[Corrections were made to this article on June 11, 2019. Click here to access CORRECTED version with list of changes.]
J Clin Aesthet Dermatol. 2019;12(2):12–18
 by Mark S. Nestor, MD, PhD; Brian Berman, MD, PhD; David Goldberg, MD, JD; Armand B. Cognetta, Jr., MD; Michael Gold, MD; William Roth, MD; Clay J. Cockerell, MD; and Brad Glick, DO, MPH
by Mark S. Nestor, MD, PhD; Brian Berman, MD, PhD; David Goldberg, MD, JD; Armand B. Cognetta, Jr., MD; Michael Gold, MD; William Roth, MD; Clay J. Cockerell, MD; and Brad Glick, DO, MPH
Drs. Nestor and Berman are with the Center for Clinical and Cosmetic Research in Aventura, Florida and the Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine in Miami, Florida. Dr. Goldberg is with Skin Laser & Surgery Specialists of NY/NJ, Icahn School of Medicine at Mount Sinai, and Fordham Law School in New York, New York. Dr. Cognetta is with Dermatology Associates of Tallahassee in Tallahassee, Florida. Dr. Gold is with Gold Skin Care Center, the Tennessee Clinical Research Center, Vanderbilt University School of Nursing, and Meharry Medical College School of Medicine in Nashville, Tennessee. Dr. Roth is with Dermatology and Dermatological Surgery in Boynton Beach, Florida. Dr. Cockerell is with Cockerell Dermatopathology in Dallas, Texas. Dr. Glick is with the Glick Skin Institute in Margate, Florida.
FUNDING: Sensus Healthcare (Boca Raton, Florida) provided financial support for the preparation of this article.
DISCLOSURES: Drs. Nestor, Berman, Goldberg, and Gold are consultants, speakers, and/or members of the advisory board for Sensus Healthcare, a manufacturer of superficial radiation therapy devices. The other authors report no conflicts of interest relevant to the content of this article.
Abstract: Background. The use of superficial radiation therapy (SRT) has experienced a renaissance for treating nonmelanoma skin cancers (NMSCs) and recurrent keloids; however, published treatment guidelines are lacking.
Objective.The objective of this work was to provide consensus guidelines on the use of SRT for treating NMSC and recurrent keloids based on a review of the literature and expert opinion.
Methods and Materials.A search of the medical literature was performed to obtain published information on the use of SRT for review. A group of qualified dermatologists convened to discuss their views on the use of SRT for the treatment of NMSCs and recurrent keloids. The various guidelines were considered to have consensus based on a supermajority two-thirds vote. The final consensus guidelines are thus based on the medical literature, when available, and expert opinions.
Results.Agreement on consensus guidelines was reached for numerous aspects of SRT use, including appropriate tumor types for SRT; anatomical areas suitable for SRT; energy, fractions, and scheduling recommendations for SRT; use of SRT in the presence of comorbidities; safety factors; and treatment recommendations for recurrent keloids, based the literature and on both the opinions of the expert group and a survey of experienced users.
Conclusion. Consensus was reached that SRT is a safe and effective treatment for basal cell and squamous cell carcinomas and should be considered as the first-line form of radiation treatment. Postsurgical treatment of keloid excision suture lines with SRT significantly reduces keloid recurrence rates.
KEYWORDS: Superficial radiation therapy, nonmelanoma skin cancer, literature review, consensus guidelines
Introduction
Nonmelanoma skin cancer (NMSC), which includes basal cell carcinoma (BCC) and squamous cell carcinoma (SCC),1 is the most frequently diagnosed type of cancer in humans,2 with an incidence that continues to increase. The mean annual number of adults treated for NMSC grew from approximately 3.0 million from the years 2002 to 2006 to 4.3 million from the years 2007 to 2011 (p<0.001).2 Well-known risk factors for developing NMSC include chronic exposure to ultraviolet radiation, fair complexion, advancing age, and immunosuppression.1,2 While most cases of NMSC are relatively benign and respond to treatment, a small number of SCCs result in metastasis and death (mortality ratio estimates are 1.25 to 1.30).3,4
The economic burden of skin cancer treatment is substantial and continues to rise with increasing incidence. The mean annual total cost for treating NMSC skin cancer in the United States increased from $2.7 billion from the years 2002 to 2006 to $4.7 billion from the years 2007 to 2011 (p<0.001). During this time, the mean cost per patient rose from $882 to $1,105 (p=0.04).5
The range of available treatment options for NMSC includes surgery, cryotherapy, curettage and electrodessication, and radiation therapy (RT). RT includes superficial radiation therapy (SRT), photodynamic therapy, various forms of brachytherapy, and chemotherapeutic agents.6–12 Treatment decisions are based on tumor type, anatomical location, patient age, tumor stage and classification, physician preference, and/or treatment setting.1 The choice of treatment becomes more complex among the elderly due to frailty, limited life-expectancy, and comorbidities.1 Anatomical location also becomes an important factor, as NMSC lesions commonly occur on the ears, eyes, and nose13 where treatment can have significant cosmetic consequences.
