 J Clin Aesthet Dermatol. 2020;13(12):32–37.
J Clin Aesthet Dermatol. 2020;13(12):32–37.
by Anne Coakley, BS and Matthew J. Wu, BS; Jayanth Kumar, BS; Farinoosh Dadrass, MS; Joy Tao, MD; Lauren Moy, MD; Kirsten Webb, MD; and Kristin Lee, MD
Ms. Coakley, Mr. Wu, Mr. Kumar, and Ms. Dadrass are with Loyola University Chicago Stritch School of Medicine at Loyola University Medical Center in Maywood, Illinois. Drs. Tao, Moy, Webb, are with the Division of Dermatology, Department of Internal Medicine at Loyola University Medical Center in Maywood, Illinois. Dr. Lee is with the Department of Dermatology at DuPage Medical Group in Lombard, Illinois.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background: Acrochordon (skin tag) removal by snip excision is a routine dermatologic procedure. Bleeding is a common sequelae of snip excision that requires hemostatic control. Chemical cautery is a common means of achieving hemostasis in this procedure.
Objective: The aim of this study was to evaluate three different chemical cautery solutions for their time to hemostasis, pain upon application, and associated pigmentary changes.
Methods: Twelve patients with six or more skin tags on the bilateral neck and/or axilla were enrolled. Two skin tags were cauterized with ferric subsulfate solution, two with silver nitrate, and two with aluminum chloride hexahydrate solution. Time to hemostasis and pain with application of each cautery solution to the skin tag was recorded. At a two-week follow-up appointment, patient satisfaction was assessed with a survey, and pigmentary changes were documented with digital photography.
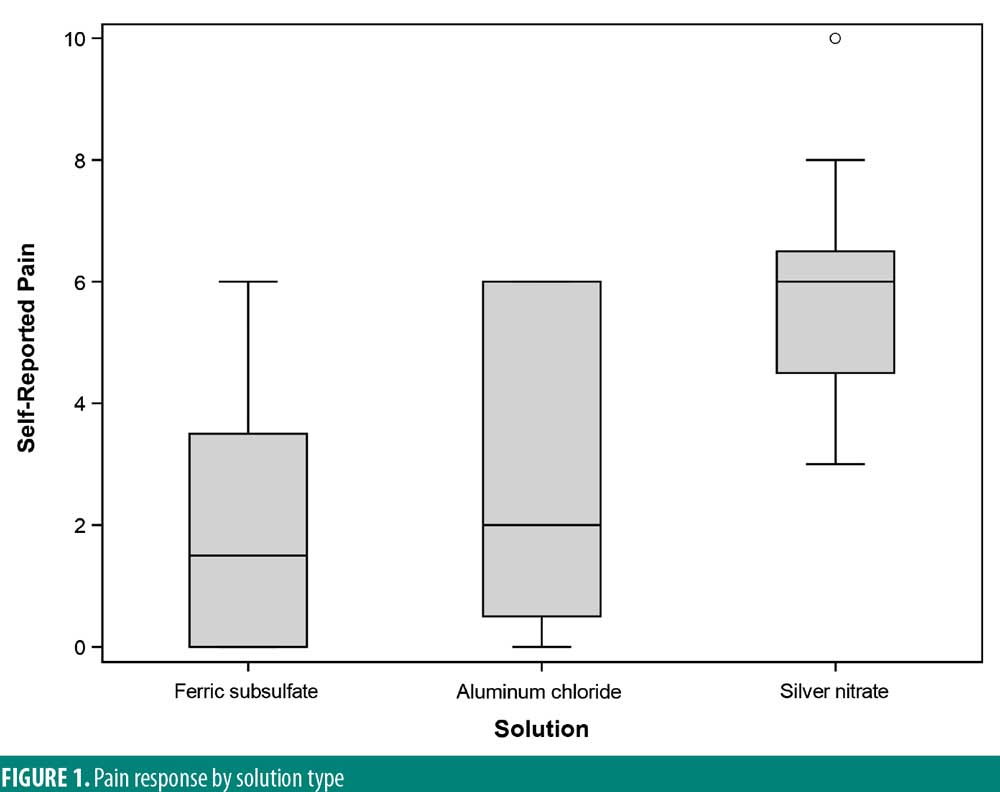
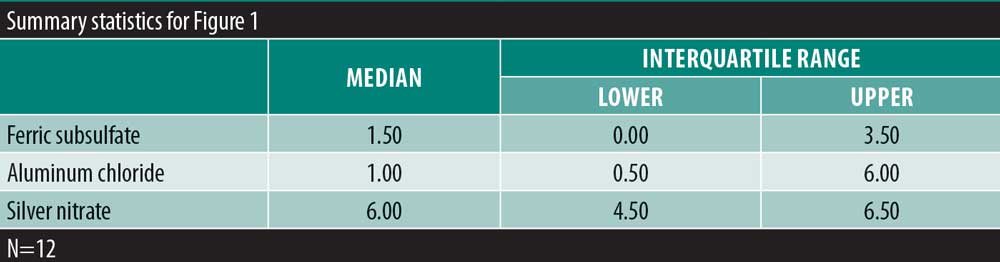
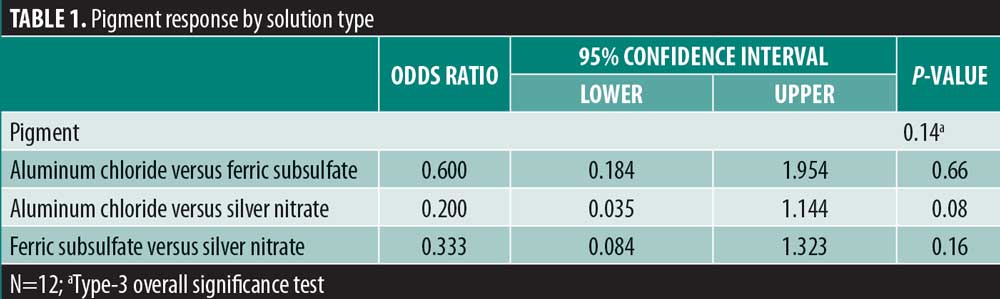
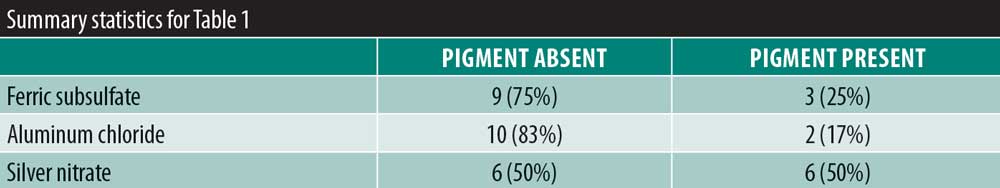
Results: There was no significant variability in the time to hemostasis among the three chemical cautery solutions (p=0.57). Pain response was significantly different among the three solutions (p=0.003). Compared to silver nitrate (median=6.00, interquartile range [IQR]: 4.50–6.50), aluminum chloride hexahydrate (median=1.00, IQR: 0.50 to 6.00; Sidak p=0.02) and ferric subsulfate (median=1.50, IQR: 0.00–3.50; Sidak p=0.01) had a significantly lower pain response. Among participants, three (25%) experienced a pigmentary change with ferric subsulfate, two (17%) with aluminum chloride, and six (50%) with silver nitrate (overall p= 0.14).
Conclusion: These results indicate that the three standard chemical cautery solutions for skin tag snip excision have significant differences in pain upon application and pigmentary changes. This might be a relevant consideration when selecting a chemical cautery solution.
Keywords: General dermatology, surgical dermatology, chemical cautery
