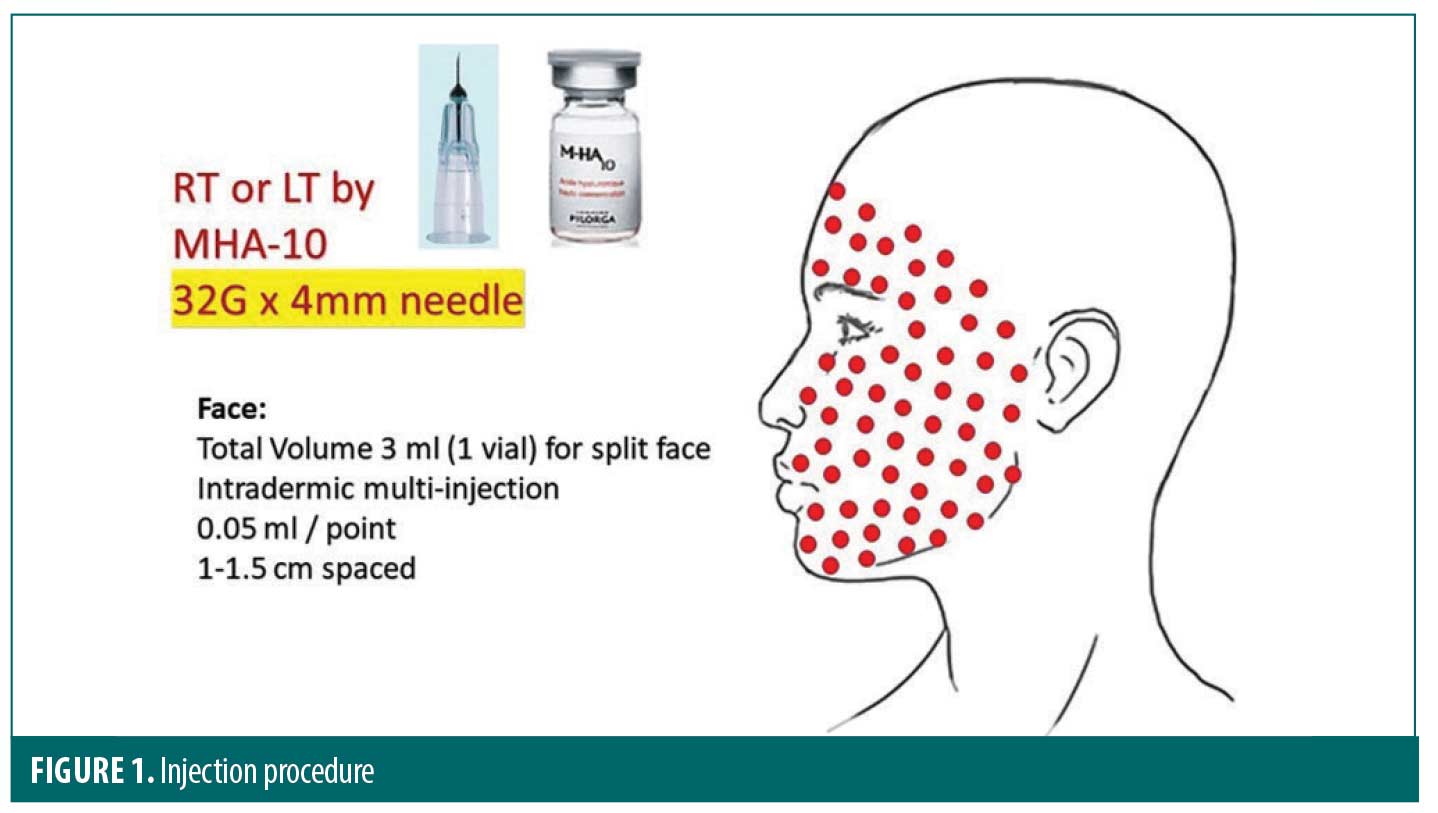
Evaluation methods. There were two evaluation times: at baseline (Day 1), before the first injection session and at Day 53, 10 days after the third injection session.
Silicon replicas of Crow’s feet: skin texture and more specifically, quality of facial lines can be evaluated by making negative impressions of the skin. These replicas are made by positioning a locating ring on the sampling area which is then filled with Silflo® impression material.
Replicas are analyzed using an image analysis system developed by Monaderm (98000, Monaco). The replica is lit with incident light at 35° which produces shadowing behind each wrinkle or hollow. Digitized resultant images are recorded by a CCD camera and then analyzed by dedicated software (Quantirides® software, Monaderm, Monaco). Parameters such as mean depth and mean length of wrinkles are calculated. Parameters are also analyzed by class of wrinkle based on their initial depth: Class 1 (0 – 55µm, very superficial lines), Class 2 (55 – 110µm, superficial lines) and Class 3 (110 – 1000µm, medium to deep lines).
Clinical evaluation of crow’s feet and cheek fold depths were performed for each side of the face using Bazin’s validated photographic scales.15
Skin radiance on each side of the face was evaluated by the Investigator using a 5-point scale (from 0: very dull skin to 4: very radiant skin); lighting conditions and position of the subject were standardized.
Skin hydration was measured using a Corneometer® CM825 (Courage-Khazaka, Köln, Germany). This instrument measures the hydration status of superficial skin layers using electrical capacitance measurements. The probe is placed perpendicularly on the skin surface and measurements are taken in less than one second. Subjects were instructed to abstain from washing the face for a period of at least two hours prior to evaluation visits and were acclimatized for 20 minutes in a humidity-controlled environment prior to making hydration measurements. Measurements were performed in triplicate on the mid-cheek on each side of the face.
Skin mechanical properties were evaluated using the Cutometer® DUAL probe (Courage and Kazhaka, Köln, Germany). Using a suction method, this instrument measures the deformation in a plane perpendicular to the skin surface. A cycle of 400 mbar negative pressure is applied to the skin through the probe for a period of two seconds, followed by a relaxation period of two seconds; this cycle is repeated five times. The induced deformation of the skin is measured by an optical system housed in the probe which has an aperture of 2mm diameter. Different biomechanical parameters are calculated from the skin deformation curve including Uf, the total distensibility of the skin; Ue, the elastic deformation; Ur, the elastic back deformation; Ua, the total deformation recovery; Uf – Ua the residual deformation. Three measurements of five cycles were performed on the mid-cheek on each side of the face. Mechanical deformation and hydration measurements were made on separate sites.
Skin assessments. The Investigator GAIS (IGAIS) was assessed in relation to baseline and has seven levels (from +3 for very much improved to -3 for very much worse). This score represents the overall impression of the expert and was assessed by the Investigator for each half-face, at every visit from Day 22 to Day 53. A Subject GAIS (SGAIS) was also performed by the subjects for each half-face, at every visit from Day 22 to Day 53. The IGAIS and the SGAIS use identical scales.
Standardized photographs (face, two profiles, two obliques) were taken at Day 1 and Day 53 visits to illustrate the effects of the treatment. Photographs were taken using a CANON EOS20 camera equipped with a 60mm lens f1/2.8. The settings of the camera, the lighting (LED tubes, T 4000°K) and the distance were standardized to ensure that comparison of photographs of before and after the three sessions of mesotherapy was reliable.