Among the available treatments, superficial radiation therapy (SRT) is one of the oldest, having been developed more than 100 years ago.14 For many years, SRT played a major role in the practice of dermatology; by 1975, 55.5 percent of dermatology offices in North America were equipped with SRT or Grenz ray devices and 44.3 percent of dermatologists reported using them regularly.15 At that time, dermatologists were routinely trained on the use of SRT and were familiar with its indications and treatment protocols.16 Despite the abundance of clinical trial data demonstrating the effectiveness of SRT for treating NMSC,17–24 including long-term studies,25–27 the use of SRT declined as new surgical techniques gained popularity and replacement SRT devices became unavailable. Dermatology residency programs no longer provide training on the use of SRT.
Recently, SRT has experienced a renaissance as advocates strive to fill knowledge gaps regarding its use and demonstrate again its efficacy with large studies.14 One retrospective study assessed the results of 1,715 histologically confirmed, nonaggressive BCCs (n=712), SCCs (n=994), and tumors with combined features (n=9) that were treated with SRT over a 10-year period.25 The mean patient age was 79 years, and the mean duration of follow-up was 31.5 months (range: 1–120 months). The overall five-year recurrence rate was 5.0 percent. Specific five-year recurrence rates were also determined for BCC (4.2%), SCC (5.8%), invasive SCC (6.7%), and SCC in situ (5.5%). Treated areas included the cheeks, nose, forehead, lips, chin, and scalp, with excellent cosmetic results.
Similarly to NMSC, an enormous body of literature has demonstrated the effectiveness of SRT as an adjunctive therapy for the treatment of recurrent keloids; nevertheless, SRT also remains an underutilized therapy for this distressing condition.28–30
In addition to decades of clinical data on the use of SRT, the recent increase in its use has also been greatly aided by the commercial availability of a new and advanced SRT device that has been cleared for treating NMSC and recurrent keloids (SRT-100™; Sensus Healthcare, Boca Raton, Florida).
A group of qualified and experienced dermatologists convened in South Beach, Florida, on March 1, 2018, to discuss their views on the use of SRT for the treatment of BCC, SCC, and recurrent keloids. In the absence of guidelines on the use of SRT for these conditions, the goal was to provide consensus guidelines regarding the use of SRT for the treatment of NMSC and recurrent keloids. The eight participants had a combined 250 years of dermatology practice and 78 years of experience with SRT and had treated approximately 8,750 patients with SRT. The various guidelines were considered to have consensus based on a supermajority two-thirds vote.
Methods
Literature search. Several weeks prior to the meeting, searches of the Cochrane Library (http://www.cochranelibrary.com) and PubMed (https://www.ncbi.nlm.nih.gov/pubmed) databases were performed to identify the available information regarding treatment of BCC, SCC, and recurrent keloids. PubMed searches included articles published from January 1, 1998, to June 30, 2018, with inclusion restricted to articles dealing with human patients and in the English language. As the subject matter was broad, numerous searches were performed using combinations of medical subject heading terms: nonmelanoma skin cancer, basal cell skin cancer, squamous cell skin cancer, squamous cell skin cancer in situ, Bowen’s disease, treatment, therapy, radiation therapy, superficial radiation therapy, superficial X-ray therapy, soft X-ray therapy, surgery, Mohs surgery, Mohs micrographic surgery, brachytherapy, electronic brachytherapy, electron beam radiotherapy, photodynamic therapy, radiation dermatitis, and clinical trials.
Consensus guideline development. One author (MSN) identified clinical topics for group discussion, which included the following:
- Available forms of radiation therapy for NMSC and recurrent keloids
- SRT versus other radiation therapies
- Electronic surface brachytherapy
- Tumor types for SRT
- Appropriate patients for SRT
- Suitable anatomical areas for SRT
- Measuring tumors and tumor margins for SRT
- Energy, fractions, and scheduling for different tumor types and anatomical areas
- Topical treatments for radiation dermatitis
- Patient safety factors
- Pausing treatment for radiation dermatitis
- Treating recurrent keloids using SRT.
Each topic included several discussion points. These topics and discussion points were sent to each meeting participant for review and generation of comments prior to the planned discussion and were included in the final document prior to the group meeting. Following the expert opinion meeting, the discussion points were revised and resent to all participants until a consensus was reached. The guidelines were considered to have consensus based on a supermajority two-thirds vote. The following guidelines statements are based on the medical literature and expert consensus opinions. A summary of these statements is provided in Table 1.