Skin tags, or acrochordons, are common benign skin lesions. They are hypertrophied redundant skin folds, frequently occurring on the neck, axilla, and groin region.1 Skin tags are typically found on flexural body surfaces more commonly in individuals who are older, female, or who have obesity.2 They can become irritated when they rub against clothing or jewelry. Indications for removal include ongoing irritation and patient preference for aesthetic reasons.1 Skin tags can be treated with snip excision and hemostasis, electrosurgery, chemical cautery, and cryosurgery.3 Treating skin tags with excision and hemostasis is an efficient and inexpensive means of removal and carries minimal risk of infection compared to electrosurgery or cryosurgery.2
Bleeding is a common sequela of many dermatologic procedures. Ferric subsulfate solution, silver nitrate, and aluminum chloride hexahydrate are commonly used chemical cautery solutions to control bleeding after minor surgical procedures. They cause hemostasis by precipitating proteins in the tissues and occluding smaller vessels.4,5 Application of ferric subsulfate solution for 15 seconds to a wound site has been shown to produce hemostasis.6 Similarly, silver nitrate has been shown to produce rapid hemostasis when applied to the wound.5 Aluminum chloride hexahydrate solution at varying concentrations demonstrated a significant reduction in hemostasis time compared to the control group.7 However, there is limited research directly comparing the time to hemostasis among these three chemical cautery solutions.
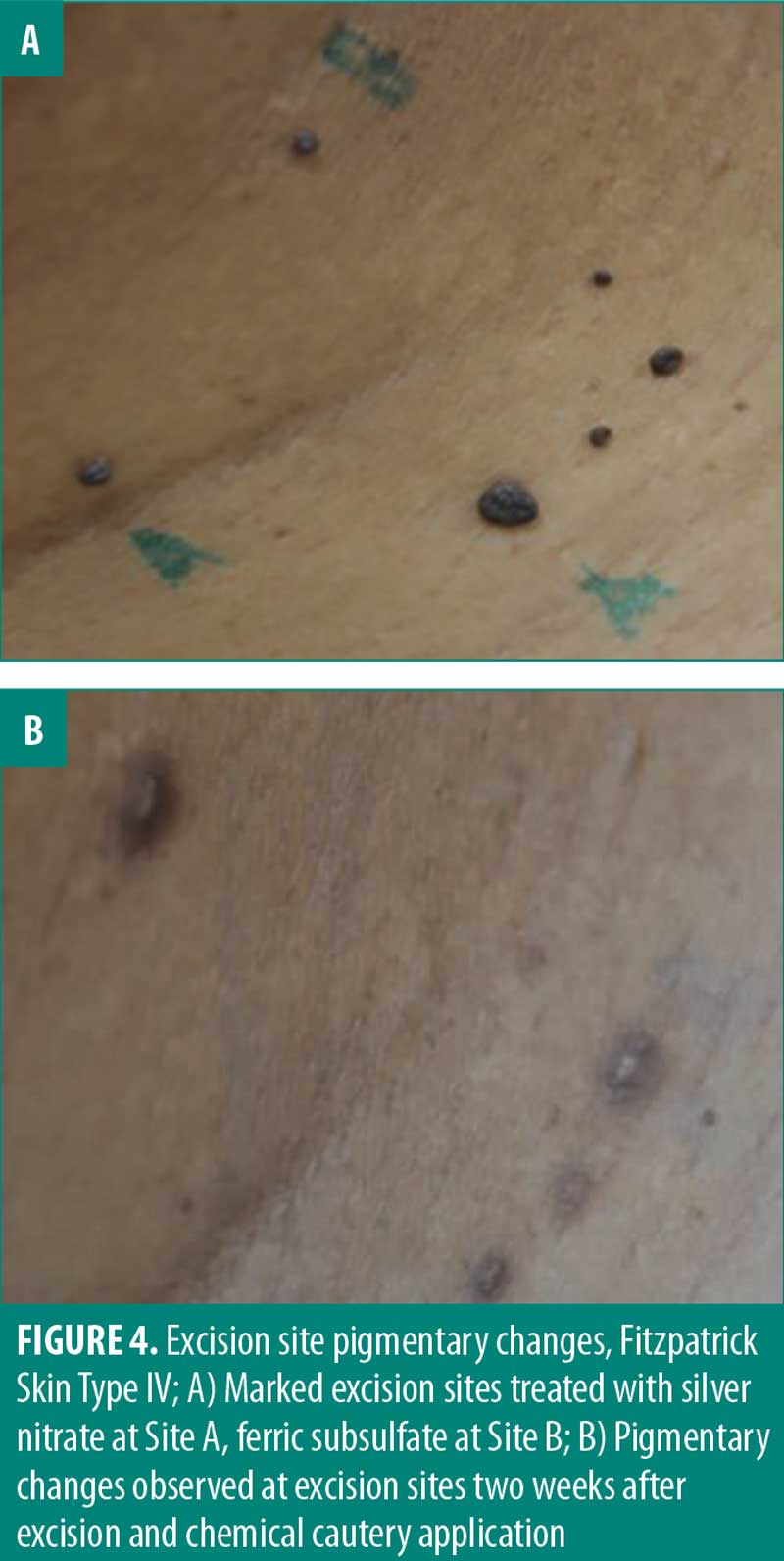
Pigmentary change is an important consideration for any dermatologic procedure, particularly in cosmetically sensitive areas. Silver nitrate and ferric subsulfate solution have been shown to cause hyperpigmentation at the lesion site due to deposition of silver and iron particles, respectively.5 Silver nitrate is associated with a small risk of tattoo.8 Aluminum chloride hexahydrate has not been shown to carry the same risk of hyperpigmentation or tattoo. This study aimed to assess pigmentary changes because it is a relevant consideration in the choice of one chemical cautery solution over another.
There is also limited research to assess patient comfort and satisfaction with each of these chemical cautery solutions. Mild-to-moderate discomfort is anticipated with many dermatologic procedures. However, minimizing pain is always an important consideration for elective clinical procedures, especially if choosing between multiple chemical cautery solutions of equal efficacy.