At Day 53, a SAQ was used to evaluate subjects satisfaction with the treatment in terms of reduction of skin wrinkles, as well as increase of skin radiance, hydration, firmness and elasticity.
Throughout the study, the Investigator had to inquire about any adverse event (AE) occurrence, by interviewing the Subject using an open question taking care not to influence the Subject’s answer and, if appropriate, by directed questioning and clinical examination.
Aspect and sensitivity of treated areas was assessed separately from other AEs. These reactions were scored by the investigator at each visit from the first injection until the end of the study. Concerning aspect, the following parameters were assessed: erythema, ecchymosis, hematoma, edema, dyschromia, irregularity at palpation (nodule/papule), necrosis, Tyndall effect and over-correction. Concerning sensitivity, the following variables were scored: pain at palpation, pruritus and spontaneous pain after injection.
Statistics. Statistical analyses were performed using R statistical Software (version 4.0.2).
For “wrinkle depth” (primary efficacy criterion), the data were analyzed using a 2-factor repeated measure mixed ANOVA with Treatment, Time and Time x Treatment interaction as fixed effects and Subject as random effect. If the model assumptions were not met (residue distribution), the analysis had to be performed on the transformed data (ranks). Concerning the secondary criteria, for continuous variables (e.g., hydration, biomechanics, relief and elasticity parameters), the analyses were identical to those used for the primary criterion.
For the score variables (Bazin cheek fold, Bazin crow’s feet and radiance), treatments were compared using the Wilcoxon rank sum test.
Concerning the SGAIS and IGAIS scores, for each side the change from baseline was transformed into a binary endpoint as follows:
- Improvement of at least one point
- No change or worsening
The data was analyzed using a mixed model for repeated ordinal data with treatment and time as fixed factors and subject as random factor. For each treatment, a linear mixed model analysis with one within subject factor “Time” (repeated measures) was performed. Post-hoc comparisons were done between each evaluation time and baseline (Day 0) using a Dunnett test.
For the SAQ, the frequency of responses to questions was analyzed by the Khi-2 test. If the number of responses to one or several categories was less than five, categories were grouped (i.e., positive responses on one side, and negative responses + no opinion on the other).
Results
Thirty healthy subjects (29 females and 1 male, mean age 57 ± 7 years) were randomized and 28 completed the study. Two subjects did not complete the study (one consent withdrawal, one serious AE unrelated to the study content). Four subjects were Fitzpatrick Skin Type II and 24 were Skin Type III. The Per Protocol (PP) analysis set, included the 28 subjects and was used for efficacy analysis. All female subjects of childbearing potential (n=8) were using an approved method of contraception and had a negative urinary pregnancy test at Screening, Day 1, Day 22 and Day 43.
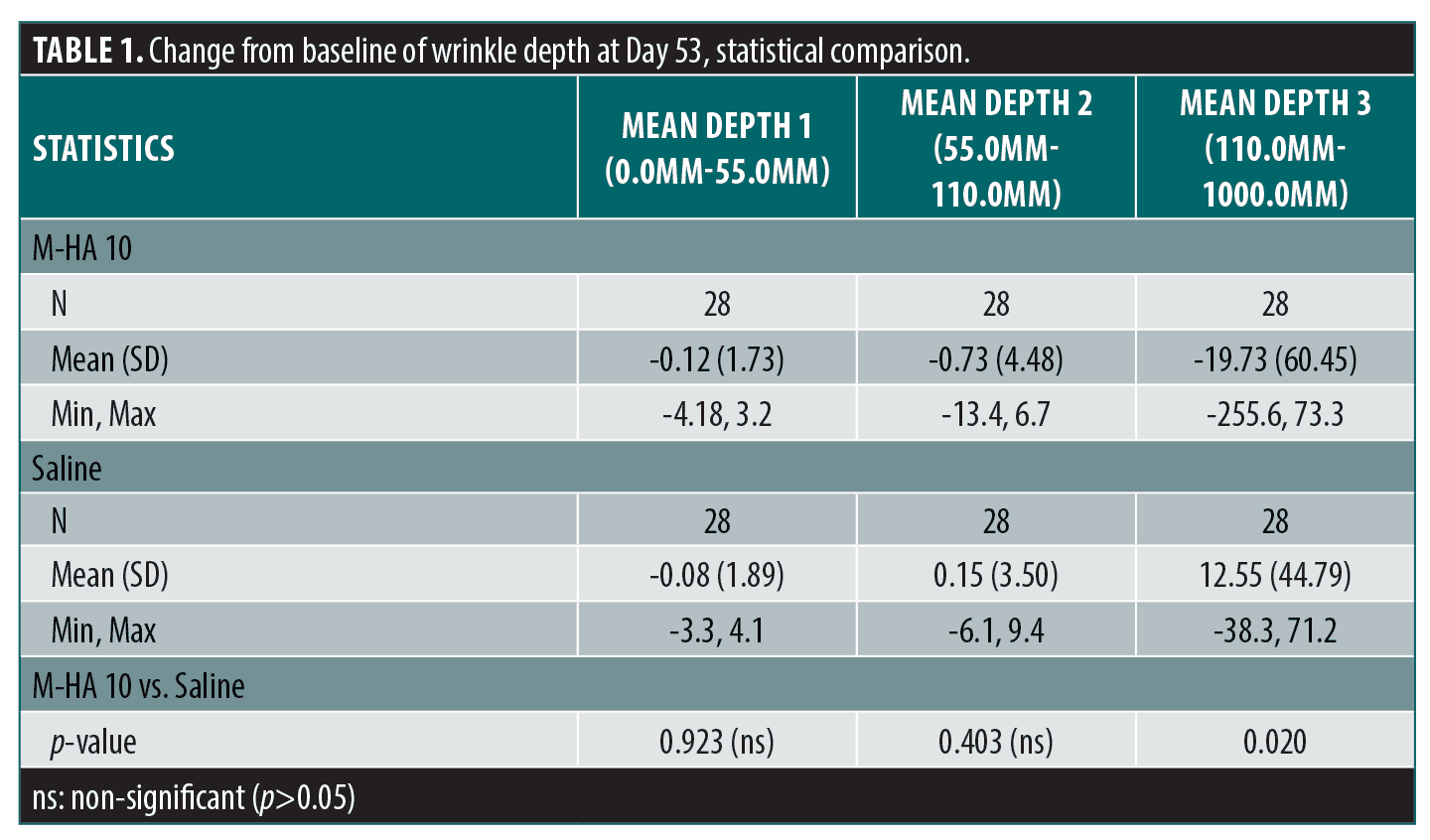
Instrumental parameters. Concerning the primary criterion, crow’s feet wrinkle depth, there was a significant difference in change from baseline between M-HA®10 and saline for the wrinkles in the range 110 to 1000µm (see Table 1). It can be noted that for M-HA®10, the majority of variations were negative (reduced wrinkle) whereas for saline the majority of variations are close to zero or positive (no effect).
Descriptive statistics of clinical scores for crow’s feet, cheek folds and radiance are summarized in Table 2; statistical comparisons are also shown.