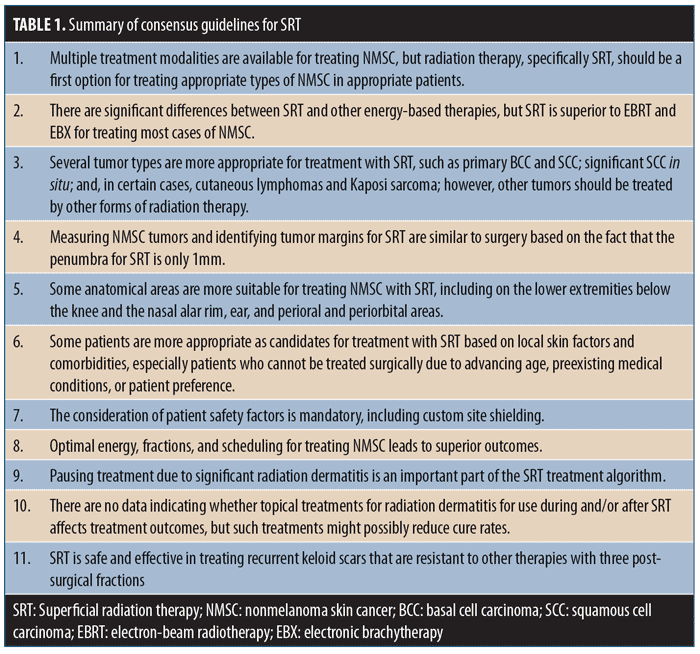
Consensus Guidelines for Superficial Radiation Therapy
1.0—Multiple treatment modalities are available for treating NMSC, but radiation therapy, specifically SRT, should be a first option for treating appropriate types of NMSC in appropriate patients.
1.1 Currently available treatments for NMSC include destruction, surgery, photodynamic therapy, topical therapies, and several energy-based therapies and various forms of RT, although no specific treatment choice algorithm exists.31–33
1.2 In many cases, cure rates using RT, and specifically SRT for the treatment of MSC, are similar to surgical options.34 Cosmesis with appropriate energies and fractions might be superior to surgery for NMSC in certain anatomic locations.6,10,34,35
1.3 Radiation therapy, specifically SRT, should be a first option for treating appropriate types of NMSC tumors (see 3.1).7,20,25,35 Patient consent for NMSC should include a discussion of all treatment options including SRT.36
1.4 Contraindications to the use of SRT include aggressive tumor histology or deep tumor invasion, previously irradiated lesions, and some types of NMSC occurring on organ transplant recipients.14
2.0—There are significant differences between SRT and other energy-based therapies, but SRT is superior to electron-beam radiotherapy (EBRT) and electronic brachytherapy (EBX) for treating most cases of NMSC.
2.1 EBRT utilizes electrons to treat NMSC37 and EBX involves the application of short-contact X-rays to treat NMSC lesions.9,38
2.1.1. There are significant differences in the physical and clinical properties of these treatment modalities, such as beam profile and depth of penetration. The beam and delivered dose of SRT have dramatically less lateral edge beam drop-off (1mm) in the penumbra at the treatment site compared with EBRT (8–10mm).39 EBRT requires higher energy to successfully encompass a superficial lesion and is associated with lower overall cure rates for NMSC.
2.1.2. SRT is therefore superior to EBRT for treating most NMSC and results in better cosmesis.40,41
2.1.3. To some extent, different energy-based therapies can be optimal for different tumor types and anatomical areas.38,42 For example, EBRT has an established role as adjunctive therapy in tumors with perineurial invasion, treatment of cutaneous T-cell lymphomas, Merkel cell carcinoma, dermatofibrosarcoma protuberans, and select melanomas of the head and neck that demonstrate extracapsular spread in lymph nodes or are spindle cell subtypes.43
2.2 EBX should be considered short-contact SRT, since the energy source is the same and the technology is virtually identical to short-contact SRT devices.44,45
2.2.1. SRT is superior to electronic surface EBX based on its abilities to vary energies from 50 to 100cGy and employ larger spot sizes. In contrast to EBX, clinical data on thousands of patients support long-term cure rates and cosmesis with SRT.10,25
2.2.2. Although the energy source is the same, SRT is currently more cost-effective in terms of equipment and patient cost.14
3.0—Several tumor types are more appropriate for treatment with SRT, such as primary BCC, SCC, significant SCC in situ, and certain cases of cutaneous lymphomas and Kaposi sarcoma. However, other tumor types should be treated using other forms of radiation therapy.
3.1 SRT is a viable nonsurgical option and chief indication for primary BCC and SCC and for significant SCC in situ.25,46 However, similar to surgical options, using SRT to treat large, deep tumors can have lower cure rates than smaller tumors, except for superficial ones.47,48
3.2 SRT can also be used in certain cases to treat cutaneous lymphomas and Kaposi sarcoma.49–51 However, other tumor types (e.g., tumors with perineurial invasion, cutaneous T-cell lymphomas, Merkel cell carcinoma, dermatofibrosarcoma protuberans, and select melanomas of the head and neck that demonstrate extracapsular spread in lymph nodes or spindle cell subtypes) should be treated by other forms of radiation therapy such as EBX.43
4.0—Measuring NMSC tumors and identifying tumor margins for SRT are similar to surgery—the penumbra for SRT is only 1mm.
