 J Clin Aesthet Dermatol. 2020;13(7):32–35
J Clin Aesthet Dermatol. 2020;13(7):32–35
by Fathia M. Khattab, MD
Dr. Khattab is with the Faculty of Medicine, Zagazig University in Zagazig, Egypt.
FUNDING: No funding was provided for this study.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Botulinum toxin (BTX) A has different biological activities, including anti-inflammatory and antipruritic behavior. Studies on humans and animals have shown that BTX is efficient in treating itch caused by histamine, lichen simplex chronicus, psoriasis, rosacea, allergic rhinitis, and scar avoidance.
Objective. This study sought to assess the impact of BTX-A in patients with atopic dermatitis using scores of SCORAD and to identify parameters linked to greater improvements.
Methods. This was a prospective, intrapatient, left-to-right, randomized, placebo-controlled study of BTX-A for the treatment of atopic dermatitis. The study included 26 patients with atopic dermatitis (12 males and 14 females) with an average age of 37.8 years. Responses to therapy were assessed using SCORAD, Dermatology Quality of Life Index (DLQI), and the worldwide clinical reaction score evaluation.
Results. Mean SCORAD values dropped from 50.5 to 11 points (p<0.001); meanwhile, 64.1 percent of patients reported an excellent response, including 78.9 percent of patients with severe AD. The DLQI score fell by 10.15 points (43.5%) in patients treated with BTX-A. A statistically significant reduction in SCORAD and DLQI scores occurred relative to in the placebo group (p<0.001).
Conclusion. Based on the results of this study, BTX-A appears to be a safe and effective therapy for atopic dermatitis of all grades (mild, moderate, and severe). However, BTX-A appears to be best suited for patients with severe atopic dermatitis.
Keywords: Atopic dermatitis, BTX-A, Botox, onabotulinum toxin A
Atopic dermatitis (AD) is a chronic inflammatory skin disease followed by serious itching.1 Itch is a feeling that provokes a desire to scratch, where continuous scratching further aggravates the symptoms of skin affected by AD.2 It is known that AD and atopic itch involve a complicated interplay of secreted factors, inflammatory cells, resident skin cells, and neural networks.3
Botulinum toxin is a Clostridium botulinum-generated exotoxin. It disturbs signal transmission at the neuromuscular and neuroglandular intersection by inhibiting the release of acetylcholine from the presynaptic nerve terminal. It has been commonly used in illnesses of the neuromuscular, hypersecretory, and autonomic nerve systems.4
Botulinum toxin A (BTX-A) blocks acetylcholine by cleaving the synaptosome-associated protein-25, which contributes to the creation of the exocytic soluble N-ethyl-maleimide-sensitive protein receptor complex (SNARE), which is vital for the fusion of vesicles containing acetylcholine with the presynaptic membrane.5 Local peripheral BTX-A injection can reduce the number of different drugs that sensitize nociceptors.6 This antinociceptive impact is connected with the inhibition of formalin-induced glutamate release and a possible decrease in peripheral nociceptive input by inhibiting the release of P substance and calcitonin-related peptide, which play an important role in neurogenic inflammation.7 However, in cases of AD, the effect of BTX-A has not yet been determined.
Therefore, this study sought to assess the results of BTX-A in patients with chronic, mild, and moderate-to-severe AD by comparing SCORAD values between before and after treatment and to detect the number of changes in SCORAD after treatment.
Patients and Methods
This was a prospective intrapatient, left-to-right, randomized placebo-controlled study conducted on 26 patients with bilateral AD lesions. AD was diagnosed based on the revised criteria of Hanifin and Rjika.10 Demographic data, including age, sex, and the nation of origin, as well as clinical information, including onset of disease, progress, recurrence, duration of disease, family history of AD, and prior treatments were collected from patients.
Patients who were immunocompromised or received immunosuppressive therapy one month prior to the study or topical therapy three months prior were excluded from this research. Also excluded were pregnant or lactating women, patients with neuromuscular disease, or patients with intake of aminoglycoside-type antibiotics. An ethics committee approved the research protocol and the patients provided written consent for participation.
