 J Clin Aesthet Dermatol. 2018;11(12):30–39
J Clin Aesthet Dermatol. 2018;11(12):30–39
by Joan Vandeputte, MD, FCCP (Belgium)
Dr. Vandeputte is a Fellow of the European Board of Plastic, Reconstructive, and Aesthetic Surgery and a plastic surgeon in private practice and at Oudenaarde General Hospital in Belgium.
FUNDING: Merz Pharmaceuticals GmbH provided a restricted grant to support data collection and analysis.
DISCLOSURES: Dr. Vandeputte is a consultant for Merz Pharmaceuticals GmbH (Frankfurt am Main, Germany).
Abstract: Objective.Hyaluronic acid (HA) volumizer injection into subcutaneous and deep tissue layers is increasingly used for nonsurgical volume loss correction. Information on optimal techniques, safety, and medium- to long-term satisfaction is limited. This study evaluated cohesive polydensified matrix® HA volumizer CPM-26 (Modélis® Shape or Belotero® Volume) across different indications.
Design.This study was a retrospective chart review and included a patient questionnaire given at six months posttreatment and during long-term assessment (mean: 3.2 years).
Setting.This study was conducted in a Belgian single-site plastic surgery practice.
Participants.Consecutive patients (n=110, aged 19–74 years, 81% female) receiving single/multiple injections with CPM-26 between 2010 and 2016 were included.
Measurements.Procedural details and safety outcomes, patient estimates of satisfaction and adverse events, and Global Aesthetic Improvement Scale (GAIS) score were considered.
Results.Participants received 601 injections over 189 sessions. The most common sites for volumizing injection were the lateral midface, deep prejowl sulcus, and anterior midface. In six participants, the injection site was the penis (8, 1.3%). Questionnaire data were provided by 81 patients. At six months, 94.9 percent (74/78) were satisfied with outcomes, while, at long-term assessment (mean: 3.2 years), 74.9 percent (54/73) were satisfied.
Conclusion. Deep CPM-26 injection improved appearance and was reported as beneficial by two-thirds of patients at long-term assessment. Local adverse events were minor, transient, and infrequently required treatment.
KEYWORDS: Belotero, Modélis, CPM, volumizing gel, volumizer, aesthetics, patient satisfaction
Introduction
Soft-tissue and bone volume loss contribute significantly to facial appearance changes with age.1–3 Treatment options have expanded with the availability of soft-tissue fillers and associated developments in injection technique. Dermal fillers were first used to improve skin tone, turgor, and texture as part of superficial line and wrinkle management.4 Later, the treatment of volume deficits by subcutaneous and deep injection, using a range of specifically designed products, emerged.5 Despite the increasing popularity of volumizing procedures,6 however, uncertainties remain regarding product choice, technique of administration at different sites, and long-term performance.
Among available biodegradable volumizing products, hyaluronic acid (HA) fillers predominate. HA products generally have a favorable performance and safety profile.7 Because HA is a naturally occurring biopolymer component of the skin, it is readily degraded by members of the hyaluronidase enzyme family.8 To improve longevity, manufacturers have developed a range of gels with a variety of crosslinking technologies that slow down hyaluronidase breakdown and offer different mechanical properties.9 A further consideration is that successful volumizers should safely integrate into soft tissues and preserve a natural appearance both at rest and during alterations in facial expression.
Cohesive polydensified matrix (CPM®) HA volumizer Modélis® Shape (Anteis S.A., Geneva, Switzerland, a wholly owned subsidiary of Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) was first launched in 2010 and is now marketed as Belotero Volume® (CPM-26). It has received a CE mark and has been approved in the European Union and countries worldwide to restore facial volume loss.10 CPM-26 consists of cross-linked sodium hyaluronate (NaHA) formulated to a concentration of 26mg/mL and suspended in a physiological phosphate buffer with or without local anesthetic (0.3% w/w lidocaine hydrochloride). The unique CPM® manufacturing technology results in a gel matrix of various densities. The denser areas contribute to longevity and resist deformation. The less dense areas facilitate administration and aid product remodeling after injection by firm, local pressure.11, 12 Postmarketing experience suggests that CPM-26 has an excellent safety and efficacy profile and is successful for midface augmentation.13 Furthermore, recent data indicate that results are still visible after 18 months.14 The product can also be used to treat human immunodeficiency virus–associated lipoatrophy.15 Examination of the literature suggests that additional practical information on safe and effective injection techniques would be beneficial.16
The aim of this study was to gain real-world insight from one European practice regarding CPM-26 (with or without lidocaine) application for deep tissue volumization across a range of indications (i.e., facial volume restoration and penile girth enhancement) over a longer observation period, focusing on performance, safety, and medium- to long-term patient satisfaction.
Methods
Overview. This was a retrospective, observational study conducted at a single site, a plastic surgery practice in Belgium. The study population included consecutive patients requesting aesthetic correction who received volumizing injections between March 2010 and January 2016. All patients were older than 18 years of age and provided informed consent and photoconsent.
Treatment. The decision to treat and choice of needle/cannula, access point, technique, and injection volume were made at the discretion of the treating physician, with consideration of patient goals and expectations. Treatment involved one or more deep administrations of CPM-26 during one or more treatment sessions. The product was delivered under aseptic conditions by bolus or linear retrograde injection using a 27-gauge (G) needle or a 22-G or 25-G cannula.
Different versions of CPM-26 were used according to local supply. A 2mL dose without lidocaine was launched in 2010. Subsequently, a 1mL syringe became available in 2014, with and without the local anesthetic.
Eleven subjects who received 9.0 percent (17/189) of the total injection sessions later on experienced subdermal injection of Radiesse® (calcium hydroxyapatite, CaHA; Merz North America, Inc., Raleigh, NC, USA) mixed with various amounts of 2% lidocaine in combination with adrenaline (1:200,000), aiming to stimulate subcutaneous and dermal collagen synthesis.17–19
Data collection. Baseline information, including standardized facial photographs, was collected prior to treatment. Details of each session were recorded, with safety outcomes and treatment of adverse events retrieved from patient charts. These details included injection location, needle/cannula size, technique, and volume of product used. Details of any subsequent, nonvolumizing treatment by the investigator were also reviewed.