This study aimed to evaluate and compare the pain intensity, time to hemostasis, and pigmentary changes among the three commonly used chemical cautery solutions of silver nitrate, ferric subsulfate, and aluminum chloride hexahydrate. The overall goal was to better understand the benefits and drawbacks of each of these chemical cautery techniques to help physicians address the needs of individual patients.
Methods
Patient Selection. After approval by the Loyola Institutional Review Board, 12 patients were enrolled in this study. Inclusion criteria consisted of patients with six or more skin tags on the bilateral axilla or neck. Exclusion criteria consisted of patients on anticoagulation and antiplatelet therapy, current smokers, and patient who were pregnant or might become pregnant during the study. Participants were selected by voluntary participation and were consented prior to the excision procedure.
Six skin tags were removed by snip excision on each patient, and each skin tag was cauterized with one chemical cautery solution. Prior to the procedure, participants were asked to not wear any deodorants because they could potentially contain aluminum chloride. Skin tags were removed by grasping the body of the skin tag with Debakey forceps while cutting the pedicle with a single move. Two of the skin tags were cauterized with ferric subsulfate solution (Monsel’s solution, Premier Medical Co, Racine, Wisconsin), two with silver nitrate (GF Health Products Inc., Atlanta, Georgia), and two with aluminum chloride hexahydrate solution (Drysol, Merck &Co., Kenilworth, New Jersey). Participants were blinded to the chemical cautery solution applied to each excision site. Participants were not pre-treated with topical or subcutaneous lidocaine or lidocaine with epinephrine. Time to hemostasis was recorded in seconds with time zero being the application of solution to the excision site. Immediately after the excision of all skin tags, participants were asked to fill out a numeric scale rating the pain they experienced with each chemical cautery technique, ranging from no pain (0) to the most severe pain they have ever experienced (10). Patients were instructed to keep the area clean and dry and to not apply any bandages or deodorant to the area before the two-week study follow-up appointment.
At the two-week follow-up appointment, participants were asked to fill out a survey using a standardized numerical scale (0 to 10) to assess satisfaction with the outcome of excision at each site. A participant survey was also conducted to determine if participants noticed any pigmentary changes at excision sites. Participants’ excision sites were then evaluated for safety. Any pigmentary changes at snip excision sites were recorded and documented with digital photography. Participants were also contacted 6 to 12 months after their snip excisions to assess long-term pigmentary change based on the participant’s self-reported observations.