Study design. This was a randomized, placebo-controlled study with medication kits numbered sequentially. An autonomous nonblinded doctor conducted the dilution of BTX-A and the preparation of syringes. In terms of appearance, placebo syringes were the same as syringes containing the active drug. The medication was given blindly with intradermal injection. In the first group, a total of 100 IU of BTX-A (5 U/route), diluted with 4mL of sodium chloride (0.9%), was injected intradermally throughout the affected region using 30-gauge needles. A total of 40 injections were received by each patient, with a minimum distance of 1cm between the injection points.8 The placebo group received normal saline, which was dispensed the same way. The participants, the doctors who administered therapy, and the evaluators were blinded to which treatment was conducted. A single physician, who was not an assessor, conducted drug administration. For three months, patients who showed a complete response were followed up with. Pictures were taken making using a digital camera (Canon, Tokyo, Japan).
Clinical assessment. The result of efficacy was a shift in the severity of AD. The SCORAD index was used by the same physician at each visit to measure the seriousness of the disease before BTX-A therapy. The severity of the disease was classified as mild (<25 points), moderate (25–50 points) and severe (>50 points).9 Each patient also underwent Dermatology Quality of Life Index (DLQI) assessment.
The global assessment of the clinical reaction evaluation was conducted with a score of 0 to 4 points for SCORAD and DLQI, where zero points indicated no improvement (0%), one point indicated a slight improvement (1%–25%), two points indicated a moderate improvement (26%–50%), three points indicated a marked improvement (51%–75%), and four points indicated an excellent response (76%–100%).10