All patients were sent questionnaires between June 2016 and December 2016, requesting details of any posttreatment adverse events they might have experienced. They were asked to estimate their satisfaction with the cosmetic outcome at six months after injection and again at the time of questionnaire receipt (mean interval: 3.2 years). To grade appearance, a modified Global Aesthetic Improvement Scale (GAIS)20 was used, incorporating seven categories, as follows: 3= very much improved; 2= much improved; 1= improved; 0= no change; ?1=worse; ?2=much worse; or ?3= very much worse. Exploratory analyses were performed to investigate possible relationships between treatment parameters (e.g., volume injected, number of treatment sessions, and interval between injections) and adverse events. Patients were also asked about any subsequent aesthetic treatments they may have received.
Statistics. Statistical analyses were performed using SAS® 9.4 (SAS Institute, Cary, NC, USA). The analysis was mainly descriptive, with quantitative variables expressed as means and standard deviations and qualitative variables expressed as frequencies and percentages. Quality control was performed by a statistician not involved in the analysis.
Results
Overview of participants. A total of 110 patients were included. Their mean age at first treament was 47.9 years (range: 18.6–74.4 years). The majority (80.9%) were women and Caucasian (Table 1). During 189 treatment sessions, 601 injections were administered. Most patients had one (n=62; 56.4%) or two treatment sessions (n=32; 29.1%), with patients undergoing a mean of 1.7 sessions (range: 1–7 sessions). On average, 3.2 sites were injected per session. The mean interval between treatment sessions was 26.1 weeks. A questionnaire was completed by 81 patients (74%) at a mean of 38.9 months (3.2 years) after their last CPM-26 injection. Of 81 patients, 24 (29.6%) subsequently underwent additional aesthetic treatments with different products after their last CPM-26 injection (Table 1).
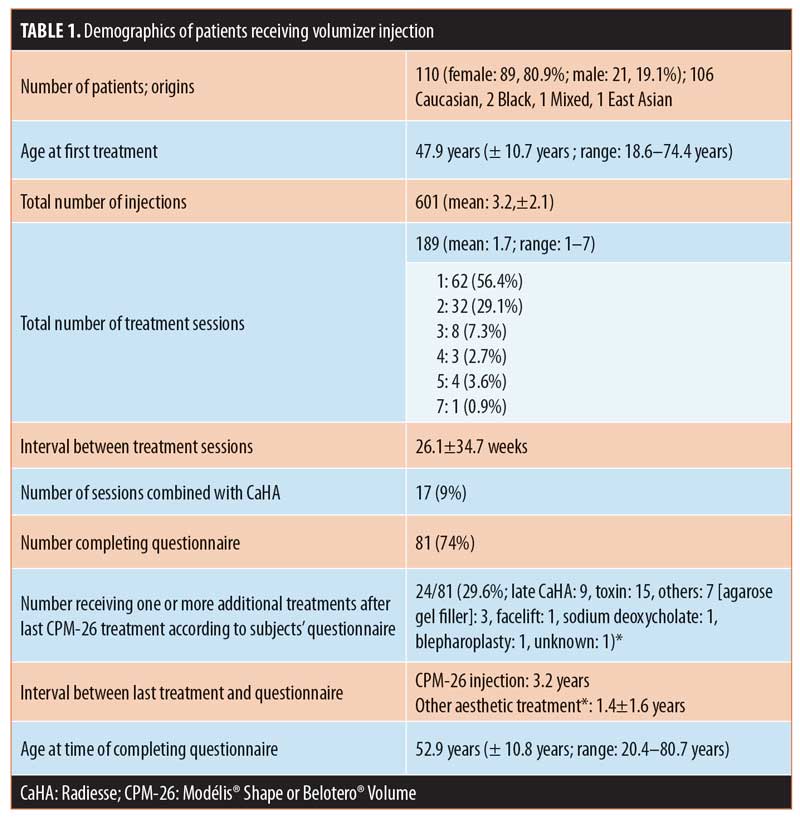
Injection sites and combination treatment. The most common injection sites were the lateral midface (zygomaticomaxillary and zygomatic arch; n=103 injections; 17.1%), deep prejowl sulcus (n=74; 12.3%), and anterior midface (maxilla; n=66; 11.0%; Figure 1). For six patients, the injection site was the penis (n=8 injections; 1.3%). Overall, the most popular treatment areas were the midface (n=226 injections; 37.6%) and jawline (prejowl sulcus, mandibular angle, and chin; 158 injections; 26.3%). Reinjection was frequent in the nose (6/15), zygomyticomaxillary and zygomatic arch area (36/103), and deep prejowl sulcus (20/74).
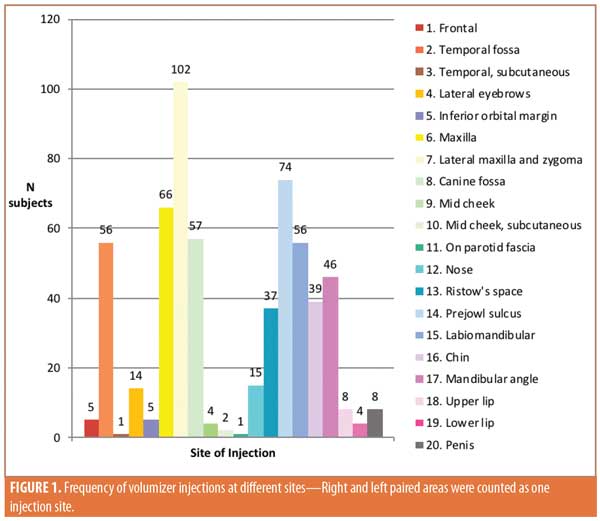
It was common to inject multiple areas in the same session: in 134 sessions, injections were administered to more than one target site (Table 2). The midface (maxilla + zygomaticomaxillary and zygomatic arch + canine fossa) was most frequently targeted area at 9.7 percent (13/134).