4.1 Tumor margins are similar to those used in surgery.52 The most appropriate method for establishing the margin is to measure the tumor using the same margins necessary to achieve adequate cure rates if it were treated surgically.
4.2 The initial measurement for the NMSC should include all clinical areas that could have tumor present, similar to the way drawn surgical excision margins are estimated. The maximum diameter of this measured area should be reported as tumor size. Additional SRT treatment margins can then be 5mm or less of clinically normal skin due to the fact that the penumbra for SRT is only 1mm. Older literature based on EBRT, which had a penumbra of over 6mm, estimated that the radiation field should extend 5 to 10mm (the umbra) beyond the tumor into clinically normal skin.25
5.0—Some anatomical areas are more suitable for treating NMSC with SRT. These include extremities below the knee, nasal alar rim, ear, perioral, and periorbital areas.
5.1 In areas where tissue-sparing is important, SRT might be better suited than surgery, which can have undesirable cosmetic and functional consequences requiring reconstructive surgery.20,53,54
5.2 SRT has particularly favorable cosmesis on the nasal alar rim, ear, and perioral and periorbital areas.19,20,22,26,34,47,48,55,56 SRT has been shown to be particularly beneficial for certain cases of NMSC on the lower extremities below the knee with site-specific radiation planning, dosimetry, and fractionation.57
5.3 Surgical site infection rates for Mohs micrographic surgery and wide local excisions performed below the knee range from 2.3 to 8.3 percent, respectively.58 Among 151 lower-extremity NMSC lesions treated with SRT, there were no treatment-related infections.57
6.0—Some patients are more appropriate for treatment with SRT based on local skin factors and comorbidities, especially patients who cannot be treated surgically due to advancing age, preexisting medical conditions, or patient preference.
6.1 Patients with NMSC present with varying ages,59,60 medication use patterns, and comorbidities.60 SRT is beneficial and cost-effective for NMSC on the lower extremities, which might otherwise be associated with cellulitis and infection—especially among frail, elderly patients.14,25
6.2 SRT is especially indicated for treating patients who cannot be treated surgically due to advancing age; preexisting medical conditions such as diabetes, stasis dermatitis, chronic edema, and circulatory compromise;54,61,62 concomitant drug therapy (e.g., anticoagulation); or patient preference.63,64
6.3 As there is no anesthesia or cutting associated with the use of SRT, making it is ideal for patients who fear surgery.42
7.0—The consideration of patient safety factors is mandatory, including custom site shielding.
7.1 To reliably and safely deliver the dose to the tumor bed, proper patient positioning, immobilization, and shielding should be repeatedly tested and finely tuned during treatment simulation. Shielding of the eyes and nontreated areas including the torso and thyroid should be used routinely as well as specific shields for intraoral and intranasal locations when those areas are associated with treatment.25
7.2 Custom lead shielding should be fabricated for the specific tumor site in all patients undergoing SRT.65 Any variance in shield can cause position or radiation therapy cone contact, which might result in undertreatment of a tumor.
7.3 Patients should be informed about expected short-term and long-term side effects,66 which include various degrees of radiation dermatitis and the occurrence of postinflammatory hypo- or hyperpigmentation among patients with dark skin.
8.0—Optimal energy, fractions, and scheduling for treating NMSC leads to superior outcomes.
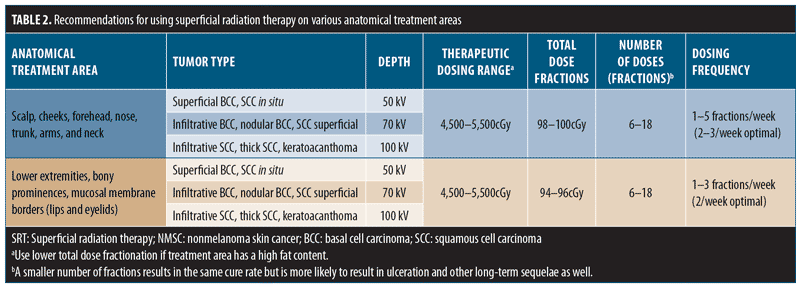
8.1 Treatment recommendations are specific for anatomical locations. Altering the fraction size and the overall total dose leads to acute (e.g., radiation dermatitis and ulceration) and latent (e.g., atrophy, telangiectasia, and pigmentation changes) reactions.18,67 Data indicate that changes in SRT fractionation schemes by increased number and time between treatments have led to better outcomes.55,56,68 Additionally, the total dose fraction (TDF) should be between 90 and 110, especially when treating low-vascular areas such as the lower limbs.