Statistical analysis. Data were presented as mean ± standard deviation. The statistical analysis of values before and after treatment was performed using the Wilcoxon signed-rank test. Differences in parameters before and after treatment were analyzed using the Mann-Whitney U test, where p<0.05 was considered to be statistically significant.
Results
Twenty-six patients participated in the study with a mean age of 37.8±14.6 years. Other demographic and clinical data are presented in Table 1. There was no statistically significant difference between the different age or sex subgroups with respect to treatment efficacy. The global assessment of the clinical response in SCORAD showed no improvement in four patients, mild improvement in four patients, moderate improvement in three patients, marked improvement in five patients, and excellent response in 10 patients. The mean SCORAD score decreased in the BTX-A therapy group by 39.5 points (76.7%), from 50.5 to 11 points. Most patients achieved a 50-percent improvement, with 64.1 percent of patients showing an excellent response (Table 2).
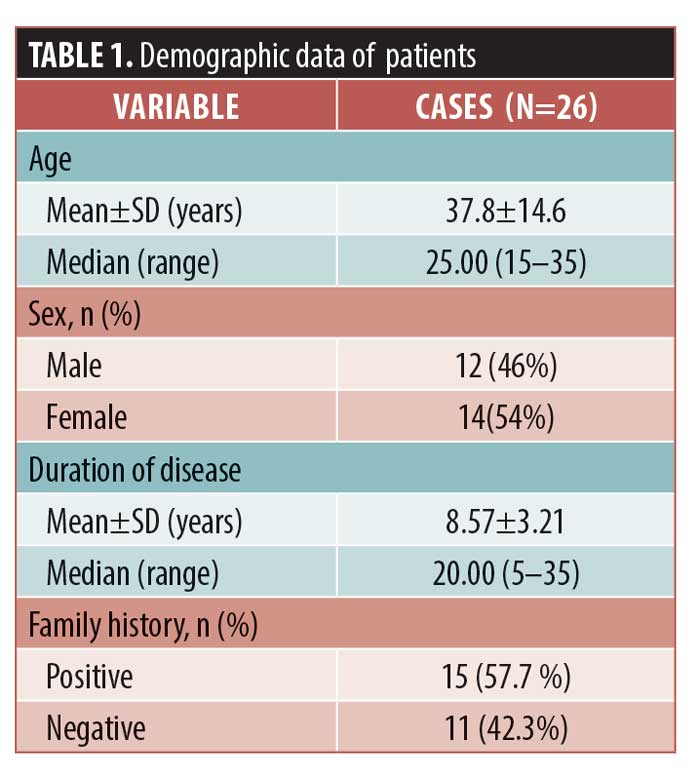
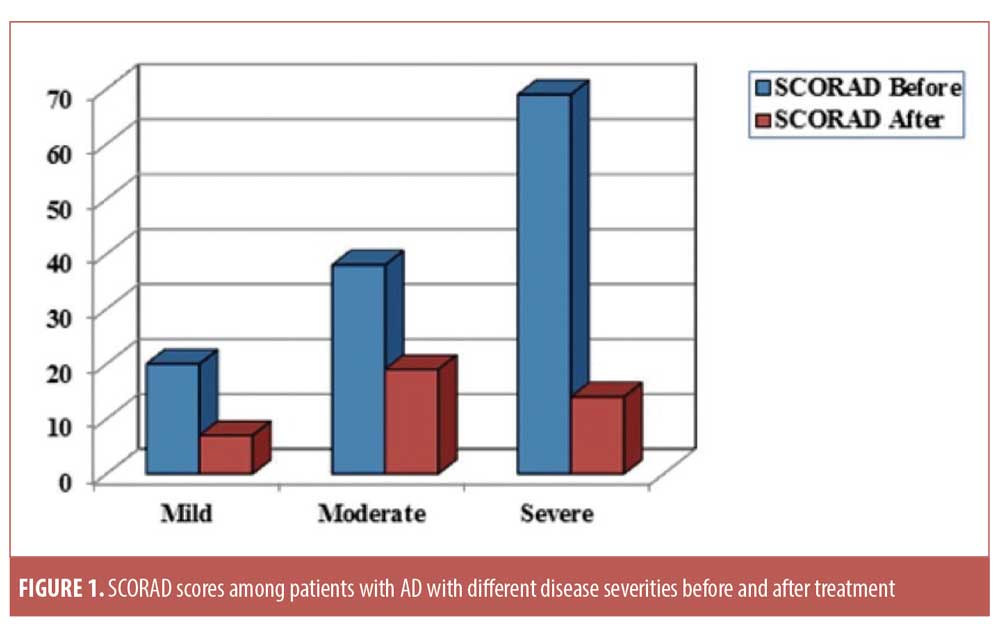
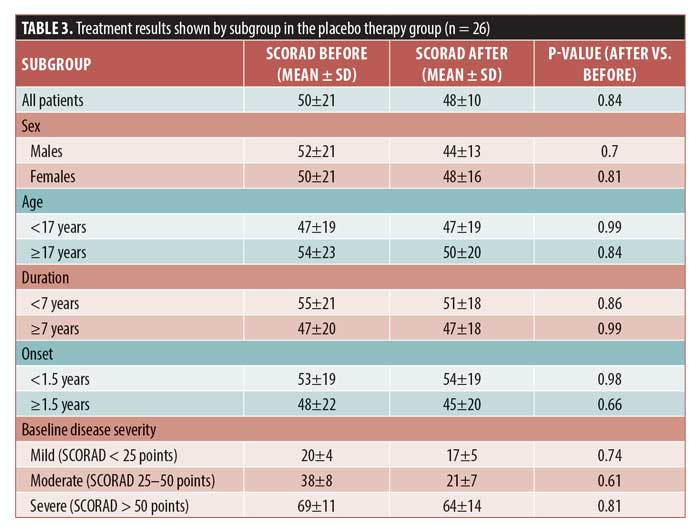
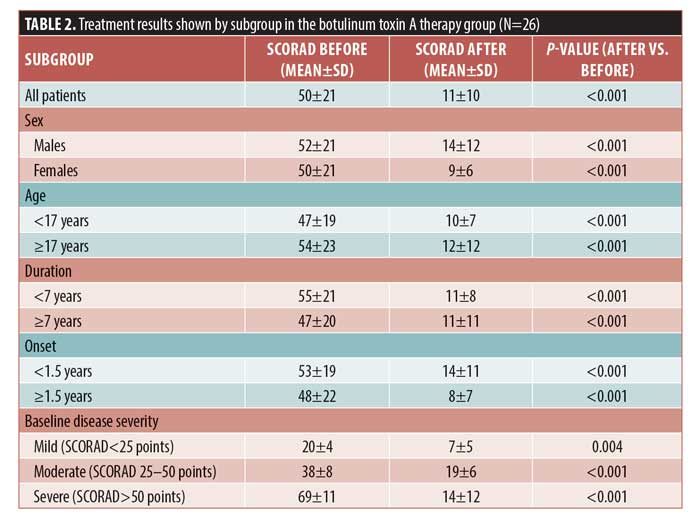 Significant improvements in the SCORAD score were detected in all subgroups after treatment. According to SCORAD scores, four patients (15.3%) were classified as having mild disease, 10 (38.4%) were classified as having moderate disease, and 12 (46.3%) were classified as having severe disease. In the mild, moderate, and severe disease subgroups, the percentages in SCORAD score improvements were 64.1%, 9%, and 80.2%, respectively (p=0.004, p<0.001, and p<0.001, respectively). An excellent response was achieved in 54.5%, 48.3%, and 78.9% of patients with mild, moderate, and severe disease, respectively (Figure 1). In the placebo group, the mean SCORAD score did not change before and after therapy (Table 3).