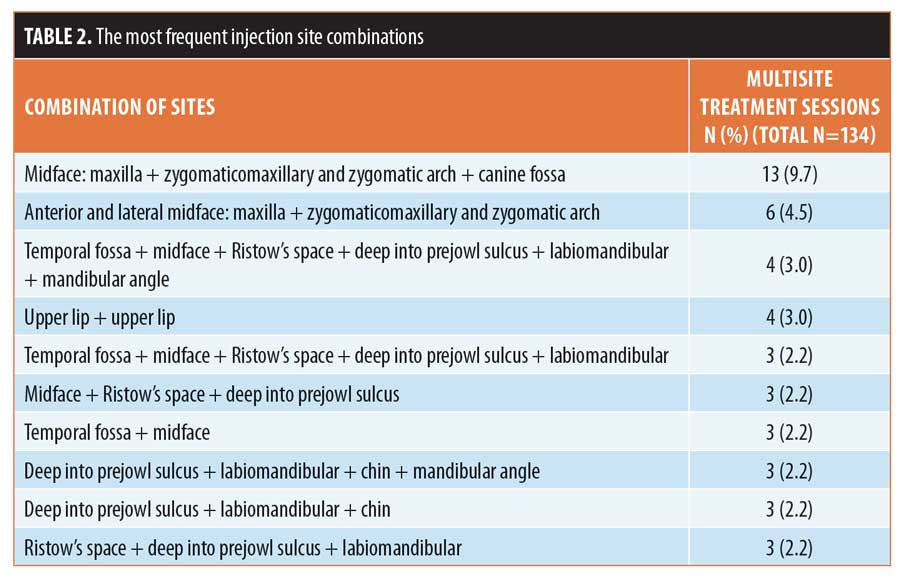
In 11 patients who completed 9.0 percent (17/189) of sessions, treatment was later supplemented by one (n=7), two (n=3), or three sessions (n=1) of subdermal CaHA injection, of which one was during the same session. Retrograde injection with CaHA was performed using a 25-G cannula inserted into the superficial fat compartments in the following sites: temporal hollow (n=6), midface over maxilla and zygoma (n=6), cheek hollow (n=7), superficial labiomandibular fat compartment (n=12), chin (n=2), and inferior mandibular border between the angle and jowl (n=5).
Technique and injection volume. Figure 2 and Table 3 provide details of the injection sites, frequency of needle/cannula use, access points, and injection volume at each treatment area.
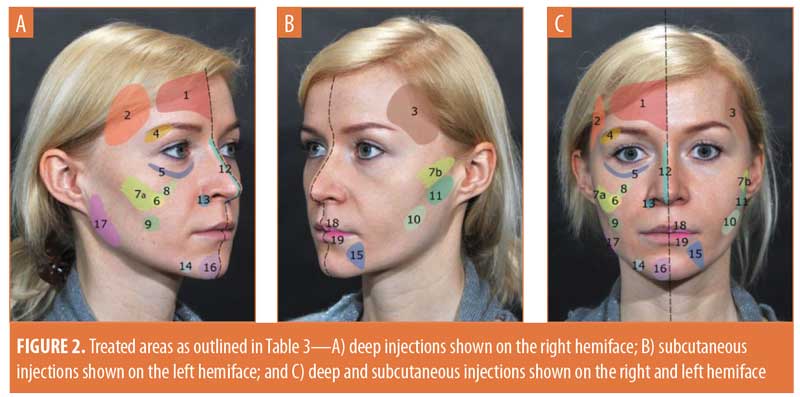
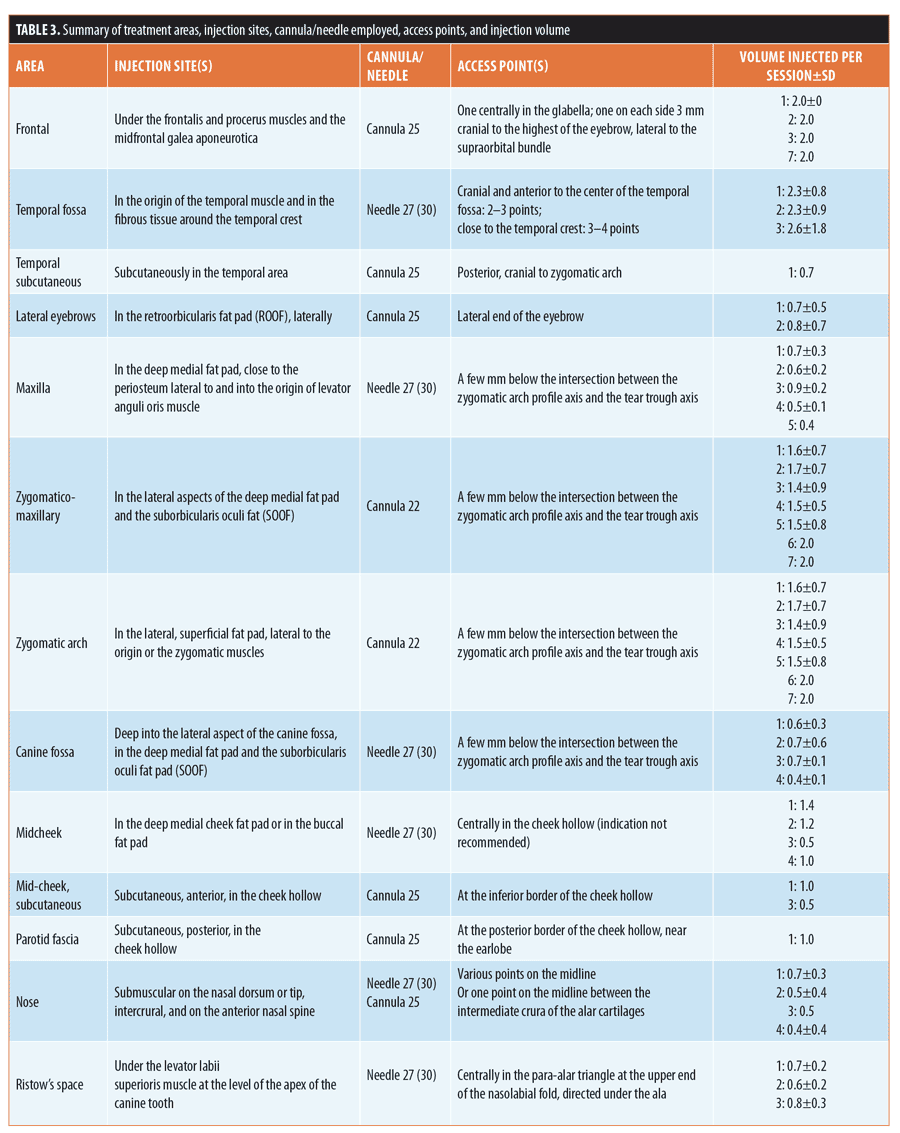
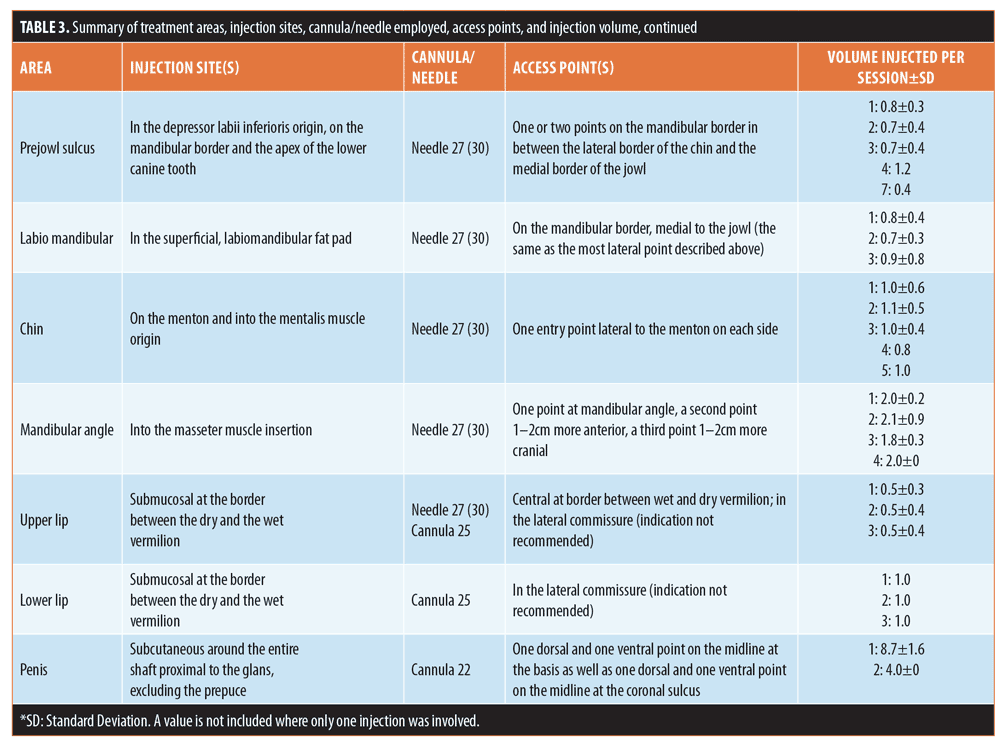
Treatment typically involved injection into deep dermis and/or in subcutaneous fat compartments using a 27-G needle (n=367; 61.1%) or 22-G or 25-G cannula (n=159; 26.5%). Following initial aspiration, bolus injection (n=337; 56.1%) was accompanied by small, side-to-side needle movements aimed at reducing the risk of direct vascular embolization. In other areas, a linear retrograde technique was considered appropriate (n=264; 43.9%). The most frequent combinations were bolus injection with a 27-G needle (50.4% of injections) or a linear retrograde approach using a 22-G cannula (26.3% of injections).
The mean injection volume for face areas across the 189 treatment sessions was 3.6mL±2.6mL. Injection volume was generally greater in Sessions 1 and 2 (3.9±2.8mL and 3.5±2.5mL, respectively) than in Sessions 3, 4, or 5 (3.0±2.3mL, 2.1±1.3mL, and 1.6±0.6mL, respectively). A greater mean volume (7.5±2.6mL) was injected in the penis (Session 1: 8.7±1.6mL and Session 2: 4.0±0mL).