8.2 The range of available energy with SRT permits the use of higher energy for deeper NMSC lesions. Cure rates are similar for different fraction numbers provided the TDF is similar, but short- and long-term adverse events can be significantly fewer in number with a larger number of fractions.
8.3 The ideal number of fractions involves discussion with patients and family regarding outcome and cosmesis (more fractions) versus convenience (less fractions). The treatment recommendations in Table 2 are deemed appropriate for each area.68,69
9.0—Pausing treatment due to significant radiation dermatitis is an important part of the SRT treatment algorithm.
9.1 Constant evaluation of the treatment site is necessary throughout the course of treatment. There can be minimal pain, swelling, or moist desquamation at the treatment area. There should be a pause (decay) in treatment at the first sign of significant ulceration, swelling, or pain.
9.2 Subsequently, a reassessment and dosimetry calculation should be performed to determine whether a change in treatment parameters is necessary.
10.0 —There are no data indicating whether use of topical treatments for radiation dermatitis during and/or after SRT affects treatment outcomes, but such treatments can possibly reduce cure rates.
10.1 Numerous topical products are promoted for preventing or treating radiation dermatitis. Although there is a hypothesis that inflammation associated with radiation therapy is a mechanism of curing NMSC,70 there is no clinical study evidence regarding whether reducing inflammation associated with radiation dermatitis impacts cure rates.
10.2 Similarly, there is insufficient evidence to support or refute the use of specific therapies for the prevention or management of radiation-induced skin changes. Additional studies are needed.71–73
10.3 The post-SRT management of radiation dermatitis is based principally on the severity of damaged skin.
11.0 —SRT is safe and effective in treating recurrent keloid scars that are resistant to other therapies with three postsurgical fractions.
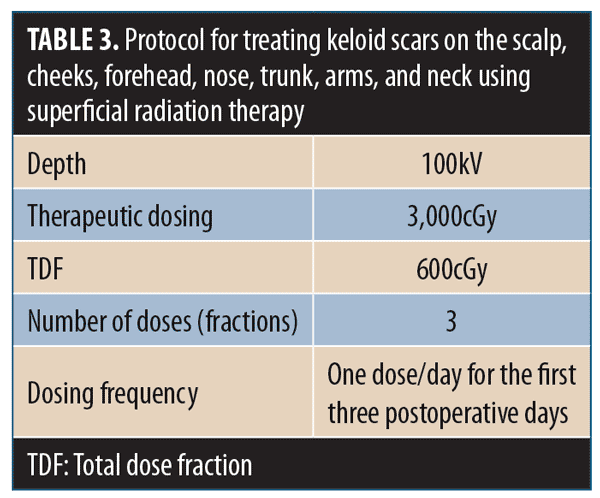
11.1 There is substantial evidence that SRT is effective for treating recurrent keloid scars that are resistant to other therapies.74–76 Postsurgical treatment of keloid excision suture lines with several fractions of SRT significantly reduces keloid recurrence rates.77–82 Although effective outcomes can be achieved with single doses of SRT, long-term sequelae are improved with three doses.83
11.2 Fractionation of the SRT dose reduces the risk of hyperpigmentation and other adverse events. The optimal treatment protocol is a biologically effective dose of 3,000cGy in three fractions of 600cGy on the first three postoperative days.84 The treatment recommendations in Table 3 are appropriate for keloids.76,85–88
11.3 There is little evidence that exposing keloids or surrounding healthy skin to SRT at a 3,000-cGy dose causes skin cancer.89–92
Conclusion
Superficial radiation therapy should be the first option for treating appropriate types of NMSC tumors in suitable patients, as cure rates are similar to most surgical options and there exists superior cosmesis in certain anatomic locations. SRT is also a safe and cost-effective option for treating NMSCs on the lower extremities that might otherwise be associated with medical complications in elderly patients and those with existing comorbidities. Postsurgical treatment of keloid excision suture lines with SRT significantly reduces keloid recurrence rates with no evidence that exposing surrounding healthy skin causes skin cancer.
Acknowledgment
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., with the preparation of this manuscript.
References
- Garcovich S, Colloca G, Sollena P, et al. Skin cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis. 2017;8(5):643–661.
- Didona D, Paolino G, Bottoni U, et al. Non-melanoma skin cancer pathogenesis overview. Biomedicines. 2018;6(1):E6.
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–247.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243–251.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48:183–187.
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:6153.
- Hernández-Machin B, Borrego L, Gil-García M, et al. Office-based radiation therapy for cutaneous carcinoma: evaluation of 710 treatments. Int J Dermatol. 2007;46(5):453–459.
- Alam M, Nanda S, Mittal BB, et al. The use of brachytherapy in the treatment of nonmelanoma skin cancer: a review. J Am Acad Dermatol. 2011;65(2):377–388.