Significant improvements in the SCORAD score were detected in all subgroups after treatment. According to SCORAD scores, four patients (15.3%) were classified as having mild disease, 10 (38.4%) were classified as having moderate disease, and 12 (46.3%) were classified as having severe disease. In the mild, moderate, and severe disease subgroups, the percentages in SCORAD score improvements were 64.1%, 9%, and 80.2%, respectively (p=0.004, p<0.001, and p<0.001, respectively). An excellent response was achieved in 54.5%, 48.3%, and 78.9% of patients with mild, moderate, and severe disease, respectively (Figure 1). In the placebo group, the mean SCORAD score did not change before and after therapy (Table 3).
 Regarding the DLQI score after treatment, in the BTX-A therapy group, the mean was 13.2±10.2 points and the mean decrease was 10.15±8.44 units. The global assessment of clinical response in DLQI score showed no improvement in five patients, mild improvement in one patient, moderate improvement in four patients, marked improvement in six patients, and excellent response in 10 patients, with a statistically significant difference before and after treatment (p<0.05). However, in the placebo group, the DLQI score showed no difference before and after therapy (p=0.68) and the global assessment of clinical response in DLQI score indicated no improvement in 26 patients. There were no local or systemic serious side effects except for in six patients (29.2%) who reported injection site pain and three patients who experienced injection site swelling in the BTX-A therapy group. Photo documentation is presented in Figures 1 to 3.
Regarding the DLQI score after treatment, in the BTX-A therapy group, the mean was 13.2±10.2 points and the mean decrease was 10.15±8.44 units. The global assessment of clinical response in DLQI score showed no improvement in five patients, mild improvement in one patient, moderate improvement in four patients, marked improvement in six patients, and excellent response in 10 patients, with a statistically significant difference before and after treatment (p<0.05). However, in the placebo group, the DLQI score showed no difference before and after therapy (p=0.68) and the global assessment of clinical response in DLQI score indicated no improvement in 26 patients. There were no local or systemic serious side effects except for in six patients (29.2%) who reported injection site pain and three patients who experienced injection site swelling in the BTX-A therapy group. Photo documentation is presented in Figures 1 to 3.