Treatments with calcium hydroxyapatite were performed when additional benefit was expected from precise sculpting of contours by subcutaneous injection, from decreasing mid-cheek hollowness (lacking bony support), as well as from treating important volume deficits by two-level filling.
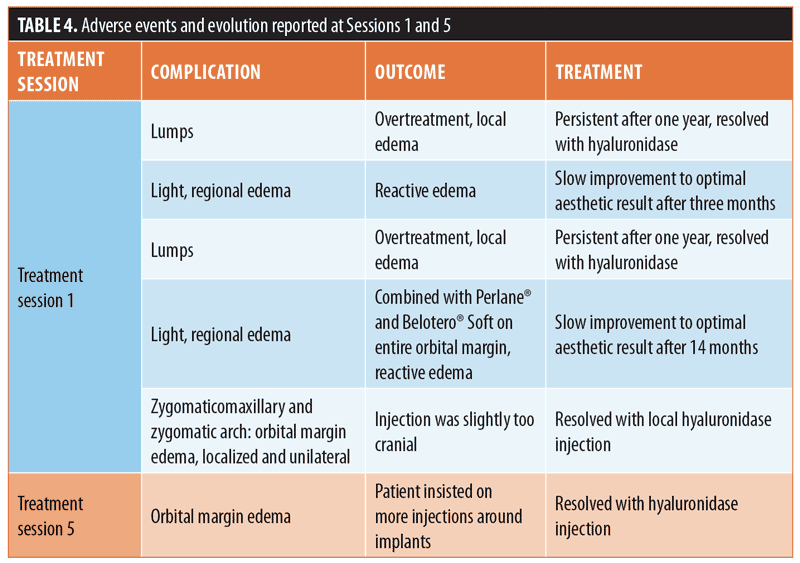
Safety data. There were no reports of serious adverse events such as visual disturbance, tissue ischemia, necrosis, or allergy. Review of the physician’s charts indicated that five patients reported adverse effects after injection. After Session 1, two subjects experimented lumps, and three experienced edema, including one who noted further edema after Session 5 (Table 4). Two patients’ edema gradually resolved without treatment. By comparison, three subjects required hyaluronidase injection (likely etiology: overtreatment, n=2; persistant edema, n=1).
Questionnaire responses indicated that 56.8 percent (46/81) of patients remembered having one or more local/general adverse effects (Figure 3). Seven (15.2%) sought medical advice. Management included the injection of hyaluronidase (n=4), manual redistribution of volumizer (n=1), and application of vitamin K cream for bruising (n=1). Notably, there were no infections, scars, or granulomas. Information on final outcome was provided by 58.7 percent (27/46) of patients. Resolution was reported in 77.8 percent (21/27) of patients. In 22.2 percent (6/27), complications were reported on subject questionnaires as “not resolved” (missing response: 41.3% [19/46]). Exploratory analyses did not show any statistically significant relationship between injection volume, number of treatment sessions, and interval between injections and the occurrence of adverse events.