Statistical Methods. A generalized estimating equation (GEE model) was used to compare the odds of the presence of a pigment among the three solution types, and an exchangeable working correlation matrix was used to account for the repeated (dependent) responses within each patient. A nonparametric Friedman rank test was used to test for overall variability in the satisfaction response among the three solution types. Because the overall null hypothesis test was rejected, post-hoc tests for all pairwise comparisons were conducted using exact Wilcoxon signed rank tests. Significance values from these post-hoc tests were adjusted for inflated Type 1 error using a Sidak correction. A nonparametric Friedman rank test was used to test for overall variability in the hemostasis duration among the three solution types.
Results
There was significant overall variability in the pain response among the three chemical cautery solutions (overall p=0.003). Compared to the pain response for silver nitrate (median=6.00, interquartile range [IQR]: 4.50–6.50), post-hoc tests revealed that the pain response was significantly lower for both aluminum chloride hexahydrate (median=1.00, IQR: 0.50 to 6.00; Sidak p=0.02) and ferric subsulfate (median=1.50, IQR: 0.00–3.50; Sidak p=0.01). No significant difference was found in the pain response between aluminum chloride hexahydrate and ferric subsulfate (Sidak p=0.37) (Figure 1). Among the 12 participants, three (25%) experienced pigmentary changes when exposed to ferric subsulfate, two (17%) experienced pigmentary changes when exposed to aluminum chloride hexahydrate, and six (50%) experienced a pigment when exposed to silver nitrate. However, there was no difference in the odds of experiencing a pigmentary change among the three solution types (overall p=0.14) (Table 1).
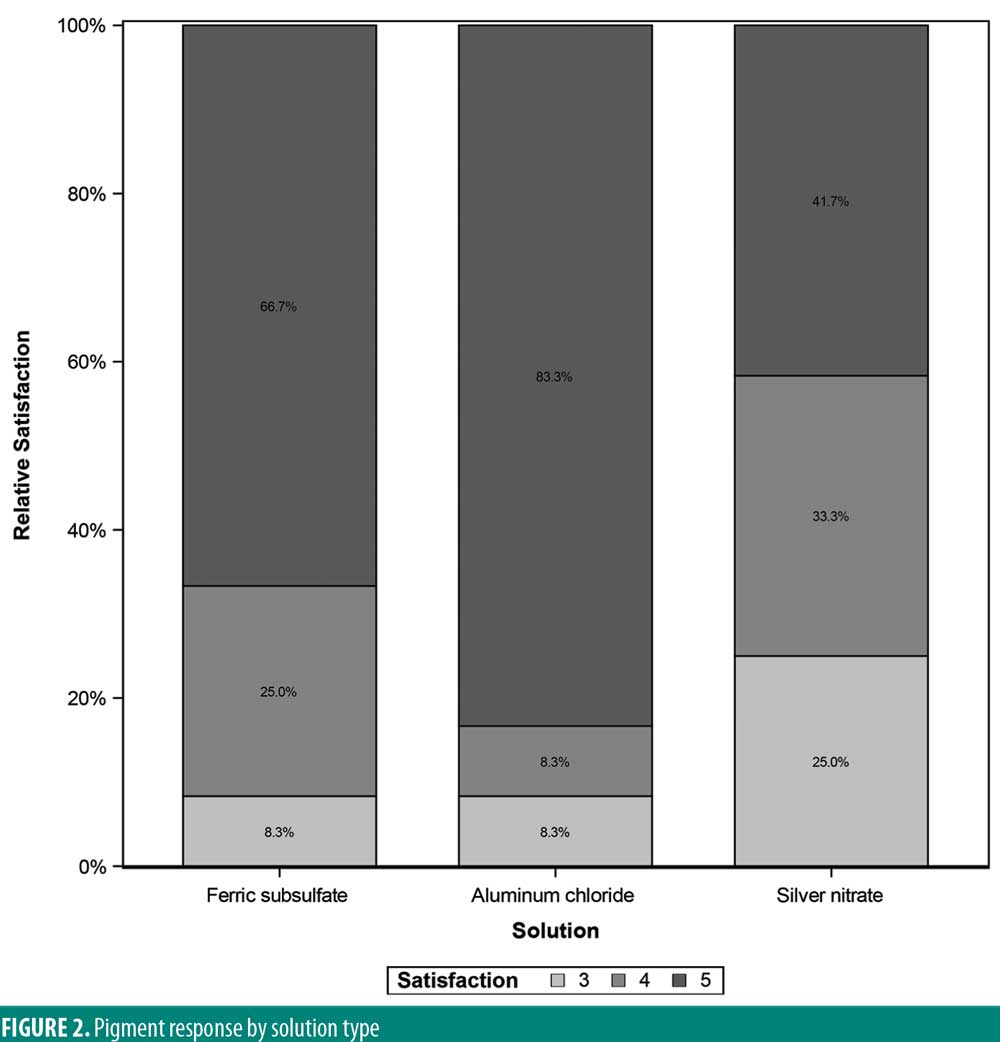
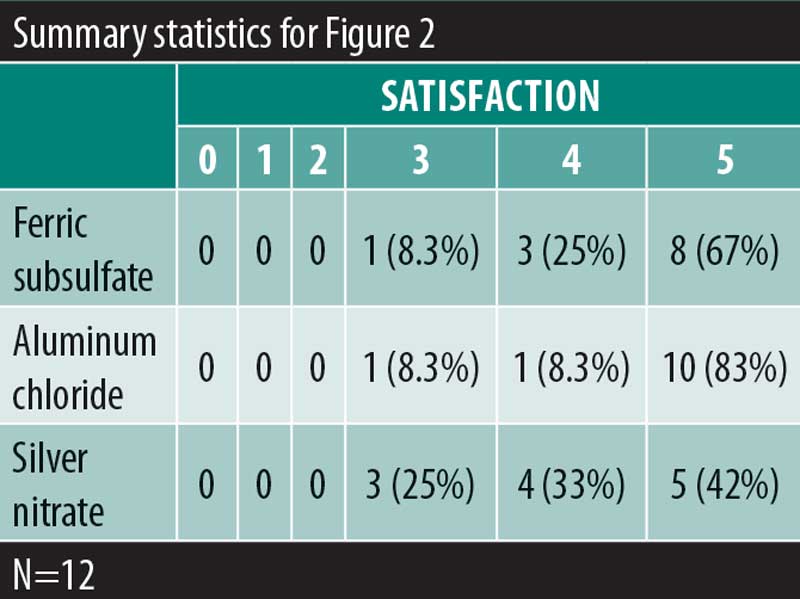
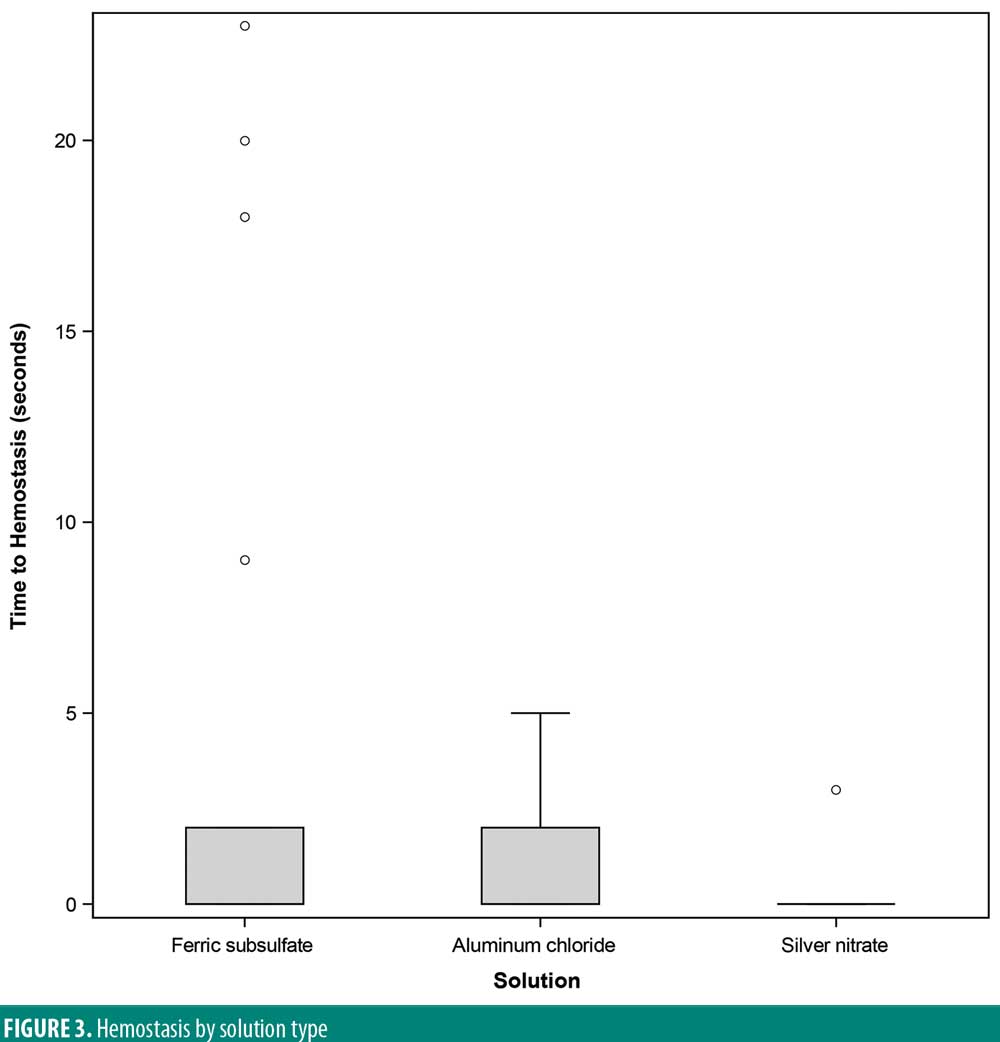
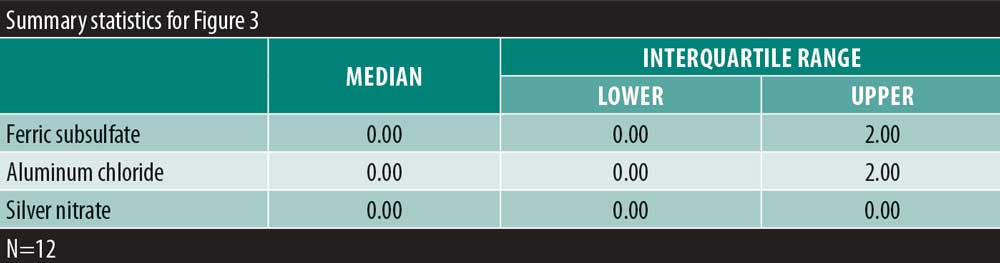
A significant overall variability in the satisfaction response was found among the three chemical cautery solutions (overall p=0.042). After adjusting each pairwise comparison for inflated Type 1 error though, there was no significant difference in the satisfaction response between aluminum chloride hexahydrate and ferric subsulfate (Sidak p=0.88), between aluminum chloride and silver nitrate (Sidak p=0.18), and between ferric subsulfate and silver nitrate (Sidak p=0.58) (Figure 2). Finally, in this sample, there was no significant overall variability in the time to hemostasis among the three chemical cautery solutions (overall p=0.57) (Figure 3).