- Ballester-Sánchez R, Pons-Llanas O, Candela-Juan C, et al. Electronic brachytherapy for superficial and nodular basal cell carcinoma: a report of two prospective pilot trials using different doses. J Contemp Brachytherapy. 2016;8(1):48–55.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53(6):993–1001.
- Piccolo D, Kostaki D. Photodynamic therapy activated by intense pulsed light in the treatment of nonmelanoma skin cancer. Biomedicines. 2018;6(1):E18.
- Metterle L, Nelson C, Patel N. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): a review. J Am Acad Dermatol. 2016;74(3):552–557.
- Pearl DK, Scott EL. The anatomical distribution of skin cancers. Int J Epidemiol. 1986;15(4):502–506.
- Cognetta AB Jr, Wolfe CM, Goldberg DJ, et al. Practice and educational gaps in radiation therapy in dermatology. Dermatol Clin. 2016;34(3): 319–333.
- Goldschmidt H. Ionizing radiation therapy in dermatology. Current use in the United States and Canada. Arch Dermatol. 1975;111(11):1511–1517.
- Pomper ME, Schlam EH. Rediscovering radiation therapy for skin cancers. Prac Derm. 2007; 25–27.
- Bodner WR, Hilaris BS, Alagheband M, et al. Use of low-energy X-rays in the treatment of superficial nonmelanomatous skin cancers. Cancer Invest. 2003;21(3):355–362.
- Abbatucci JS, Boulier N, Laforge T, et al. Radiation therapy of skin carcinomas: results of a hypofractionated irradiation schedule in 675 cases followed more than 2 years. Radiother Oncol. 1989;14(2):113–119.
- Caccialanza M, Piccinno R, Percivalle S, et al. Radiotherapy of carcinomas of the skin overlying the cartilage of the nose: our experience in 671 lesions. J Eur Acad Dermatol Venereol. 2009;23(9):1044–1049.
- Rodriguez JM, Deutsch GP. The treatment of periocular basal cell carcinomas by radiotherapy. Br J Ophthalmol. 1992;76(4):195-197.
- Fitzpatrick PJ. Skin cancer of the head—treatment by radiotherapy. J Otolaryngol. 1984;13(4):261–266.
- Olschewski T, Bajor K, Lang B, et al. [Radiotherapy of basal cell carcinoma of the face and head: Importance of low dose per fraction on long-term outcome.] J Dtsch Dermatol Ges. 2006;4(2): 124–130. Article in German.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):748–755.
- Wilder RB, Kittelson JM, Shimm DS. Basal cell carcinoma treated with radiation therapy. Cancer. 1991;68(10):2134–2137.
- Cognetta AB, Howard BM, Heaton HP, et al. Superficial x-ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol. 2012;67(6):1235–1241.
- Childers BJ, Goldwyn RM, Ramos D, et al. Long-term results of irradiation for basal cell carcinoma of the skin of the nose. Plast Reconstr Surg. 1994;93(6):1169–1173.
- Zagrodnik B, Kempf W, Seifert B, et al. Superficial radiotherapy for patients with basal cell carcinoma: recurrence rates, histologic subtypes, and expression of p53 and Bcl-2. Cancer. 2003;98(12):2708–2714.
- Song C, Wu HG, Chang H, et al. Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. J Radiat Res. 2014;55(5): 912–916.
- Emad M, Omidvari S, Dastgheib L, et al. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids: a prospective clinical trial. Med Princ Pract. 2010;19(5):402–405.
- van de Kar AL, Kreulen M, van Zuijlen PP, et al. The results of surgical excision and adjuvant irradiation for therapy-resistant keloids: a prospective clinical outcome study. Plast Reconstr Surg. 2007;119(7):2248–2254.
- Fahradyan A, Howell AC, Wolfswinkel EM, et al. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare (Basel). 2017;5(4):E82.
- Eskiizmir G, Cingi C. Nonmelanoma skin cancer of the head and neck: current diagnosis and treatment. Facial Plast Surg Clin North Am. 2012;20(4):415–417.
- McGregor S, Minni J, Herold D. Superficial radiation therapy for the treatment of nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2015;8(12):12–14.
- Leshin B, Yeatts P, Anscher M, et al. Management of periocular basal cell carcinoma: Mohs’ micrographic surgery versus radiotherapy. Surv Ophthalmol. 1993;38(2):193–212.
- Grossi Marconi D dCRB, Rauber E, de Cassia Soares P, et al. Head and neck non-melanoma skin cancer treated by superficial x-ray therapy: an analysis of 1021 cases. PLoS One. 2016;11(7):e0156544.
- Shriner DL, Wagner RF Jr, Weedn VW, et al. Informed consent and risk management in dermatology: to what extent do dermatologists disclose alternate diagnostic and treatment options to their patients?. J Contemp Health Law Policy. 1992;8:137–162.
- Hogstrom KR, Almond PR. Review of electron beam therapy physics. Phys Med Biol. 2006;51(13):R455–R489.