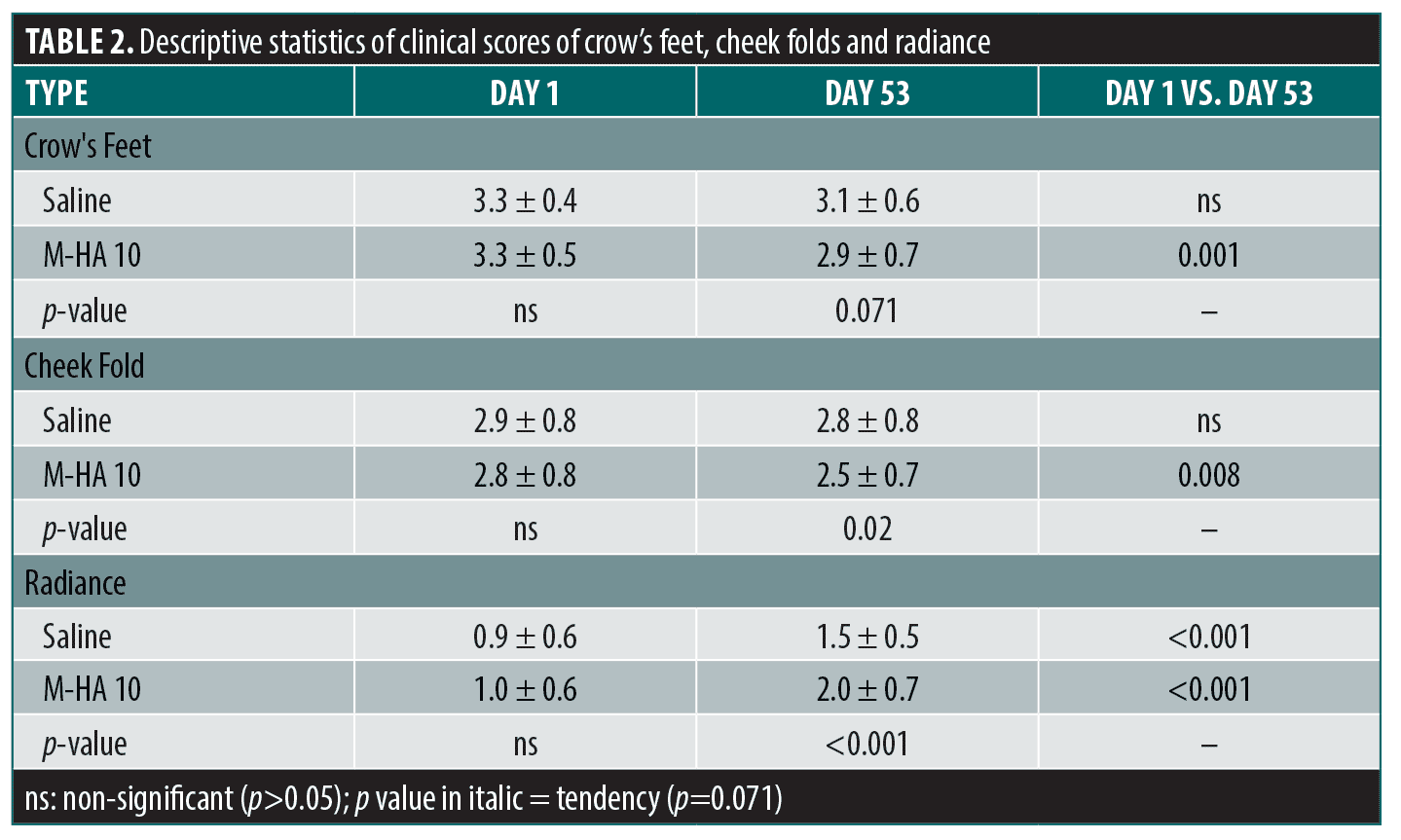
Compared to baseline, the Bazin crow’s feet wrinkle scores decreased significantly for M-HwA®10 (p=0.001) whereas no significant difference was detected for saline. Results of comparative statistics on the Bazin crow’s feet wrinkle scores between two sides show that the difference between M-HA®10 and saline is close to being significant (p=0.071) at Day 53 in favor of M-HA®10.
Concerning the cheek folds, a significant difference was observed between M-HA®10 and saline at Day 53 (p<0.02) and a significant decrease in depth compared to baseline at Day 53 for M-HA®10 (p=0.008).
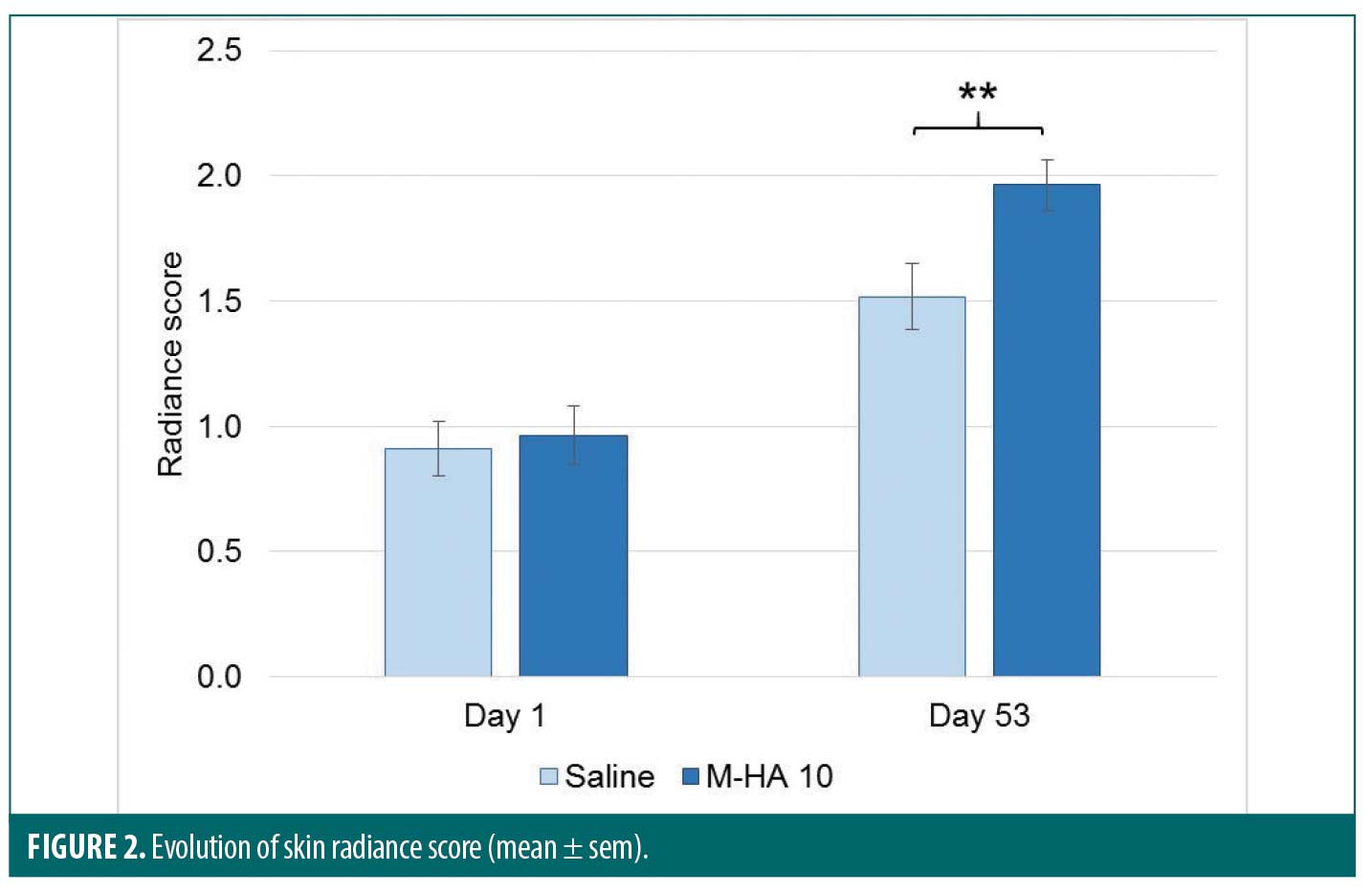
Figure 2 shows the evolution of radiance score for both treatments. The skin radiance increased significantly for both products. Nevertheless, radiance score was significantly higher at Day 53 for M-HA 10 compared to saline (p<0.001).