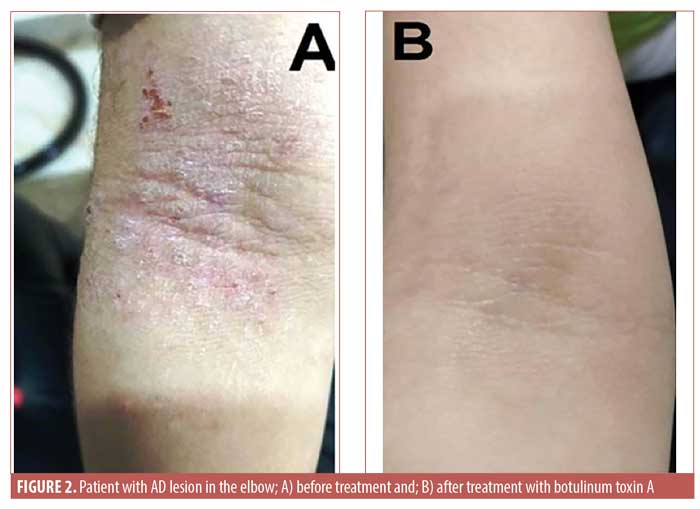
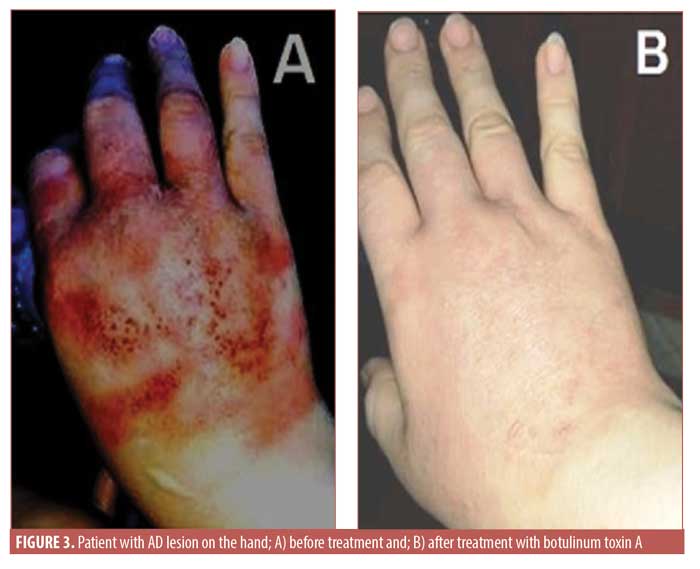
Discussion
To the author’s knowledge, this is the first study to assess the effect of BTX-A in patients with AD. The biology of the neuronal cholinergic scheme in humans has been studied.11 Acetylcholine has recently been found to induce itching in patients with AD and can thus be involved in the development of AD symptoms. Human keratinocytes synthesize nonneuronal acetylcholine, which acts as a local molecule-signaling cell, regulating tasks such as proliferation, cell adhesion, cell contact (barrier function), and glandular activity.12 Wessler et al13 showed a significant rise in acetylcholine content in the skin of patients with AD, potentially contributing to the pathogenesis of AD.
BTX-A consists of a heavy chain with a receptor binding site and a translocation domain as well as a light endopeptidase chain. By interfering with the exocytotic release, BTX-A inhibits the vesicular release of neurotransmitters.14 The anti-itch effect of BTX-A is also the consequence of inhibiting the release of acetylcholine and other itch mediators. Scientists were also inspired by proof of the analgesic characteristics of BTX-A in pain and nociception.15
Research in AD mouse models has suggested that AD is followed by weakening itch and complicated interactions between immune cells and nerve fibers. The NC/Nga mouse is an appropriate animal model to study AD because, under standard circumstances, these animals experience spontaneously AD-like skin lesions.16 Han et al17 examined the protective impact of BTX-A on lesions of the NC/Nga mouse (via intradermal injection on the rostral back). The primary outcome was skin thickness and transepidermal water loss. The authors also evaluated skin thickness, waterlessness, skin severity scores, skin histological changes (e.g., mast cell count, interleukin-4 messenger RNA and protein expression), and complete IgE serum levels. This research showed that BTX-A could considerably reduce the seriousness of AD, the level of interleukin-4 expression, and the number of infiltrated mast cells.
Overall, these studies indicate the ability for BTX-A to alleviate symptoms in patients with localized chronic itch caused by brachioradial pruritus, lichen simplex, partial- to full-thickness burns, inverse psoriasis, and Hailey-Hailey disease that are refractory to conventional therapy.18
Botulinum inhibits the nerved release of CGRP, P, and VIP substances; decreases the infiltration of eosinophils, mast cell activity, and vessel dilation; and relieves itch and neurogenic inflammation.19 Such an impact of BTX-A on itch, inflammation, and fibrosis is particularly important and has given mechanistic insight into the published assessment of remission of itch in patients with allergic rhinitis burning and alleviation20,21 and lichen simplex chronicus.22
Conclusion
BTX-A is a safe and effective therapy for atopic dermatitis of all grades (mild, moderate, and severe). However, BTX-A is ideal for patients with severe atopic dermatitis.