- Kasper ME, Chaudhary AA. Novel treatment options for nonmelanoma skin cancer: focus on electronic brachytherapy. Med Devices (Auckl). 2015;8:493–502.
- Korevaar EW, van Vliet RJ, Woudstra E, et al. Sharpening the penumbra of high energy electron beams with low weight narrow photon beams. Radiother Oncol. 1998;48(2):213–220.
- Khan L, Breen D, Zhang L, et al. Predictors of recurrence after radiotherapy for non-melanoma skin cancer. Curr Oncol. 2014;21(2):e326–e329.
- Lovett RD, Perez CA, Shapiro SJ, et al. External irradiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys. 1990;19(2):235–242.
- Telfer NR, Colver GB, Morton CA, et al. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159(1):35–48.
- Ahamad A, Weed DT, Pruett DD, et al. Definitive radiotherapy for a head and neck Merkel cell carcinoma and comprehensive nodal volumes: a case for using a computer-designed variable-thickness compensator to reduce risk and severity of mucositis. Med Dosim. 2018;43(1):69–73.
- Eaton DJ. Electronic brachytherapy—current status and future directions. Br J Radiol. 2015;88(1049):20150002.
- Lukens JN, Gamez M, Hu K, et al. Modern brachytherapy. Semin Oncol. 2014;41(6):831–847.
- Newlands C, Currie R, Memon A, et al. Non-melanoma skin cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S125–S32.
- Mazeron JJ, Chassagne D, Crook J, et al. Radiation therapy of carcinomas of the skin of nose and nasal vestibule: a report of 1676 cases by the Groupe Europeen de Curiethérapie. Radiother Oncol. 1988;13(3):165–173.
- Silva JJ, Tsang RW, Panzarella T, et al. Results of radiotherapy for epithelial skin cancer of the pinna: the Princess Margaret Hospital experience, 1982–1993. Int J Radiat Oncol Biol Phys. 2000;47(2):451–459.
- Kandaz M, Bahat Z, Guler OC, et al. Radiotherapy in the management of classic Kaposi’s sarcoma: a single institution experience from Northeast Turkey. Dermatol Ther. 2018;31(4):e12605.
- Ramírez K, Zavala J, Morán D, et al. Classic Kaposi’s sarcoma–complete response to radiation therapy: a case report. J Med Case Rep. 2016;10(1):322.
- Tsao MN, Sinclair E, Assaad D, et al. Radiation therapy for the treatment of skin Kaposi sarcoma. Ann Palliat Med. 2016;5(4):298–302.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89(1):38–43.
- Mendenhall WM, Amdur RJ, Hinerman RW, et al. Radiotherapy for cutaneous squamous and basal cell carcinomas of the head and neck. Laryngoscope. 2009;119(10):1994–1999.
- Barysch MJ, Eggmann N, Beyeler M, et al. Long-term recurrence rate of large and difficult to treat cutaneous squamous cell carcinomas after superficial radiotherapy. Dermatology. 2012;224(1):59–65.
- Fitzpatrick PJ, Thompson GA, Easterbrook WM, et al. Basal and squamous cell carcinoma of the eyelids and their treatment by radiotherapy. Int J Radiat Oncol Biol Phys. 1984;10(4):449–454.
- Tsao MN, Tsang RW, Liu FF, et al. Radiotherapy management for squamous cell carcinoma of the nasal skin: the Princess Margaret Hospital experience. Int J Radiat Oncol Biol Phys. 2002;52(4):973–979.
- Roth WI, Shelling M, Fishman K. Superficial radiation therapy is a highly effective modality for the treatment of basal and squamous cell carcinomas of the lower extremities. Presented at the Fall Clinical Dermatology Conference; October 18–21, 2018; Las Vegas, Nevada.
- Omar B, Eilers Jr RE, Rubin AG, et al. Clinical characteristics of lower extremity surgical site infections in dermatologic surgery based upon 24-month retrospective review. J Drugs Dermatol. 2018;17(7):766–771.
- Dacosta Byfield S, Chen D, Yim YM, et al. Age distribution of patients with advanced non-melanoma skin cancer in the United States. Arch Dermatol Res. 2013;305(9):845–850.
- Linos E, Parvataneni R, Stuart SE, et al. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006–1012.
- Zygogianni A, Kouvaris J, Tolia M, et al. The potential role of radiation therapy in Bowen’s disease: a review of the current literature. Rev Recent Clin Trials. 2012;7(1):42–46.
- Mitsuhashi N, Hayakawa K, Yamakawa M, et al. Cancer in patients aged 90 years or older: radiation therapy. Radiology. 1999;211(3):829–833.
- Dundar Y, Cannon RB, Hunt JP, et al. Radiotherapy regimens in patients with nonmelanoma head and neck skin cancers. Int J Dermatol. 2018;57(4):441–448.