A long-term assessment of pigmentary change was conducted by phone call follow-up. The 12 participants were contacted after snip excision to determine whether they had noticed any long-term pigmentary change at the skin tag excision sites. The snip excisions had been a minimum of six months to a maximum of 12 months prior to the time of follow-up. Of the 12 participants, eight responded (67%). All eight respondents (100%) reported no evidence of long-standing pigmentary change at any of the excision sites.
Discussion
An ideal hemostatic agent for skin tag excision should be easy to use, obtain hemostasis rapidly, produce minimal pain, and have little to no pigmentary change. Chemical cautery is ubiquitous in dermatological procedures because it is a simple and safe method for controlling bleeding. The chemical cautery solutions investigated in this study have all been validated as an effective means of achieving hemostasis.5–9 However, there are risks and benefits to each of these cautery solutions that should assist in clinical decisions.
Ferric subsulfate is composed of 10 to 20 percent ferric subsulfate and is capable of occluding small wounds by protein precipitation.5,10 Benefits to using ferric subsulfate include ease of application, bacteriostatic properties, and low cost. The major drawback of using ferric subsulfate is the small risk of permanent brown pigmentation due to iron particle deposition.11–13 Ferric subsulfate also promotes hyperpigmentation at the application site by increasing inflammation.5,9 Other potential drawbacks to ferric subsulfate include increased erythema, dermal fibrosis, and wound site re-epithelialization.13,14 Similarly, in our study 25 percent of participants experienced a pigmentary change when exposed to ferric subsulfate. The pain upon application with ferric subsulfate was also found to be greater than the pain of aluminum chloride hexahydrate application though less than silver nitrate application (median=1.50, IQR: 0.00–3.50; Sidak p=0.01). Given no significant differences in the satisfaction response between the three solutions, the increased pain and pigmentary changes are clinically relevant considerations in the use of ferric subsulfate as a hemostatic agent.
Silver nitrate is also used to achieve hemostasis in surgical procedures.5 Silver nitrate is advantageous in ease of use, can be stored at room temperature, and is cost effective. Application of silver nitrate produces a dark eschar, which, although temporary, can be alarming to the patient. The main drawback of silver nitrate is its potential to tattoo the skin as a result of silver particles embedding into the epidermis.8 In this study, 50 percent of participants experienced a pigmentary change after exposure to silver nitrate. Additionally, the pain upon application was significantly higher for silver nitrate (median=6.00) compared to both aluminum chloride hexahydrate (median=1.00) and ferric subsulfate (median=1.50). The increased pain severity and pigmentary changes associated with silver nitrate are potential limitations to its use in clinical practice.
Aluminum chloride hexahydrate is similar to ferric subsulfate and silver nitrate in that it is easy to use, cost-effective, and easy to store.5 However, aluminum chloride hexahydrate is different in that it does not carry a risk of hyperpigmentation or tattoo. Two participants in our study reported pigmentary change (17%) after exposure to aluminum chloride hexahydrate. This pigmentary change could be explained by post-inflammatory hyperpigmentation given aluminum chloride hexahydrate’s negligible risk of tattoo. Excessive application of aluminum chloride hexahydrate has also been shown to delay healing.5,9,13 Though the amount of aluminum chloride hexahydrate was standardized by using the same size cotton tip applicator across all excisions, there remains the possibility of excessive application due to technical error. The pigmentary change experienced by the two participants could then be explained by delayed wound healing leading to prolonged inflammation that the participant perceived as pigmentary change. In terms of pain, aluminum chloride hexahydrate was found to have the lowest pain upon application among the three solutions (median=1.00, IQR: 0.50 to 6.00; Sidak p=0.02). Both the reduced pain upon application and minimal risk of hyperpigmentation suggest aluminum chloride hexahydrate is a convenient hemostatic agent for clinical use.
Rapid hemostasis is the primary goal of using chemical cautery in dermatologic procedures. Time to hemostasis was found to be equivalent among these three chemical cautery solutions. One challenge in this study was the limited number of excisions that bled. This made it difficult to truly assess the time to hemostasis with each chemical cautery solution. The minimal bleeding with excision could be due to technique, small skin tag size, or not pre-medicating with lidocaine. The average diameter of the skin tags included in this study was small (2mm) with a small associated pedicle (1.5mm). The excision technique used in this study involved cutting the skin tag as close to the base as possible. However, an unintended cut farther from the base of the skin tag with its associated vasculature could potentially explain the minimal residual bleeding. Bleeding could have also been reduced because participants were not pre-medicated with lidocaine. The vasoactive effects of lidocaine have been well documented, particularly in higher concentrations, and it is thought to be mediated by the release of nitric oxide.15–17 Given these properties of lidocaine, bleeding from excision sites could be less without premedication than with lidocaine pre-treatment. However, the doses of lidocaine necessary to achieve this effect are far greater than the doses used for standard snip excision, making this explanation unlikely. Further, a more common premedication for snip excision is lidocaine and epinephrine. This standard combination would also be expected to reduce bleeding compared to the absence of premedication given epinephrine’s known vasoconstrictive effects.16,17
Pain is an important consideration for all dermatologic procedures, especially in procedures such as skin tag excision where patients are not always premedicated, and the procedures are elective. Silver nitrate has been previously shown to have a strong burning sensation with application.5 Aluminum chloride also has the potential to cause painful paresthesia at the site of application.5,9,13 A comparison of ferric subsulfate solution to ball electrode demonstrated that ferric subsulfate was associated with significantly less pain, though only in the context of gynecologic surgeries.18 This study found a significant difference in pain intensity was found between silver nitrate and aluminum chloride hexahydrate (Sidak p=0.02) and silver nitrate and ferric subsulfate (Sidak p=0.01). The increased pain severity and pigmentary changes associated with silver nitrate suggests it might not be an ideal chemical cautery for skin tag removal.