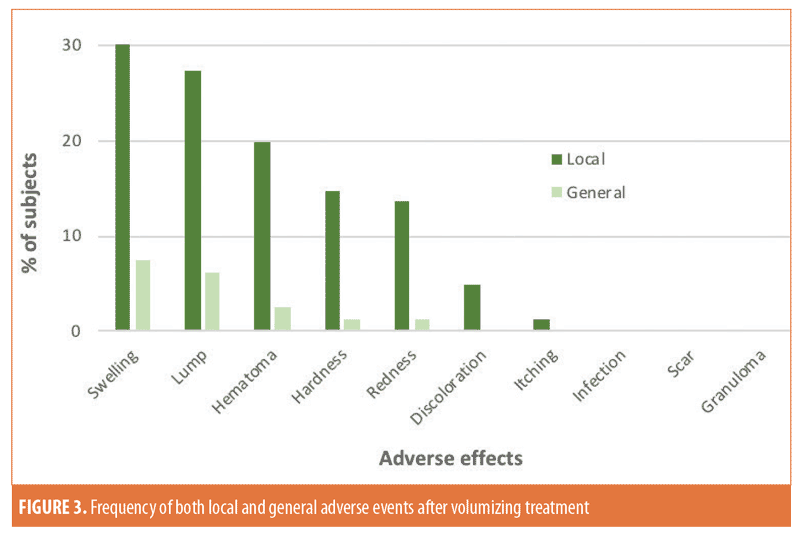
Performance. A total of 94.9 percent (74/78) of patients were satisfied with their treatment results, four (5.1%) were dissatisfied, and three were missing responses. In three patients, reasons for dissatisfaction were poor appearance (e.g., asymmetry, swelling, irregularity). The fourth subject was initially satisfied but reported that the effects had waned after six months.
Among 81 respondents, outcomes generally matched expectations (fully: n=57, 70.4%; partially: n=20, 24.7%; not met: n=4, 4.9%). Eight provided reasons for their expectations not being met, which included anticipation of better results, an unnatural/puffy look, failure to achieve tighter contours, volume loss, and limited durability.
Using the GAIS, 96.2 percent (75/78) of patients considered their appearance improved and three reported no change or worsening. Three responses were missing (Figure 4). Additionally, 74.0 percent (57/77) of patients were willing to repeat treatment and 76.6 percent (59/77) would recommend it. Fifteen patients gave reasons for their unwillingness to repeat, which included cost (e.g., “too expensive”), swelling, bruising, no further need, appearance (e.g., “unnatural, swollen”), preference for another technique, lack of effect (e.g., “I did not see a big difference between before and after”), and limited durability.

Similar reasons for not recommending volumizing treatment were provided by 14 patients, with several preferring not to recommend for personal reasons.
During long-term assessment at a mean of 3.2 years after the last treatment, results had shifted somewhat, with 74.0 percent (54/73) patients remaining satisfied and 66.2 percent (51/77) maintaining a positive GAIS score. Approximately 33.8 percent rated their results as unchanged or worse than at baseline. Lack of satisfaction was generally due to durability.
Comments on individual treatment sites and recommendations. Based on his personal experience, the investigator now suggests several recommendations, as follows.
For the forehead, softer facial expression can be achieved by making the forehead more rounded, and deep injection above the periosteum outside the glabella can compensate for the effects of a frontal sinus impaction fracture. Given the vascularity of this region, injecting under the frontalis muscles is advisable.21 The areolar plane, above the periosteum, is less vascular and allows for good remodeling.
The temporal fossa can appear too deep or deepen after zygomatic arch enhancement. In view of local vascularity, injection into the temporal muscle origin, close to the bone, is considered less risky than subcutaneous injection. First making bone contact reduces the chance of embolizing the middle and deep temporal artery branches. Administration above and in front of the central part of the fossa while keeping distance from the part of the temporal fossa close to the arch minimizes the likelihood of injecting under the muscle into the temporal extension of the buccal fat pad, where a volumizer is likely to be ineffective.22
It is easier to mold volumizers after placement in the temporal muscle than in the fibrous tissue surrounding the temporal crest. If the crest is visible, it is recommended to perform a series of small injections along its course. When significant restoration is required, treating various intramuscular levels deep in the face and above the bone is preferable to administering a large bolus. For subcutaneous injection in the temples, products such as CaHA might be preferable to hyaluronic acid gels as, in the investigator’s opinion, there is less risk of irregularity and unsightly venous congestion.
Flattening of the lateral aspect of the eyebrows can be treated by injecting 0.2mL to 0.4mL of CPM-26 in the retro-orbicularis oculi fat pad (ROOF), and can provide some lift.23 This procedure is to be avoided where there is upper eyelid skin descent. Any injection-related edema may accentuate this sign of aging. Also, patients without a clear pretarsal skin crease often present with hollow upper eyelids and an “A-frame” appearance of the superomedial orbital margin. Here, injection risks a more “bony” or skeletonized appearance.
For the inferior orbital margin, because of the risk of swelling, the investigator abandoned CPM-26 injection over the inferior orbital margin. Deep injection of other HA products intended as soft dermal fillers is likely to cause less swelling.24 A retrograde injection technique is recommended.
Midface augmentation might require placement of the filler in one to three specific areas25 (Figure 5), all performed through a single injection point (X in Figure 5).
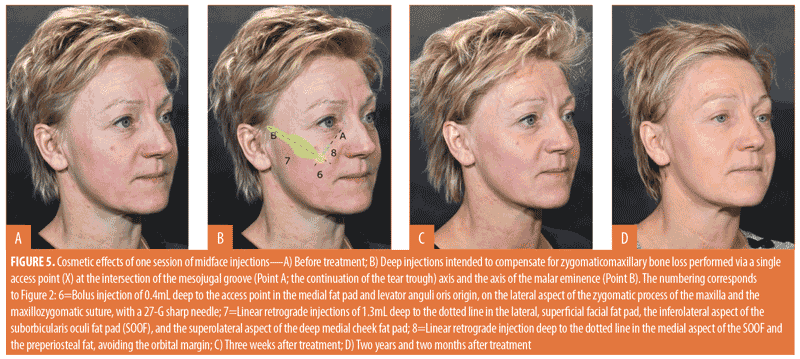
The first maneuver, injection at the anterior aspect of the maxilla deep to the access point (X in Figure 5), is intended to compensate for decreased anterior support and a more downward tilt of the maxillary surface. It might involve the levator anguli oris muscle origin. Filler should be injected as close to the periosteum as possible to avoid adding weight to the cheek. East Asian patients tend to have a flatter anterior midface and may benefit from more filler in this region.