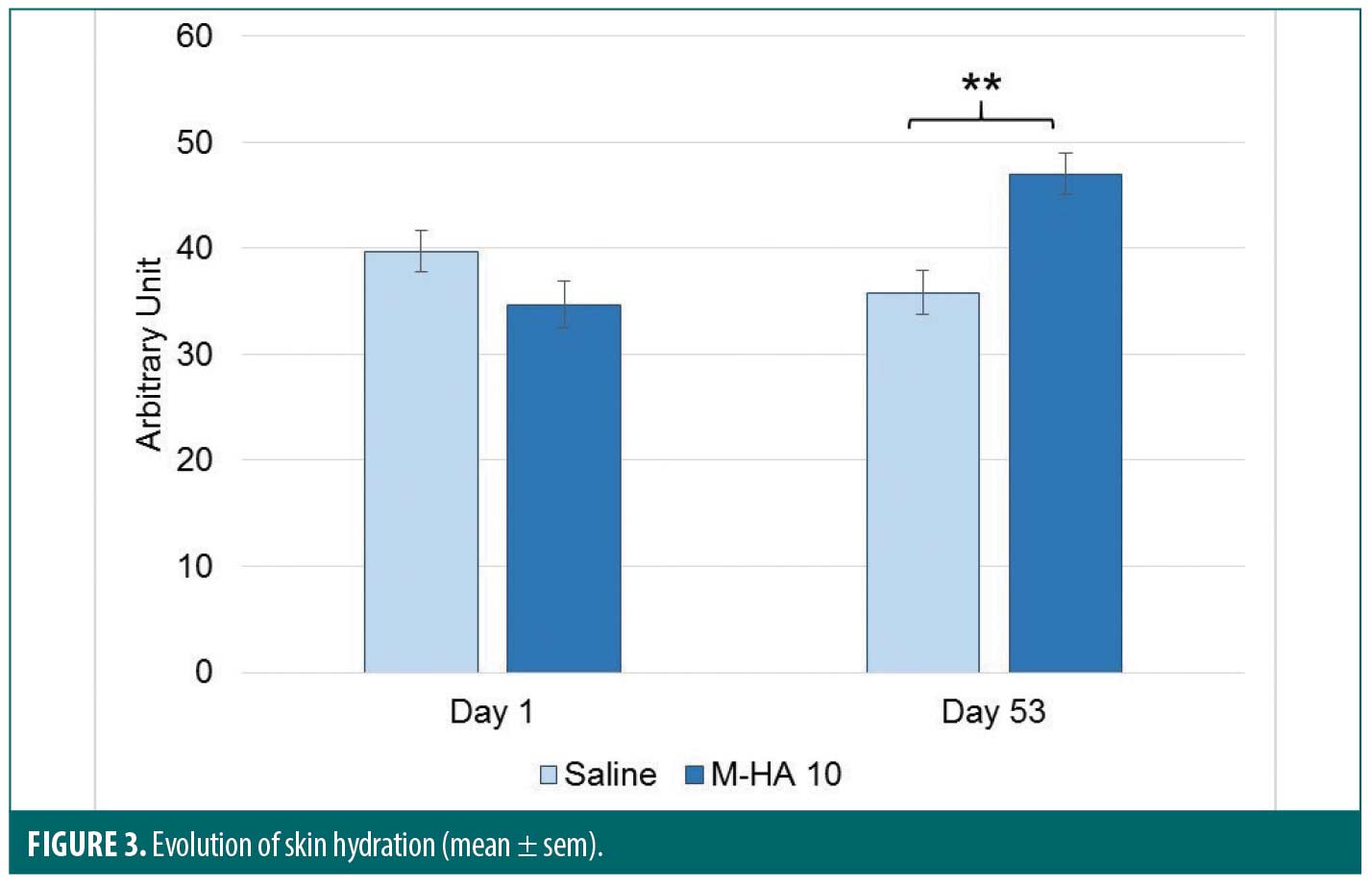
The evolution of skin hydration is shown in Figure 3 for both treatments. Results of the global statistical analysis, performed on skin hydration measured by Corneometer®, show a significant Visit effect (p=0.044) and a significant difference between M-HA®10 and saline (p=0.049). At Day 53, a significant difference was noted in change from baseline hydration values between M-HA®10 and saline (p<0.01).
Concerning the skin bio-mechanical parameters, a significant decrease of the residual deformation (R1) after one aspiration-release cycle of was observed in favor of M-HA 10 (p=0.018) as shown in Figure 4.
