 J Clin Aesthet Dermatol. 2021;14(6):18–20.
J Clin Aesthet Dermatol. 2021;14(6):18–20.
by Kevin M. Adams, MD; Philip Milam, MD; Alejandro Gru, MD; and Benjamin H. Kaffenberger, MD
Drs. Adams, Milam, and Kaffenberger are with The Ohio State University College of Medicine in Columbus, Ohio. Dr. Gru is with the University of Virginia School of Medicine in Charlottesville, Virginia.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Kaposi sarcoma (KS) is characterized by cutaneous lesions that are often papular and present variably depending on the stage of progression. We describe a patient with a fibroma-like nodule, a lesion not commonly associated with KS. Previously observed in lymphedematous human immunodeficiency virus-associated KS, the occurrence of this nodule in the absence of chronic lymphedema suggests an alternative etiology.
Keywords: Kaposi sarcoma, Kaposi’s sarcoma, human herpesvirus-8, Kaposi’s sarcoma-associated fibroma, fibroma, fibroma-like nodule, fibroma-like proliferation, fibrous proliferation, CD34
Kaposi sarcoma (KS), a type of cancer caused by human herpesvirus-8 (HHV-8), affects the differentiation of infected endothelial cells, causing them to assume a spindle morphology.1 Microscopy reveals inflammatory cell infiltrates and marked proliferation of spindle cells, which become more numerous as the lesion progresses.1 Grossly, these tumors typically present as red or violaceous patches, which progress to thickened plaques before developing into the classic nodules, which can ulcerate. KS is definitively diagnosed by viral polymerase chain reaction for HHV-8 by the detection of latency-associated nuclear antigen (LANA), which is present in HHV-8-infected cells.2
Fibromas are common benign tumors composed of connective tissue, often named based on their location in the body.3 In the case of a subset of digital fibromas, spindle cells found within lesions commonly express the hematopoietic progenitor cell antigen CD34,3 a transmembrane phospho-glycoprotein that can be used as a general marker for progenitor and stem cell activity.4 We present a patient with KS with a fibroma-like proliferation consisting of CD34+ spindle cells which were devoid of HHV-8 infection.
Case Presentation
A man in his 60s with a history of KS following a heart transplant who was previously treated with immunosuppression reduction, radiotherapy, sirolimus, and paclitaxel presented with a three-month history of skin lesions located on his fingers, toes, and feet. A physical examination was conducted and one of the lesions was biopsied.
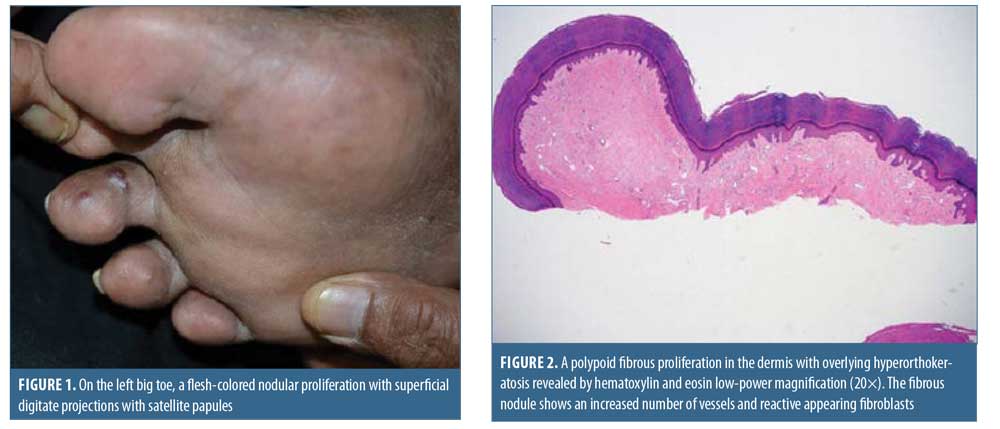
Physical examination demonstrated a lobular, ulcerated papule on the left hand, scattered vascular and dome-shaped papules on the feet, and a flesh-colored nodule with superficial digitate projections and satellite flat-topped papules on the left big toe (Figure 1).