The second maneuver (dotted line 7 in Figure 5) attempts to compensate for lateral deflation of the midface and loss of prominence of the zygomatic body and arch. The benefits of lateral correction are often underestimated. In patients with a full anterior face, this maneuver might be all that is required for enhancement. A medial access point is essential for delivering the filler deep enough in the medial part of the trajectory. One to three linear retrograde injections are delivered close to bone with a 22-G cannula. The first and longest injection delivers the greatest volume to the lateral superficial fat compartment, the inferolateral aspect of the retro-orbicularis oculi fat pad, and the superolateral aspect of the deep medial fat pad. A second, more caudal injection might compensate for a notch at the transition between the deep medial and superficial lateral plane of the first injection, at the zygomatic muscle origin level. It extends along the width of the zygomatic body but stays short of the zygomatic arch. A third, more cranial injection may result in “higher” cheekbones, a desirable outcome among female patients. As East Asian patients have more prominent cheekbones and zygomatic arches, extra volume might be less beneficial for them.
The third maneuver (dotted line 8 in Figure 5) addresses the mesojugal groove by depositing filler in the superomedial aspect of the canine fossa. Care is required not to inject over the orbital margin and to avoid irritating the infraorbital nerve. It is recommended to stay deep and not inject into the superficial inferior orbital or the superficial medial cheek fat pads, which can lead to an unnatural smile.
A lack of mid-cheek volume can be treated by injecting deep in the buccal fat pad, the lateral aspect of the deep medial fat pad, or the intermediate superficial fat pad.25 More posteriorly and caudal to the zygomatic arch lies part of the lateral superficial fat pad overlying the parotid fascia, which can also be injected.25 Consistent with Western aesthetic ideals, the cheek hollow should not be obliterated completely. Subcutaneous injection of CaHA gel might be preferred in this area to avoid water absorption.
Ideal products for nasal reshaping resist deformation by gravity, muscular action, and external forces.26 According to the investigator, the elasticity of CPM-26 makes it more suitable than dermal HA fillers designed to spread in connective tissue. Support and projection of the tip can be enhanced by CPM-26 administration on the anterior nasal spine and between the medial crura of the alar cartilages. Injecting a small volume on top of the intermediate crura can unify a bifid tip or slightly increase projection. When augmenting the dorsum and radix, it is preferable to inject in the less vascular submuscular plane, where molding is easier. Greater precision can be obtained with a 30-G needle injection or 27-G cannula. It is recommended to compress the orbital collaterals and supraorbital and supratrochlear areas with the nondominant hand during injection, to reduce the risk of embolization.27,28 Although nasal augmentation and tip support with CPM-26 are generally successful, patients often seek further touch-ups. After surgery, scar tissue prevents filler spread and carries a higher risk of embolization. For deep injection of postsurgical cases, using an HA gel designed specifically for intradermal administration is suggested.
Ristow’s space is an open compartment located under the levator labii superioris muscle.29,30 It can be accessed directly in the triangle between the nasal ala and nasolabial folds. Because of vascularity, it is recommended to first make bone contact. Administration can partially compensate for premaxillary bone loss, making the superior portion of the nasolabial folds less deep and providing the upper lip with a rounder shape in the transverse plane. Avoid overtreatment, as filler can leak into the deep medial fat compartment under the nasolabial folds, resulting in undesirable prominence.
Prejowl sulcus correction involves deep injection in and under the depressor labii inferioris muscle. The intention here is to compensate for mandibular bone loss by partially hiding the jowl using filler to plug the gap between the latter and the chin.31 Closer to the surface, the superficial labiomandibular fat compartment can also be treated. It is recommended not to cross the marionette line, as any leakage risks jowling due to the presence of filler and reactive edema.
Loss of mandibular angle width can be partially compensated for by injection in the masseter muscle insertion at three points: at the angle; 1 to 2cm above, on the ramus; and 1 to 3cm anterior, on the corpus.31 To avoid injecting the parotid gland, the external carotid artery posteromedial to the ramus, or facial vessels, bone contact should be made prior to administration. This procedure is less painful when done using a needle. A cannula, inserted parallel to the mandible, can penetrate the masseter muscle, but this risks administration at less deep in the muscle rather than at its insertion.
Chin projection can be augmented with paramedian CPM-26 injection. Dimple correction may require subcutaneous administration, as some mentalis muscle fibers join in the midline, thereby masking the effects of deeper injection. Because of chin ptosis with age, deep CPM-26 injection in the mentalis origin might be appropriate. This approach provides support and is sometimes sufficient treatment for chin “frowning” (skin dimpling on closing the mouth). Injection in the superficial chin fat compartment is not recommended, as it can worsen a sagging chin.31
Ideally, height of the mid-lower lip should be double that of the mid-upper lip. Deep, submucosal CPM-26 injection in the upper and lower lips at the junction of the dry and the wet vermilion may result in transient swelling and is now contraindicated. Though a satisfactory result was eventually achieved, use of a dermal filler (e.g., Belotero® Intense for the lip body, Belotero® Balance for contouring; Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) at these sites is more appropriate than a volumizer.32
Whereas subcutaneous injection of CPM-26 into the penile shaft can be used to increase flaccid girth, it is unlikely to change the patient’s or his partner’s sensation during intercourse. Some gel may migrate into the prepuce or around a circumcision scar, thereby interfering with enhancement. Disproportionate girth between the glans and shaft might require intradermal injection into the corona using a dermal HA filler (e.g., Belotero® Intense; Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany).
Discussion
General. This single-center study confirms the safety and effectiveness of CPM-26 for deep-tissue facial volumization. Almost all patients reported appearance improvement at six months after treatment. In two-thirds of patients, cosmetic effects were judged to be present at a mean of nearly 39 months (i.e., more than three years) after the last volumizing treatment, with an equivalent proportion reporting that their expections had been met. Also, approximately 75 percent were still satisfied with their treatment at the time of the questionnaire and a similar proportion would repeat or recommend the intervention to others. Administration of the product was also successful in achieving penile girth augmentation. CPM-26 with and without lidocaine proved easy to use, a finding endorsed by others.33
Injection site reactions, reported by more than half of patients, were generally minor, localized, and transient. Occurence was not statistically related to injection volume, number of treatment sessions, or interval between injections. These results are similar to those reported elsewhere for CPM-2616 and are comparable to those for other HA products.34–36 Due to adverse effects, some respondents sought medical attention following injection. Patients’ statements illustrate real-world experiences in this study.