- Clark CM, Furniss M, Mackay-Wiggan JM. Basal cell carcinoma: an evidence-based treatment update. Am J Clin Dermatol. 2014;15(3):197–216.
- Baily B, Coe MA, Hearnden TM. A new technique for radiation shielding in superficial X-ray therapy. Br J Radiol. 1981;54(645):805–807.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after x-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Chan S, Dhadda AS, Swindell R. Single fraction radiotherapy for small superficial carcinoma of the skin. Clin Oncol (R Coll Radiol). 2007;19(4): 256–259.
- Zaorsky NG, Lee CT, Zhang E, et al. Hypofractionated radiation therapy for basal and squamous cell skin cancer: a meta-analysis. Radiother Oncol. 2017;125(1):13–20.
- van Hezewijk M, Creutzberg CL, Putter H, et al. Efficacy of a hypofractionated schedule in electron beam radiotherapy for epithelial skin cancer: analysis of 434 cases. Radiother Oncol. 2010;95(2):245–249.
- Schaue D, Micewicz ED, Ratikan JA, et al. Radiation and inflammation. Semin Radiat Oncol. 2015;25(1):4–10.
- Chan RJ, Webster J, Chung B, et al. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:53.
- Ferreira EB, Vasques CI, Gadia R, et al. Topical interventions to prevent acute radiation dermatitis in head and neck cancer patients: a systematic review. Support Care Cancer. 2017;25(3): 1001–1011.
- Koukourakis GV, Kelekis N, Kouvaris J, et al. Therapeutics interventions with anti-inflammatory creams in post radiation acute skin reactions: a systematic review of most important clinical trials. Recent Pat Inflamm Allergy Drug Discov. 2010;4(2):149–158.
- Mohammadi AA, Mohammadian Panah M, Pakyari MR, et al. Surgical excision followed by low dose rate radiotherapy in the management of resistant keloids. World J Plast Surg. 2013;2(2):81–86.
- Norris JE. Superficial x-ray therapy in keloid management: a retrospective study of 24 cases and literature review. Plast Reconstr Surg. 1995;95(6):1051–1055.
- Ragoowansi R, Cornes PG, Glees JP, et al. Ear-lobe keloids: treatment by a protocol of surgical excision and immediate postoperative adjuvant radiotherapy. Br J Plast Surg. 2001;54(6):504–508.
- Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Dose-effect relationship. Strahlenther Onkol. 2005;181(11):717–723.
- Sclafani AP, Gordon L, Chadha M, et al. Prevention of earlobe keloid recurrence with postoperative corticosteroid injections versus radiation therapy: a randomized, prospective study and review of the literature. Dermatol Surg. 1996;22(6):569–574.
- Cheraghi N, Cognetta A Jr, Goldberg D. Radiation therapy for the adjunctive treatment of surgically excised keloids: a review. J Clin Aesthet Dermatol. 2017;10(8):12–15.
- Berman B, Bieley HC. Adjunct therapies to surgical management of keloids. Dermatol Surg. 1996;22(2):126–130.
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43:S3–S18.
- Speranza G, Sultanem K, Muanza T. Descriptive study of patients receiving excision and radiotherapy for keloids. Int J Radiat Oncol Biol Phys. 2008;71(5):1465–1469.
- Mankowski P, Kanevsky J, Tomlinson J, et al. Optimizing radiotherapy for keloids: a meta-analysis systematic review comparing recurrence rates between different radiation modalities. Ann Plast Surg. 2017;78(4):403–411.
- Kal HB, Veen RE, Jürgenliemk-Schulz IM. Dose-effect relationships for recurrence of keloid and pterygium after surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74(1):245–251.
- Dinh Q, Veness M, Richards S. Role of adjuvant radiotherapy in recurrent earlobe keloids. Australas J Dermatol. 2004;45(3):162–166.
- Gauglitz GG. Management of keloids and hypertrophic scars: current and emerging options. Clin Cosmet Investig Dermatol. 2013;6:103–114.
- Ragoowansi R, Cornes PG, Moss AL, et al. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast Reconstr Surg. 2003;111(6):1853–1859.
- Faculty of Clinical Oncology RCoR. A review of the use of radiotherapy in the UK for the treatment of benign clinical conditions and benign tumors. Keloid scarring. Available at: www.rcr.ac.uk. Accessed July 13, 2018.
- Shen J, Lian X, Sun Y, et al. Hypofractionated electron-beam radiation therapy for keloids: retrospective study of 568 cases with 834 lesions. J Radiat Res. 2015;56(5):811–817.
- McKeown SR, Hatfield P, Prestwich RJ, et al. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br J Radiol. 2015;88(1056):20150405.
- Recalcati S, Caccialanza M, Piccinno R. Postoperative radiotherapy of auricular keloids: a 26-year experience. J Dermatolog Treat. 2011;22(1):38–42.
- Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124(4):1196–1201.