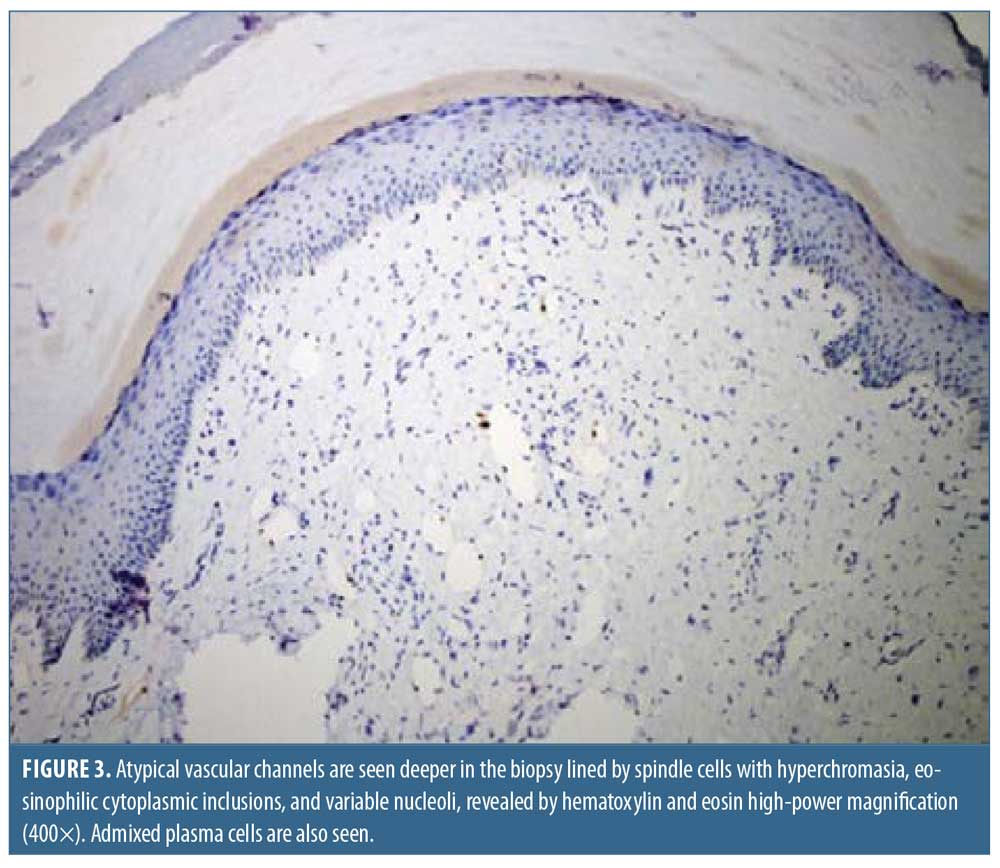
A biopsy of the lesion on the left big toe revealed polypoid lesions with fibrosis throughout the dermis (Figure 2). The superficial fibroblasts were found to be positive for CD34 and negative for HHV-8. Atypical vascular spaces lined by spindle cells with hyperchromatic nuclei were present deeper in the dermis (Figure 3). The spindle cells were noted to be positive for HHV-8 and the patient was diagnosed with KS-associated fibromas. Initial treatment consisted of valganciclovir and sirolimus.