Optimizing treatment. Successful results depend on the good selection of patients and injection technique. Undoubtedly, there is a learning curve, with some of the recommendations in this paper arising from the investigator’s prior clinical experience. An additional factor in success is accurate posttreatment volumizer remodeling by way of the application of firm, local pressure. CPM-26’s inherent plasticity allows for reshaping immediately after treatment without an alteration in physical properties, making it an appropriate volumizer for deep volume enhancement. In the investigator’s experience, CPM-26 is particularly suited for supporting tissues and increasing the projection of prominent areas such as the cheekbones.
The selected technique of administration depended on treatment site, with the most common being bolus injection with a 27-G needle or linear retrograde administration using a 22-G cannula. Cannulas are safer and less painful when linear retrograde injection is possible. By comparison, needles are less painful in thick tissue such as the temporalis or masseter muscles, and offer greater precision when injecting close to the bone.31,37 Aspiration and lateral movement of the needle are commonly employed to reduce the risk of embolization, but this might not be enough to prevent intravascular administration.38,39 Initially, CPM-26 was available in a 2mL syringe, with the smallest needle caliber that would fit being 27-G. It was only after this study that the investigator found out that a 30-G needle could be combined with the newer 1mL CPM-26 syringe. This combination, plus added lidocaine, enhanced subject comfort, especially when remodeling was required.
A stepwise injection approach is recommended so that any deficiency can be gradually compensated for, especially where large volumes are required. HA gels tend to absorb water, which can, in some patients, lead to local edema and palpable or visible lumps.40 In general, small changes in gel volume are less noticeable when they are injected beneath a thick soft-tissue layer, thereby reducing visible irregularities. Lumps usually resolve spontaneously after a few weeks. Alternatively, resolution can be facilitated by remodeling with finger pressure, even at late assessment. Completion of treatment can then be scheduled for when edema is likely to resolve, usually three or more weeks later. Delaying retreatment facilitates the optimal correction of any yet unapparent facial asymmetry. It is also recommended that wrinkle treatment using dermal filler injection be performed separately from deep volume restoration so as to decrease the risk of edema. It might be advantageous to supplement deep CPM-26 treatments with subdermal injections of CaHA, thereby combining the advantages of both products while avoiding possible interactions by direct contact. CaHA can be expected to be most effective at inducing collagenesis when injected immediately below the dermis, where fibroblasts are abundant. It might give more precision in contouring and sculpting facial prominences and hiding sharp transitions such as the temporal crest due to its low risk of absorbing water and long-term edema. Mid-cheek hollow injections should be made less deeply, as there is no bony support to inject CPM-26 onto. CaHA may compensate for a light loss of projection over time.
Managing expectations. Standardized “before-and-after” images are vital to document treatment, helping patients appreciate their results and facilitating comparison between different time points.41 Surface imperfections such as wrinkles are more obvious to those seeking aesthetic treatment than volume deficiency or lack of projection. Patients might be less aware of their profile and three-quarter views than of their frontal appearance. Simulation by injecting saline at initial consultation can help demonstrate the likely effects of volume correction.
The effects of volume augmentation can only be judged three or more weeks after treatment. Some younger patients claim a rapid loss of effect, possibly because the resolving edema gives a pleasing appearance when the overlying skin has good elasticity and surface quality. By comparison, older patients may initially appear puffy after the treatment due to their more flaccid skin, only to notice a successful outcome once the swelling has subsided. It is not uncommon for subjects to report that treatment effects have disappeared, when medical photography gives a clear indication to the contrary.
Areas to treat with precaution. The use of CPM-26 and HA volumizers are contraindicated at certain sites, such as the inferior orbital margin and lips, where less cross-linked HA dermal filler products are more appropriate.42–44 Based on the investigator’s experience with a range of CPM HA products, CPM-26 volumizing injection into the lips appears to be associated with a greater risk of edema, illustrating the importance of careful treatment selection.
Cost implications. Treatment can be expensive. In keeping with clinical experience across other products and manufacturers, some patients required multiple injections to achieve the required volume and correct asymmetry. Costs may limit what can be achieved and influence patients’ perception of the outcome, so it is important to make a treatment plan with a fixed budget before starting. Patients should be aware of the best possible outcome that can be achieved for their budget.
Study limitations. This study presents the results of one investigator performing injections at a single center, and might not be representative of the wider population. Also, the data represent “all-comers” as opposed to a defined group undergoing a standardized procedure. Data collection was retrospectively performed, from patient charts. Similarly, participants were asked to provide feedback at variable time points after treatment—their views might not reflect the treatment effectiveness and adverse events experienced several years earlier.
Conclusion
Volumizing treatment with CPM-26 is safe and effective. At six months, nearly 95 percent of patients were satisfied with treatment outcomes and rated their appearance as improved. In more than two-thirds of patients, benefits were judged to still be present at medium- to long-term assessment (i.e., more than three years later). While injection site reactions were reported by 56.8 percent of patients, these were generally minor, localized, transient, related to the injection procedure, and rarely required treatment. Working in three dimensions brings new challenges. Successful results depend on patient selection, anatomical knowledge, appropriate injection depth and volume, and matching technique to desired aesthetic outcome. An additional benefit can be expected by a combination treatment with CaHA.
Acknowledgments
Statistical analysis was performed by Delta Consultants, Eybens, France (www.delta-consultants.com). Medical writing was conducted by Dr. Steven Walker, Dr. Angela Garcia-Perez, Sarah Stinnissen, and Esther Fash at Stgilesmedical London & Berlin (www.stgmed.com), with funding from Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany.
References
- Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(1S):S4–S9.
- Mendelson B, Wong CH. Changes in the facial skeleton with aging: implications and clinical applications in facial rejuvenation. Aesthetic Plast Surg. 2012;36(4):753–760.
- Farkas JP, Pessa JE, Hubbard B, Rohrich RJ. The science and theory behind facial aging. Plast Reconstr Surg Glob Open. 2013;1(1). pii: e8-e15
- Pavicic T. Efficacy and tolerability of a new monophasic, double-crosslinked hyaluronic acid filler for correction of deep lines and wrinkles. J Drugs Dermatol. 2011;10(2):134–139.
- Muhn C, Rosen N, Solish N, et al. The evolving role of hyaluronic acid fillers for facial volume restoration and contouring: a Canadian overview. Clin Cosmet Investig Dermatol. 2012;5:147–158.