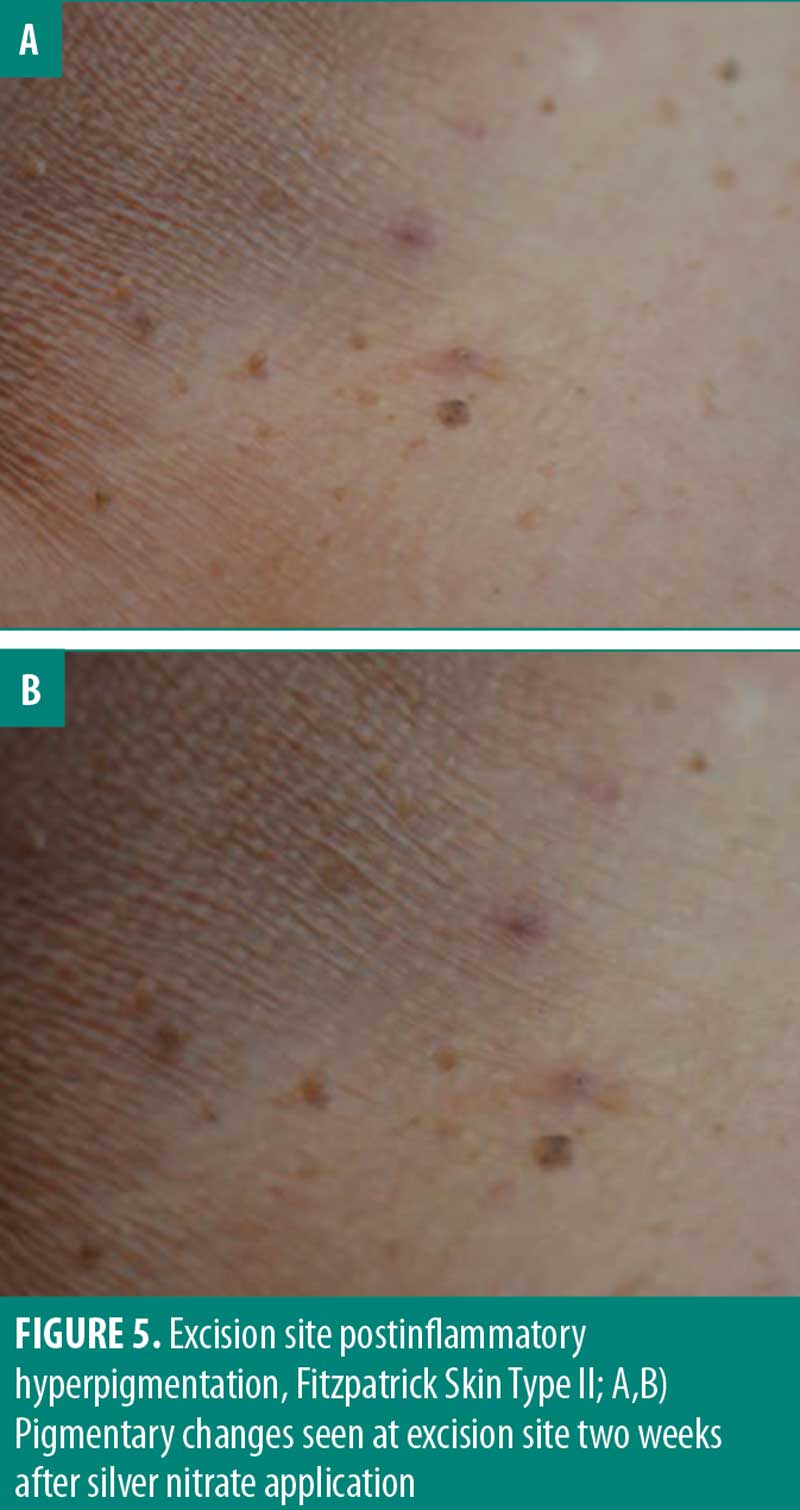
Pigmentary change is a relevant consideration for the removal of skin tags especially in cosmetically sensitive areas. Although the perceived satisfaction with each cautery solution was not significantly different, 50 percent of participants noted pigmentary changes when exposed to silver nitrate. One important consideration in determining true pigmentary change is postinflammatory hyperpigmentation (PIH). PIH is an acquired hyperpigmentation that occurs after an inflammatory event or injury.19,20 Though PIH affects all Fitzpatrick skin types, it occurs more frequently and with greater severity in more darkly pigmented skin.20 In this study, PIH was observed in patients with Fitzpatrick skin types 3 to 6 (Figure 4). However, the proportion of participants (50%) who reported pigmentary changes two weeks after exposure to silver nitrate included individuals of all Fitzpatrick skin types. This suggests the observed pigmentary change is potentially a result of the chemical cautery solution as opposed to PIH alone. Follow-up assessment of pigmentary change indicated there is minimal-to-no long-term pigmentary change, with 100 percent of participants (8/8) reporting no pigmentary change 6 months to 1 year after snip excision. These results suggest that long-term pigmentary change is equivalent among the three chemical cautery solutions. It should be noted that the pigmentary change was self-reported without clinical confirmation and therefore subject to inter-observer variation. Ultimately, the statistically significant difference in pain and perceived short-term pigmentary changes associated with silver nitrate suggest it might not be first-line treatment in clinical practice despite its effectiveness as a hemostatic agent.