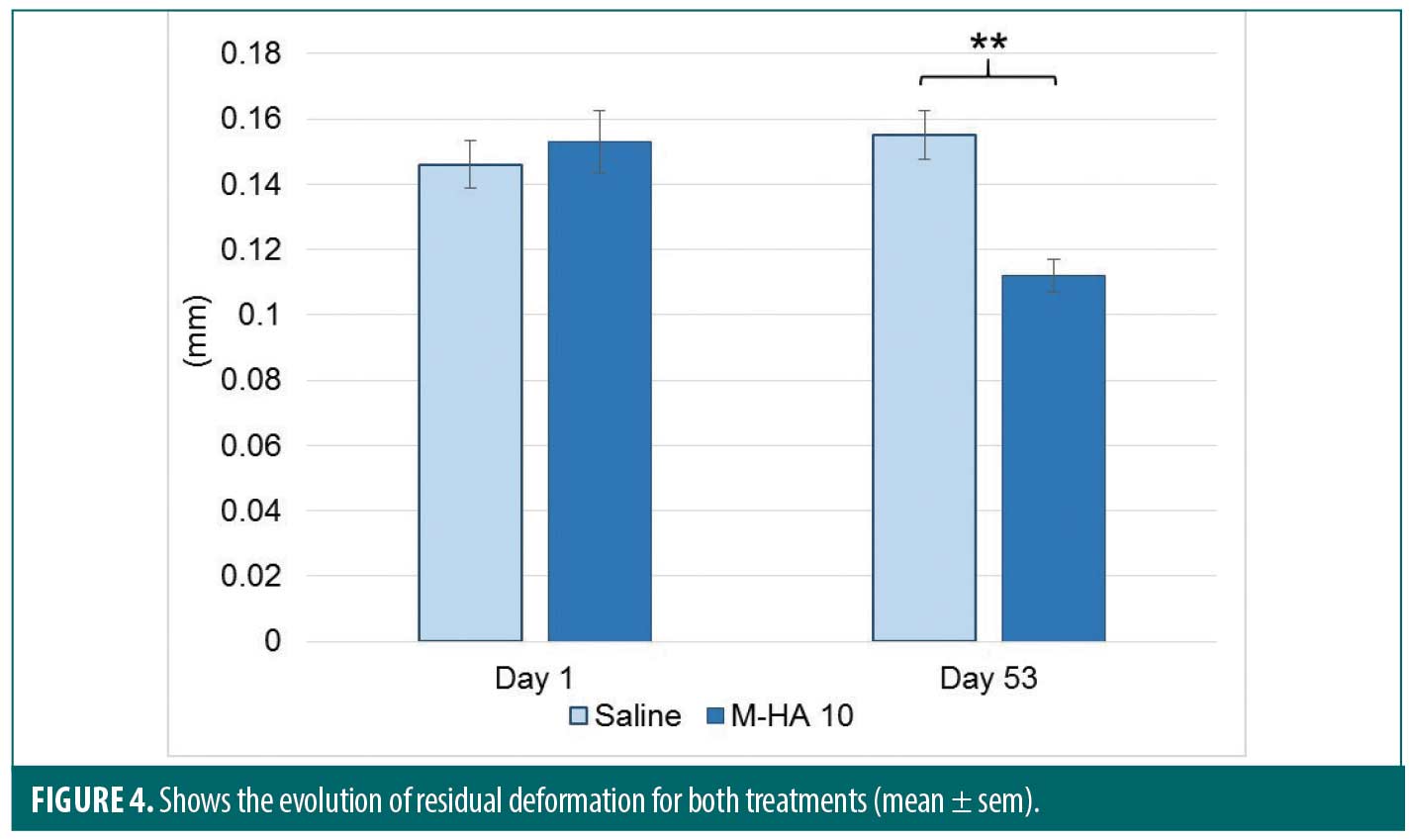
Similar results were obtained with the minimal point of the last curve (R4). For M-HA 10, R1 significantly decreased from 0.218 ± 0.065 (mean ± SD) at baseline to 0.162 ± 0.036 at Day 53 whereas for saline a non-significant evolution was observed (0.214 ± 0.048 at baseline and 0.223 ± 0.050 at Day 53). At Day 53, a significance between both products was detected in favor of M-HA®10 (p=0.03).
Both bio-mechanical parameters indicate an increase of skin firmness at Day 53.
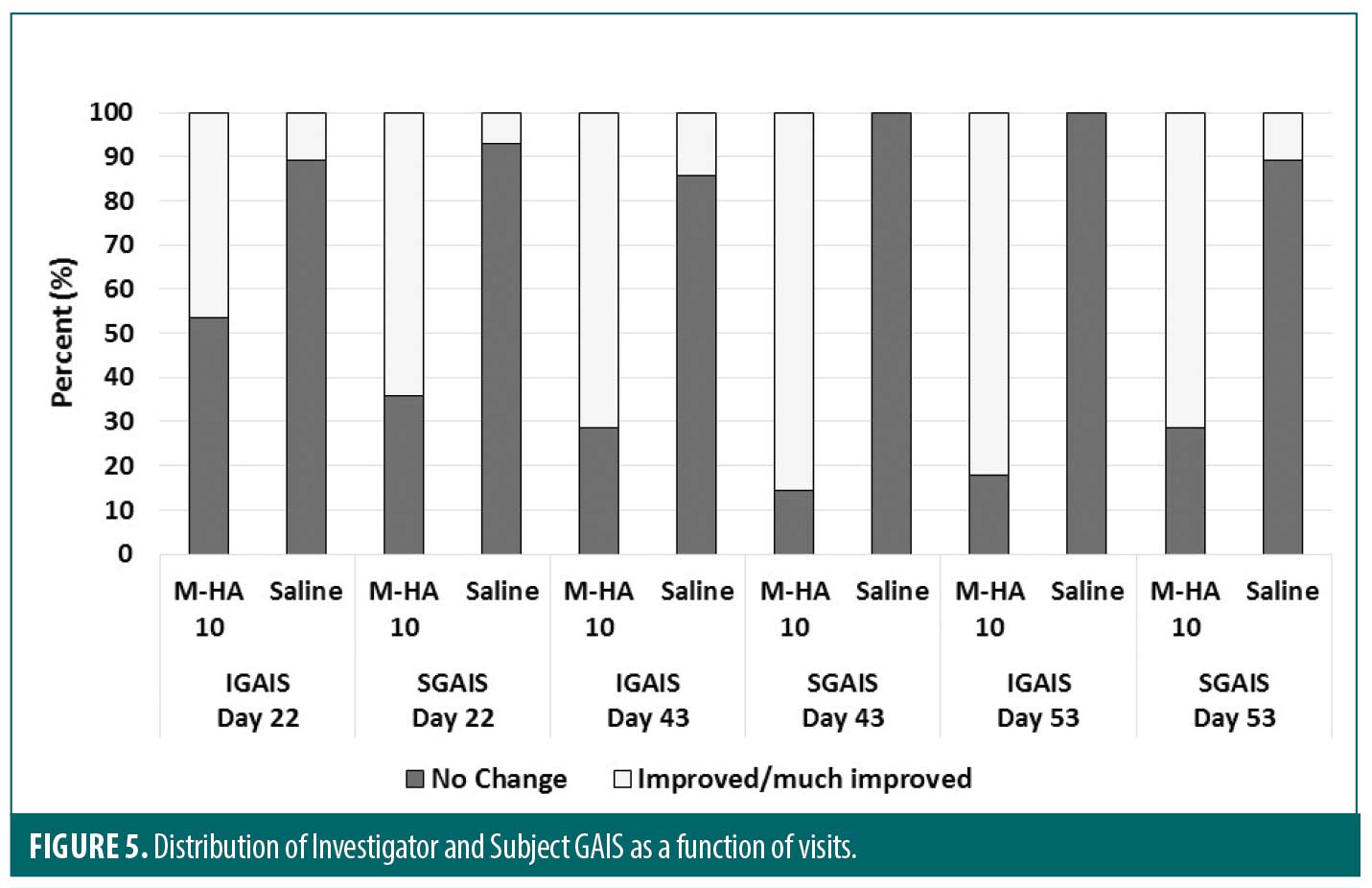
Global Aesthetic Improvement Scale and Questionnaire. The IGAIS was significantly in favor to M-HA®10 compared to saline at each visit (p<0.001). Similar results were observed for the SGAIS which was significantly in favor to M-HA®10 compared to saline at each visit (p<0.001). Figure 5 displays the distribution of Investigator and Subject GAIS scores as a function of visits.