- Cosmetic Surgery National Data Bank Statistics. Aesthet Surg J. 2016;36 Suppl 1:1–29.
- Signorini M, Liew S, Sundaram H, et al. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers—evidence- and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137(6):961e–971e.
- Salwowska NM, Bebenek KA, ??d?o DA, Wcis?o-Dziadecka DL. Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol. 2016;15(4):520–526.
- Gutowski KA. Hyaluronic acid fillers: science and clinical uses. Clin Plast Surg. 2016;43(3):489–496.
- Prasetyo AD, Prager W, Rubin MG, et al. Hyaluronic acid fillers with cohesive polydensified matrix for soft-tissue augmentation and rejuvenation: a literature review. Clin Cosmet Investig Dermatol. 2016;9:257–280.
- Micheels P, Sarazin D, Tran C, Salomon D. Effect of different crosslinking technologies on hyaluronic acid behavior: a visual and microscopic study of seven hyaluronic acid gels. J Drugs Dermatol. 2016;15(5):600–606.
- Micheels P, Besse S, Sarazin D, et al. Ultrasound and histologic examination after subcutaneous injection of two volumizing hyaluronic acid fillers: a preliminary study. Plast Reconstr Surg Glob Open. 2017;5(2):e1222.
- Micheels P, Vandeputte J, Kravtsov M. Treatment of age-related mid-face atrophy by injection of cohesive polydensified matrix hyaluronic acid volumizer. J Clin Aesthet Dermatol. 2015;8(3): 28–34.
- Kerscher M, Agsten K, Kravtsov M, Prager W. Effectiveness evaluation of two volumizing hyaluronic acid dermal fillers in a controlled, randomized, double-blind, split-face clinical study. Clin Cosmet Investig Dermatol. 2017;10:239–247.
- Becker M, Balagué N, Montet X, et al. Hyaluronic acid filler in HIV-associated facial lipoatrophy: evaluation of tissue distribution and morphology with MRI. Dermatology. 2015;230(4):367–374.
- Prager W, Agsten K, Kravtsov M, Kerscher PM. Mid-face volumization with hyaluronic acid: injection technique and safety aspects from a controlled, randomized, double-blind clinical study. J Drugs Dermatol. 2017;16(4):351–357.
- Yutskovskaya Y, Kogan E, Leshunov E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13(9):1047–1052.
- Yutskovskaya YA, Kogan EA. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and décolletage: a pilot study. J Drugs Dermatol. 2017;16(1):68–74.
- Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34 Suppl 1:S64–S67.
- Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29(6):588–595.
- Carruthers J, Carruthers A. Volumizing the glabella and forehead. Dermatol Surg. 2010;36 Suppl 3:1905–1909.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119(7):2219–2227; discussion 2228–2231.
- Lambros, V., Volumizing the brow with hyaluronic acid fillers. Aesthet Surg J. 2009;29(3):174–179.
- Huber-Vorländer J, Kürten M. Correction of tear trough deformity with a cohesive polydensified matrix hyaluronic acid: a case series. Plast Surg Nurs. 2015;35(4):171–176.
- Fundarò S. Treatment of the midface with fillers. Available at: https://www.prime-journal.com/treatment-of-the-midface-with-fillers/.
- Humphrey CD, Arkins JP, Dayan SH. Soft tissue fillers in the nose. Aesthet Surg J. 2009;29(6): 477–484.
- Bravo BS, Rocha CR2, Bastos JT2, Silva PM. Comprehensive treatment of periorbital region with hyaluronic acid. J Clin Aesthet Dermatol. 2015;8(6):30–35.
- Delorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34(4):584–600.
- Pilsl U, Anderhuber F, Rzany B. Anatomy of the cheek: implications for soft tissue augmentation. Dermatol Surg. 2012;38(7 Pt 2):1254–1262.
- Pilsl U, Rosmarin W, Anderhuber F. The premaxillary space: a location for filler injection?. Dermatol Surg. 2014;40(3):301–304.
- Salti G, Rauso R. Facial rejuvenation with fillers: the dual plane technique. J Cutan Aesthet Surg. 2015;8(3):127–133.
- Fischer TC, Sattler G, Gauglitz GG. Hyaluron filler containing lidocaine on a CPM basis for lip augmentation: reports from practical experience. Facial Plast Surg. 2016;32(3):283–288.
- Kühne U, Esmann J, von Heimburg D, et al. Safety and performance of cohesive polydensified matrix hyaluronic acid fillers with lidocaine in the clinical setting—an open-label, multicenter study. Clin Cosmet Investig Dermatol. 2016;9:373–381.
- Hoffmann K, Juvéderm Voluma Study Investigators Group. Volumizing effects of a smooth, highly cohesive, viscous 20-mg/mL hyaluronic acid volumizing filler: prospective European study. BMC Dermatol. 2009;9:9.
- Few J, Cox SE, Paradkar-Mitragotri D, Murphy DK1. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35(5):589–599.
- Wilson MV, Fabi SG, Greene R2. Correction of age-related midface volume loss with low-volume hyaluronic acid filler. JAMA Facial Plast Surg. 2017;19(2):88–93.
- van Loghem JAJ, Humzah D, Kerscher M. Cannula versus sharp needle for placement of soft tissue fillers: an observational cadaver study. Aesthet Surg J. 2017;38(1):73–88.
- Carey W, Weinkle S. Retraction of the plunger on a syringe of hyaluronic acid before injection: are we safe?. Dermatol Surg. 2015;41 Suppl 1:S340–s346.
- van Loghem JA, Fouché JJ, Thuis J. Sensitivity of aspiration as a safety test before injection of soft tissue fillers. J Cosmet Dermatol. 2018;17(1): 39–46.
- Brandt FS, Cazzaniga A. Hyaluronic acid gel fillers in the management of facial aging. Clin Interv Aging. 2008;3(1):153–159.
- Nair AG, Santhanam A. Clinical photography for periorbital and facial aesthetic practice. J Cutan Aesthet Surg. 2016;9(2):115–121.
- Goh AS, Kohn JC, Rootman DB, et al. Hyaluronic acid gel distribution pattern in periocular area with high-resolution ultrasound imaging. Aesthet Surg J. 2014;34(4):510–515.
- Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13(9):1030–1036.
- Wollina U. Improvement of tear trough by monophasic hyaluronic Acid and calcium hydroxylapatite. J Clin Aesthet Dermatol. 2014;7(10):38–43.