There are several limitations to this study. One limitation of this study is the small sample size consisting of 12 participants for a total of 72 skin tags. Larger studies would have to be conducted to validate the conclusions about each chemical cautery technique. While participants in this study self-reported their pain and satisfaction after excision using a numeric scale, a more detailed assessment using a standardized outcome survey, such as patient-reported outcome measure questionnaires, could be used to validate these self-reported values.
Conclusion
This study investigated the use of three chemical cautery solutions for skin tag excision. A direct comparison of silver nitrate, aluminum chloride hexahydrate, and ferric subsulfate was conducted to determine time to hemostasis, pain of application, pigmentary changes, and patient satisfaction with each chemical cautery solution. A significant variability in pain was found among the three solution types with a lower pain response for aluminum chloride hexahydrate and ferric subsulfate compared to silver nitrate. Though the odds of experiencing a pigmentary change were equivalent, a large proportion of participants reported pigmentary changes associated with silver nitrate (50%) compared to ferric subsulfate (25%) or aluminum chloride hexahydrate (17%). Long-term pigmentary change among the three cautery solutions appeared equivalent. These results suggest choice of a chemical cautery solution should take into consideration the differing levels of discomfort associated with application and the potential short-term pigmentary changes. Limitations to the study include a small sample size (n=12) and the self-reported values of pain, satisfaction, and long-term pigment changes. Additional studies with larger sample sizes should be conducted to further support these results. In summary, this study demonstrated the potential importance of pain and pigmentary changes in choice of a chemical cautery solution for skin tag excision.
References
- Gorgulu T, Torun M, Guler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39(4):644–645.
- Mukhtar M. Surgical pearl: tissue forceps as a simple and effective instrument for treating skin tags. Int J Dermatol. 2006;45(5):577–579.
- Glick JB, Kaur RR, Siegel D. Achieving hemostasis in dermatology-part II: topical hemostatic agents. Indian Dermatol Online J. 2013;4(3):172–176.
- Ho J, Hruza G. Hydrophilic polymers with potassium salt and microporous polysaccharides for use as hemostatic agents. Dermatol Surg. 2007;33(12):1430–1433.
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34(4):431–445.
- Baden HP. Rapid hemostasis with monsel’s solution. Arch Dermatol. 1984;120(6):708.
- Nouri S, Sharif MR, Panahi Y, et al. Efficacy and safety of aluminum chloride in controlling external hemorrhage: an animal model study. Iran Red Crescent Med J. 2015;17(3):e19714.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal “photograph,” a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16(1 Pt 2):211–217.
- Larson PO. Topical hemostatic agents for dermatologic surgery. J Dermatol Surg Oncol. 1988;14(6):623–632.
- Kuwahara RT, Craig SR, Amonette R. More on monsel’s solution. Dermatol Surg. 2000;26(10):979–980.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by monsel’s solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2(3):197–205.
- Duray PH, Livolsi VA. Recurrent dysplastic nevus following shave excision. J Dermatol Surg Oncol. 1984;10(10):811–815.
- Sawchuk WS, Friedman KJ, Manning T, Pinnell SR. Delayed healing in full-thickness wounds treated with aluminum chloride solution. A histologic study with evaporimetry correlation. J Am Acad Dermatol. 1986;15(5 Pt 1):982–989.
- Armstrong RB, Nichols J, Pachance J. Punch biopsy wounds treated with monsel’s solution or a collagen matrix. A comparison of healing. Arch Dermatol. 1986;122(5):546–549.
- Willatts DG, Reynolds F. Comparison of the vasoactivity of amide and ester local anaesthetics. an intradermal study. Br J Anaesth. 1985;57(10):1006–1011.
- Cederholm I, Evers H, Lofstrom JB. Skin blood flow after intradermal injection of ropivacaine in various concentrations with and without epinephrine evaluated by laser doppler flowmetry. Reg Anesth. 1992;17(6):322–328.
- Newton DJ, McLeod GA, Khan F, Belch JJ. Mechanisms influencing the vasoactive effects of lidocaine in human skin. Anaesthesia. 2007;62(2):146–150.
- Givens VA, Lipscomb GH, Meyer NL. A randomized trial of postoperative wound irrigation with local anesthetic for pain after cesarean delivery. Am J Obstet Gynecol. 2002;186(6):1188–1191.
- Taylor S, Grimes P, Lim J, et al. Postinflammatory hyperpigmentation. J Cutan Med Surg. 2009;13(4):183–191.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3(7):20–31.