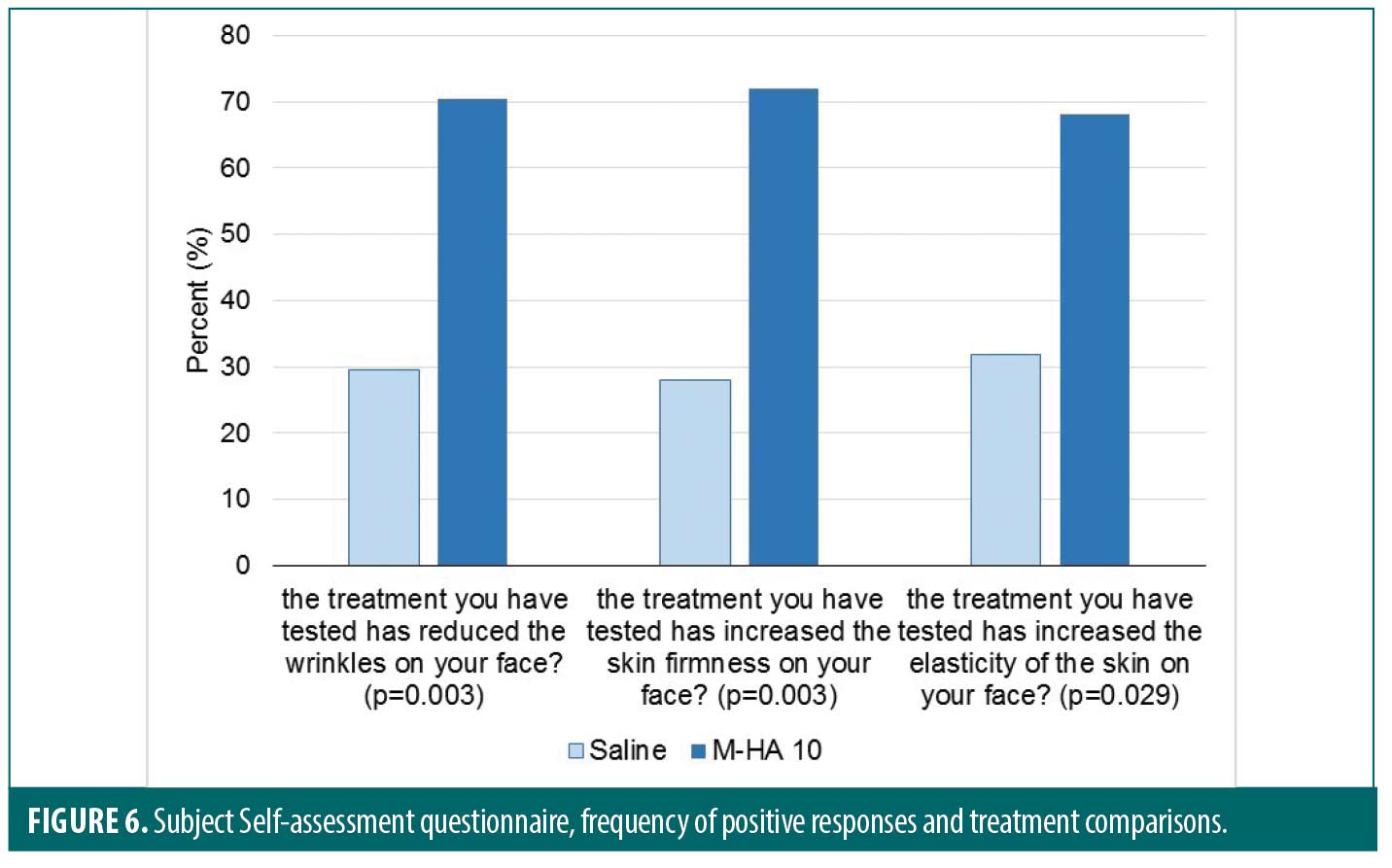
Results concerning the SAQ are displayed in Figure 6. The questions are given in the Figure.
Safety aspects. The aspect and sensitivity of treated areas were assessed separately from other AEs. The observations of expected local adverse events indicated that more edemas and irregularity at palpation were observed following the injection sessions on the M-HA®10 treated side compared to the saline side. This is a normal observation due to the consistency of the M-HA®10 which is less fluid than the saline solution. In addition, more ecchymosis were observed at the D22 and D43 visits on the M-HA®10 treated side. All of these AEs resolved in a few days. No necrosis, Tyndall effect or overcorrection was observed in this study. Very few pruritus reactions were observed on either M-HA®10 or saline treated sides.
Overall, for the safety population, 6/30 subjects (22.6%) experienced a total of nine AEs. Most of these AEs were of mild (n=4) to moderate (n=3) intensity. Only one severe intensity were reported (rhinopharyngitis) which was spontaneously resolved before the end of study. There was one Serious Adverse Event (SAE, coronary spasm) not related to the study treatment or procedure and led to the subject discontinuing the study. None of the reported AEs and SAE were judged as related to the study treatments.
Discussion
Skin bio-revitalization is a generic term used to describe a nonsurgical aesthetic treatment comprising multiple injections of vitamins, nutrients, oligo elements and HA into the skin layers. This treatment for aging skin aims to replace depleted HA levels, minerals, vitamins, and amino acids, but also to provide deep hydration. Furthermore, injecting HA into the dermal layers just below the skin surface may help stimulate collagen production and improve skin tone.16,17 M-HA10® is indicated for improvement of skin hydration and elasticity by multi-injection into the dermal/epidermal junction and into the superficial dermis to improve skin tone and reduce fine lines and wrinkles.
Several clinical studies have demonstrated that intradermal HA administration increases tissue water retention as well as fibroblast activity.18,19 In particular, it has been shown to stimulate fibroblasts to express collagen Type 1 (Col-1), matrix metalloprotease-1 (MMP-1) and tissue inhibitor of matrix metalloproteinase-1 (TIMP).20,21 A study of 50 subjects confirmed the safety and efficacy of HA mesotherapy in the prevention and treatment of skin aging; a decrease in IL1b, IL6, MMP1 and an increase in Col-121 was also demonstrated.
The primary objective of the present study was to assess the efficacy of M-HA®10 on facial fine wrinkle reduction after three sessions (Day 1, 22 and 43) of multiple intradermal injections versus a placebo (saline solution). There are few publications which show the superiority of the product over placebo, as microneedling itself has a positive rejuvenating effect. Therefore, this study design will assist in elucidating whether the observed effects are produced by the procedure itself or by the product. This was a monocentric, double-blind, randomized, placebo-controlled (saline) study performed with intra-individual comparisons.
The results demonstrated that the study product caused significant clinical and biophysical changes in the skin. Concerning the primary efficacy criterion, crow’s feet wrinkle depth measured instrumentally, a significant positive evolution from baseline was observed between M-HA®10 and saline for wrinkles in the range 110 to 1000µm. Moreover, it was observed that on the M-HA®10 side, most differences were a reduction of wrinkle depth whereas on the saline side most differences were close to zero, showing no effect. This was confirmed by the crow’s feet clinical evaluation score, where a significant decrease was observed between Day 1 and Day 53 on the M-HA 10 treated side but not for the saline treated side. Similarly, the SAQ showed that the subjects found a significant reduction of wrinkles on the side treated by M-HA®10, albeit they were unaware of which side had been treated with the product. These results align with those obtained by other authors using NCHA alone or combined with antioxidant complex.22,23
A more marked effect was observed on the cheek folds, where the difference between M-HA®10 and saline was significant at Day 53 with a significant decrease (reduction of cheek fold score) compared to baseline at Day 53 for M-HA®10. This could show that the microneedling is effective only on the thin skin areas such as crow’s feet although there was still a difference between the two sides.