References
- Leung DY, Jain N, Leo HL. New concepts in the pathogenesis of atopic dermatitis. Curr Opin Immunol. 2003;15(6):634–638.
- Eichenfield F, Ellis CN, Mancini AJ, et al. Atopic dermatitis: epidemiology and pathogenesis update. Simin Cutan Med Surg. 2012;31(3 Suppl):S3–S5.
- Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol. 2016;51(3):263–292.
- Borodic GE, Acquadro M, Johnson EA. Botulinum toxin therapy for pain and inflammatory disorders: mechanisms and therapeutic effects. Expert Opin Investig Drugs. 2001;10(8):1531–1544.
- Gazerani P, Pedersen NS, Drewes AM, Arendt–Nielsen L. Botulinum toxin type A reduces histamine-induced itch and vasomotor responses in human skin. Br J Dermatol. 2009;161(4):737–745.
- Ward NL, Kavlick KD, Diaconu D, et al. Botulinum neurotoxin A decreases infiltrating cutaneous lymphocytes and improves acanthosis in the KC-Tie2 mouse model. J Invest Dermatol. 2012;132(7): 1927–1930.
- Wen WD, Yuan F, Wang JL, Hou YP. Botulinum toxin therapy in the ovalbumin-sensitized rat. Neuroimmunomodulation. 2007;14(2):78–83.
- Heckmann M, Heyer G, Brunner B, Plewig G. Botulinum toxin type A injection in the treatment of lichen simplex: an open pilot study. J Am Acad Dermatol. 2002;46(4):617–619.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2018;121(4):464–471.
- Celakovská J, Bukac J. The severity of atopic dermatitis evaluated with the SCORAD index and the occurrence of bronchial asthma and rhinitis, and the duration of atopic dermatitis. Allergy Rhinol (Providence). 2016;7(1):8–13.
- Grando SA. Biological functions of keratinocyte cholinergic receptors. J Investig Dermatol Symp Proc. 1997;2(1):41–48.
- Heyer G, Vogelgsang M, Hornstein OP. Acetylcholine is an inducer of itching in patients with atopic eczema. J Dermatol. 1997;24(10):621–625.
- Wessler I, Reinheimer T, Kilbinger H, et al. Increased acetylcholine levels in skin biopsies of patients with atopic dermatitis. Life Sci. 2003;72(18–19): 2169–2172.
- Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43(2 Pt 1):249–259.
- Carmichael NM, Dostrovsky JO, Charlton MP. Peptidemediated transdermal delivery of botulinum neurotoxin type A reduces neurogenic inflammation in the skin. Pain. 2010;149(2):316–324.
- Martel BC, Lovato P, Baumer W, Olivry T. Translational animal models of atopic dermatitis for preclinicalstudies. Yale J Biol Med. 2017;90(3): 389–402.
- Han SB, Kim H, Cho SH, Chung JH, Kim HS. Protective effect of botulinum toxin type a againstatopic dermatitis-like skin lesions in NC/Nga mice. Dermatol Surg. 2017;43:S312–S321.
- Boozalis E, Sheu M, Selph J, Kwatra SG. Botulinum toxin type A for the treatment of localized recalcitrant chronic pruritus. J Am Acad Dermatol. 2018;78(1): 192–194.
- Park TH. The effects of botulinum toxin a on mast cell activity: preliminary results. Burns. 2013;39(4): 816–817.
- Ozcan C, Ismi O. Botulinum toxin for rhinitis. Curr Allergy Asthma Rep. 2016;16(8):58.
- Braun T, Gurkov R, Kramer MF, Krause E. Septal injection of botulinum neurotoxin a for idiopathicrhinitis: a pilot study. Am J Otolaryngol. 2012;33(1):64–67.
- Heckmann M, Heyer G, Brunner B, Plewig G. Botulinum toxin type A injection in the treatment of lichensimplex: an open pilot study. J Am Acad Dermatol. 2002;46(4):617–619.