Discussion
Fibroma-like proliferations are a rarely described clinicopathological feature of KS. Previous cases have been associated with lymphedematous human immunodeficiency virus-associated KS, the fibromatoid proliferations hypothesized to be a manifestation of chronic lymphedema.5 No clinical or histologic lymphedema was noted in our patient, suggesting an alternate etiology. While previous studies have established the possibility of HHV-8 infection in CD34+ cells,6 polymerase chain reaction was used to demonstrate the absence of HHV-8 in the superficial fibroblasts in this case. Therefore, stimulation of fibroblasts instead was likely induced by a process other than HHV-8 infection, with the cytokine milieu potentially playing a role. Alternatively, these cells may be newly recruited fibroblasts that have yet to be infected, a phenomenon that has been observed in LANA-negative endothelial cells in both early- and late-stage KS.7
The differential diagnosis included eruptive fibromas, sclerotic fibromas, pleomorphic fibromas, and cellular digital fibromas.3 Because the nodules were relatively localized, eruptive fibromas were less likely,3 and the absence of pleomorphic cells excluded pleomorphic fibroma. The uncharacteristic collagen architecture excluded sclerotic fibroma. The acral distribution and dense proliferations of CD34+ spindle cells were consistent with a diagnosis of a cellular digital fibroma.3 However, the deeper architecture as well as the presence of HHV-8-infected lymphatic structures indicated that the most accurate diagnosis was KS-associated fibromas.
Cryotherapy and/or topical alitretinoin might treat the proliferations and other cutaneous manifestations of KS. Phase III trials indicate a 37-percent response rate (defined as partial or complete clearance) of KS tumors with topical alitretinoin gel 0.1% versus a seven-percent response rate in vehicle gel patients.8 A phase II trial of cryotherapy on KS lesions indicated complete clearance in 80 percent of cases using three rounds of two freeze-thaw cycles.9 A study of 125 patients using cryotherapy indicated 63-percent complete clearance using similar methods.10
Conclusion
The presence of fibroma-like proliferations in KS is a rarely documented clinicopathological feature. The etiology for these proliferations is speculative at best, with direct infection by HHV-8 found to be unlikely as the primary process responsible in this case. Although associated with chronic lymphedema in the past, this case suggests the potential for an alternative, albeit unknown, etiology.
References
- Gramolelli S, Schulz TF. The role of Kaposi sarcoma—associated herpesvirus in the pathogenesis of Kaposi sarcoma. J Pathol. 2015;235(2):368–380.
- Schwartz EJ, Dorfman RF, Kohler S. Human herpesvirus-8 latent nuclear antigen-1 expression in endemic Kaposi sarcoma: an immunohistochemical study of 16 cases. Am J Surg Pathol. 2003;27(12):1546–1550.
- McNiff JM, Subtil A, Cowper SE, et al. Cellular digital fibromas: distinctive CD34-positive lesions that may mimic dermatofibrosarcoma protuberans. J Cutan Pathol. 2005;32(6): 413–418.
- Sidney LE, Branch MJ, Dunphy SE, et al. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014; 32(6):1380–1389.
- Ramdial PK, Chetty R, Singh B, et al. Lymphedematous HIV-associated Kaposi’s sarcoma. J Cutan Pathol. 2006;33(7):474–481.
- Henry M, Uthman A, Geusau A, et al. Infection of circulating CD34+ cells by HHV-8 in patients with Kaposi’s sarcoma. J Invest Dermatol. 1999;113(4):613–616.
- Pyakurel P, Pak F, Mwakigonja AR, et al. Lymphatic and vascular origin of Kaposi’s sarcoma spindle cells during tumor development. Int J Cancer. 2006;119(6): 1262–1267.
- Bodsworth NJ, Bloch M, Bower M, et al. Phase III vehicle-controlled, multi-centered study of topical alitretinoin gel 0.1% in cutaneous AIDS-related Kaposi’s sarcoma. Am J Clin Dermatol. 2001;2(2):77–87.
- Tappero JW, Berger TG, Kaplan LD, et al. Cryotherapy for cutaneous Kaposi’s sarcoma (KS) associated with acquired immune deficiency syndrome (AIDS): a phase II trial. J Acquir Immune Defic Syndr. 1991;4(9): 839–846.
- Kutlubay Z, Küçüktas M, Yardimci G, et al. Evaluation of effectiveness of cryotherapy on the treatment of cutaneous Kaposi’s sarcoma. Dermatol Surg. 2013;39(10):1502–1506.