For this study, skin radiance was assessed clinically under standardized conditions of lighting. In this context, skin radiance was found to be significantly improved for both sides but with a significantly higher score for M-HA 10 (p=0.007). The mean value of improvement was close to one point for M-HA®10 which can be considered as clinically relevant. This result fully agrees with the study of Baspeyras et al24 where a blinded assessment performed on calibrated, standardized photographs detected a positive significant effect of mesotherapy on skin complexion radiance after HA microinjections as well as the control (saline). An increase of skin radiance or brightness has also been observed in other studies performed with NCHA mesotherapy.21,23
Superficial skin hydration measured by Corneometer® indicated a very significant increase between Day 1 and Day 53 on the M-HA®10 treated side (+35%) whereas a small decrease was observed on the saline treated side (-10%). Using the same instrument, Lee et al25 found a lower but significant increase of skin hydration (+6%) six weeks after three sessions of a NCHA injection. In a different study Cheng et al22 found a significant increase of 9 percent in superficial skin hydration three months after five weekly sessions of pneumatic injections of NCHA.
The skin elasticity measurements made with the Cutometer® showed a significant decrease of parameter R1 (-27%) and R4 (-26%) between Day 1 and Day 53 on the M-HA 10 treated side; this indicates an increase in skin firmness. Interestingly, the Baspeyras study24 showed that intradermal microinjections of HA filler compared to a saline control induced a significant decrease of E1 and E2 parameters (combined elasticity and dermal thickness parameters), which indicates a decrease of elastin and collagen fiber stiffness which suggests that the product may significantly improve skin structure. Thus, NCHA-based injections may sustainably restore suppleness to the skin and increase collagen fiber entanglement thereby regaining the mechanical behavior of a younger skin.
The significant effect of HA-based dermal injections on skin elasticity was coupled in this study with a sustained increase in dermal thickness one and three months after the last injection session. Since dermal thickening was still significant three months after the HA treatment, it may be hypothesized that in contrast with the control, HA injections secondarily induced the synthesis of dermal components such as elastin and collagen which may contribute to the observed increase in dermal thickness and which may also be responsible for the persistence of this effect. Collagen and elastin fiber synthesis activation and their potential renewal may also explain the effect of HA on skin elasticity. The newly formed fibers may be more flexible and their greater number and entanglement may reinforce the collagen and elastin network which, embedded in a proteoglycan and glycosaminoglycan gel, forms the dermis. The observed increase in skin firmness in this study is probably due to the concomitant effect of an increase in dermal thickness and the reinforcement of the collagen and elastin network which were not measured directly. Fisher et al26 revealed that the illers by their volume actually stretch the dermis and consequently the fibroblasts respond to the stretch by producing more collagen and less MMPs, confirming the hypothesis that the effect of the fillers may result from deposition of new collagen. There are the direct evidences showing the application of mechanical tension to the dermal extracellular matrix stimulates collagen production in vivo.26
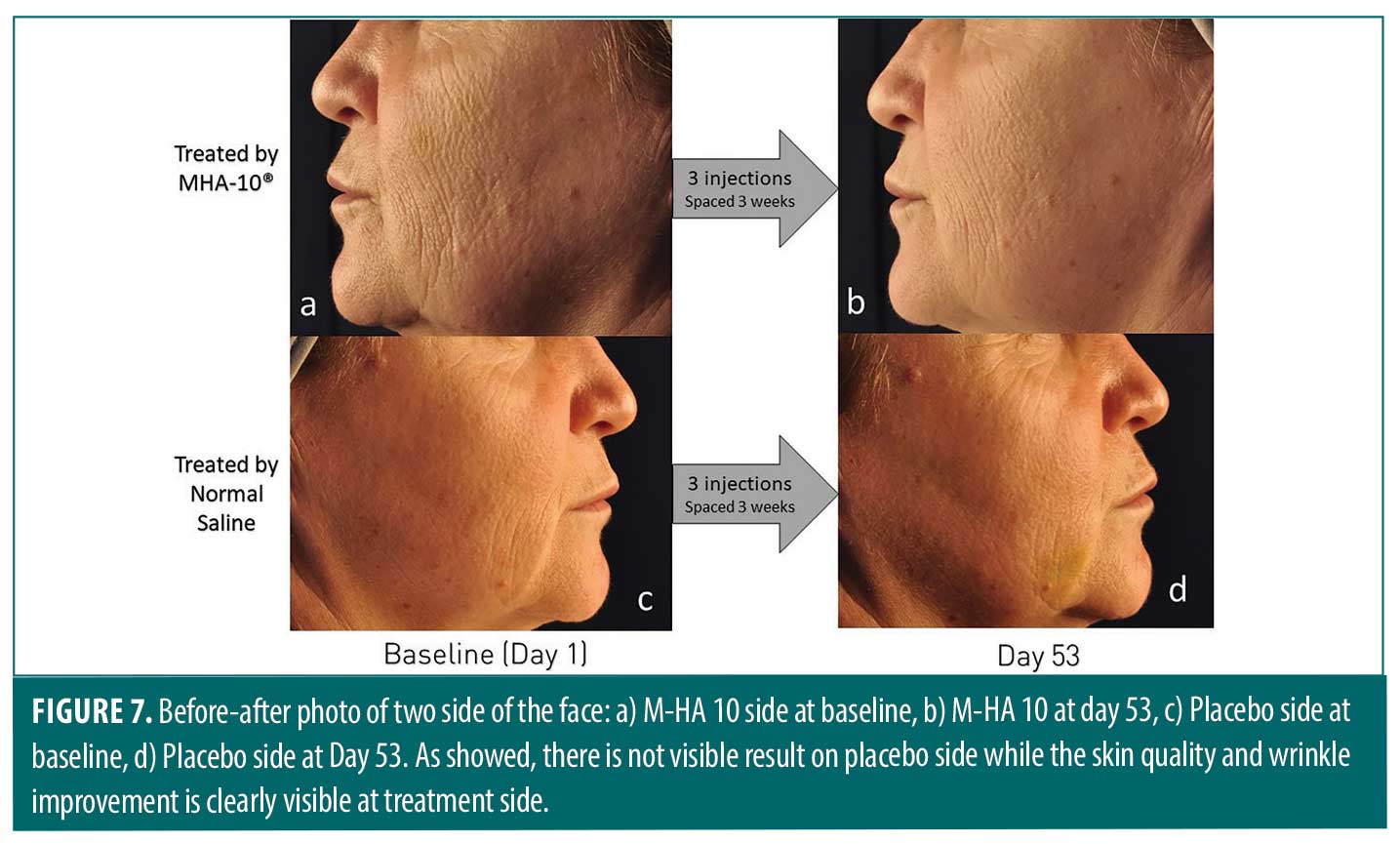
The treatment product demonstrated the visible effect on the treated side while the control side shows no visible results despite of multi-intradermal injection (Figure 7). This results could confirm the effects of the product alone excluding the positive effects of the microneedling.
In support of this hypothesis, it has been demonstrated that injections of stabilized HA into forearm skin significantly increased the synthesis of Type 1 collagen in addition to profibrotic growth factors.17 Another study, observed an increase in the ultrasound echogenicity of the subepidermal low-echogenic band (SLEB) after HA-based dermal injection every week for four weeks on the dorsum of the hands. It was concluded that these changes may be likely be due to several factors including increased density or rearrangement of dermal collagen fibers after fibroblast activation.27
The instrumental results concerning positive effect of M-HA®10 on skin wrinkles and firmness were corroborated by the SAQ. Clearly, subjects in this blind study found that compared to the saline treated side, multi-injections of M-HA®10 significantly reduced wrinkles (p=0.003), increased skin firmness (p=0.003) and increased skin elasticity (p=0.029).
The IGAIS was significantly in favor of M-HA®10 compared to saline at each visit. At Day 53, the Investigator found that M-HA®10 either improved or much improved the aesthetic aspect on a majority of subjects (82%) whereas no improvement at all was found on the saline treated side. These results were confirmed by those obtained with the SGAIS where 71 percent of subjects found that M-HA®10 either improved or much improved the aesthetic aspect compared to 89 percent of subjects who found no change on the saline treated side.
Concerning the safety assessments, expected local adverse events including aspect and sensitivity of treated areas, indicated that more edema and irregularity at palpation, and more ecchymosis was observed following the injection sessions on the M-HA®10 treated side compared to the saline side. This result concurs with previously published articles. Baspeyras et al24 observed 50 AEs among the 57 subjects included in the tolerance study population. Here AEs were characterised as expected and of mild or moderate intensity, such as haematoma, edema, papule, erythema or other transient inflammatory reactions, 46.8 percent of which occurred on the HA-treated hemi-face compared with 25.7 percent on the control side. Globally, the literature reveals that the overall safety of non-cross-linked intradermal injection is good.
Limitations. The last evaluation is planned at 10 days after the last injection session (day 53) which is at least 38 days after the first injection which is spaced enough for fibroblasts stimulation. However, the results would be more interesting if the follow up evaluation will be repeated also several weeks after the last session in order to have the cumulative effect of the three injection sessions.
Conclusion
In conclusion, this randomized, double-blind, placebo-controlled, split-face study, demonstrated the efficacy and tolerance of M-HA®10, a NCHA filler in reducing the crow’s feet mean wrinkle depth in the range of 110 to 1000µm, and in increasing skin radiance, hydration and firmness. Both the Investigator and Subject GAIS were significantly in favor of M-HA®10 compared to the control saline at each visit which concords with the instrumental measurements. Subjects found that compared to the saline treated side, M-HA®10 significantly reduced wrinkles and increased skin firmness and elasticity. This was confirmed by instrumental measurements of skin hydration and firmness. The data demonstrates that in addition to the needling effect induced by multiple intradermal injections, the NCHA can have a regenerative effect on skin aging signs and also has a good safety profile.
References
- Philipp-Dormston WG, Bergfeld D, Sommer BM, et al. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers. J Eur Acad Dermatol Venereol. 2017;31(7):1088–1095.
- Rhee do Y, Won CH, Chang SE, et al. Efficacy and safety of a new monophasic hyaluronic acid filler in the correction of nasolabial folds: a randomized, evaluator-blinded, face study. J Dermatolog Treat. 2014;25(5):448–452.
- Noh TK, Moon HR, Yu JS, et al. Effects of highly concentrated hyaluronic acid filler on nasolabial fold correction: a 24-month extension study. J Dermatolog Treat. 2016;27(6):510–514.
- Pistor M. What is mesotherapy? Chin Dent Fr. 1976;46:59–60.
- Galadari H, Al Faresi F. Mesotherapy. Skinmed. 2011;9:342–343.
- Lacarrubba F, Tedeschi A, Nardone B et al. Mesotherapy for skin rejuvenation: assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-sectional B-mode scanning. Dermato Ther. 2008 Nov-Dec;21(Suppl 3):S1–5.
- Iorizzo M, De Padova MP, Tosti A. Biorejuvenation: theory and practice. Clin dermatol. 2008;Mar-Apr;26(2):177–181.
- Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Bio Chem. 2002;277:4581–4584.
- Yoneda M, Shimizu S, Nishi Y. Hyaluronic acid-dependent change in the extracellular matrix of mouse dermal fibroblasts that is conducive to cell proliferation. J Cell Sci. 1988 Jun;90 (Pt 2):275–286.
- Turlier V, Delalleau A, Casas C et al. Association between collagen production and mechanical stretching in dermal extracellular matrix: in vivo effect of cross-linked hyaluronic acid filler. A randomised placebo-controlled study. J Dermatol Sci. 2013 Mar;69(3):187–194.
- Sutherland W. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998 Jan;16(1):41–46.
- Röck K, Fischer K, Fischer JW. Hyaluronan used for intradermal injections is incorporated into the pericellular matrix and promotes proliferation in human skin fibroblasts in vitro. Dermatology. 2010;221(3):219–228.
- Jäger C, Brenner C, Habicht J et al. Bioactive reagents used in mesotherapy for skin rejuvenation in vivo induce diverse physiological processes in human skin fibroblasts in vitro- a pilot study. Exp Dermatol. 2012 Jan;21(1):72–75.
- Fitzpatrick TB. The validity and practicality of sunreactive skin types I through VI. Arch. Dermatol. 1988;124, 869–871.
- Bazin R, Doublet E. Atlas du vieillissement cutané. Vols. Volume 1 ; Ed Med Com 2007, Paris.
- Iorizzo M, De Padova MP, Tosti A. Biorejuvenation: theory and practice. Clin Dermato. 2008 Mar-Apr;26(2):177–181.
- Wang F, Garza LA, Kang S et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Controlled Clinical Trial. 2007 Feb;143(2):155–163.
- Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: A balancing act. J Biol Chem. 2002;277:4581–4584.
- Yoneda M, Shimizu S, Nishi Y. Hyaluronic acid-dependent change in the extracellular matrix of mouse dermal fibroblasts that is conducive to cell proliferation. J Cell Sci. 1988 Jun;90 (Pt2): 275–286.
- Greesin JC, Hendricks LJ, Falkenstein PA. Regulation of collagen synthesis by ascorbic acid: Characterization of the role of ascorbate-stimulated lipid peroxidation. Arch Biochem Biophys. 1991;290:127–132.
- Savoia A, Landi S, Baldi A. A new minimally invasive mesotherapy technique for facial rejuvenation. Dermatol Ther. (Heidelb.) 2013 Jan;3(1):83–93.
- Cheng HY, Chen YX, Wang MF. Evaluation of changes in skin biophysical parameters and appearance after pneumatic injections of non-cross-linked hyaluronic acid in the face. J Cosmet Laser Ther. 2018 Nov-Dec;20(7-8):454–461.
- Sparavigna A, Tenconi B, Giori AM et al. Evaluation of the efficacy of a new hyaluronic acid gel on dynamic and static wrinkles in volunteers with moderate aging/photoaging. Clin Cosmet Investig Dermatol. 2019 Jan;12:81–90.
- Baspeyras M, Rouvrais C, Liégard L et al. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: a randomised controlled study. Arch Dermatol Res. 2013;305:673–682.
- Lee YJ, Kim HT, Lee YJ et al. Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: a randomized, double-blind, split-face trial. J Dermatolog Treat. 2020;Apr 6:1–7.
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–672. PMC2887041.
- Tedeschi A, Lacarrubba F, Micali G. Mesotherapy with an Intradermal Hyaluronic Acid Formulation for Skin Rejuvenation: An Intrapatient, Placebo-Controlled, Long-Term Trial Using High-Frequency Ultrasound. Aesthetic Plast Surg. 2015 Feb;39(1):129–133.
- Rittes PG, Rittes JC, Carriel Amary MF. Injection of phosphatidylcholine in fat tissue: experimental study of local action in rabbits. Aesthetic Plast Surg. 2006 Jul-Aug;30(4):474–478.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32: 465–480.

