 J Clin Aesthet Dermatol. 2023;16(1):47-50.
J Clin Aesthet Dermatol. 2023;16(1):47-50.
by Shane M. Swink, DO, MS; Megan A. Jones-Sheets, DO; Ankita Sinharoy, MBBS, MPH; Melissa Butt, DrPH, MPH; Anthony Gust, MD, MPH; and Andrea L. Zaenglein, MD
Dr. Swink is with the Division of Pediatrics, Section of Dermatology at The Children’s Hospital of Philadelphia in Philadelphia, Pennsylvania. Dr. Jones-Sheets is with Oakview Dermatology in Gahanna, Ohio. Ms. Sinharoy is with the Department of Public Health Sciences at Penn State College of Medicine in Hershey, Pennsylvania. Drs. Butt and Zaenglein are with the Department of Dermatology at Penn State and Hershey Medical Center in Hershey, Pennsylvania. Dr. Zaenglein is additionally with the Department of Pediatrics at Penn State Children’s Hospital in Hershey, Pennsylvania. Dr. Gust is with Advanced Dermatology Associates, Ltd., and the Division of Dermatology at Lehigh Valley Health Network, both in Allentown, Pennsylvania.
ABSTRACT: Objective. Combined oral contraceptive pills (COCs) are safe and effective therapies for females with acne vulgaris. Data is lacking regarding dermatology residents’ COCs use. We aimed to evaluate dermatology residents’ knowledge, comfort level, and prescribing practices of COCs in the management of acne vulgaris.
Methods. A cross-sectional survey study was emailed to current dermatology residents in approved training programs and descriptive statistics were performed.
Results. Most residents reported that COCs are an effective treatment for acne (160/170, 94.1%) but, less felt adequately trained on efficacy (105/170, 61.8%) and safety (72/170, 42.4%). 30 percent (51/170) of residents’ attending physicians regularly prescribed COCs for acne. Half were comfortable counseling patients on adverse effects of COCs (86/170, 50.6%) while fewer were comfortable counseling on how to properly take COCs (66/170, 38.8%). 60 percent (102/170) felt comfortable prescribing COCs to healthy adolescents while 66.5 percent (113/170) were comfortable prescribing to adults.
Limitations. include a small sample size, response bias, and inability to calculate an accurate response rate.
Conclusion. This data suggests most residents recognize COCs are an effective treatment for acne vulgaris, but less feel adequately trained. Several knowledge gaps and potential educational interventions regarding COCs, including safety, efficacy, adverse effects, and contraindications, are highlighted.
Keywords: Inflammatory conditions, acne, general dermatology, education
Combined oral contraceptives (COCs) are commonly used in the treatment of acne vulgaris and current management guidelines from the American Academy of Dermatology, European Dermatology Forum, and French Acne Guidelines all support their use in appropriate patients.1–8
Four oral contraceptive combinations are currently FDA approved for the treatment of moderate acne vulgaris in females aged 15 years or older, who have no known contraindications to oral contraceptive therapy, desire contraception, have achieved menarche and are unresponsive to topical anti-acne medications.1 While the safety and efficacy of COCs is established in the management of acne, many dermatologists are not comfortable prescribing COCs to their own patients. A survey of 116 board-certified dermatologists in the United States (U.S.) assessed their knowledge, comfort, and prescribing practices of COCs for acne treatment and showed that 95.4 percent believed that COCs were effective in treatment of acne vulgaris, but only 54 percent actually prescribed COCs.9 Reasons for not prescribing COCs were not evaluated. Another survey study found that dermatologists prescribe COCs in 2 percent of visits with patients seeking acne vulgaris treatment.10 There is no data in the literature assessing U.S. dermatology residents’ use of COCs. This cross-sectional survey study of dermatology residents aimed to identify prescribing practices, knowledge, and comfort level utilizing COCs for treatment of acne vulgaris.
Methods
A 5-option Likert scale survey evaluating demographic information, knowledge related to the use of COCs, and comfort in various clinical scenarios was sent to U.S. dermatology residents through the Association of Professors of Dermatology listserv. Strongly agree and agree were considered affirmative responses while neutral, disagree, and strongly disagree were considered negative responses for analyses. The survey responses were analyzed with descriptive statistics.
Results
A total of 170 residents completed all three sections. Incomplete (39) and duplicate surveys (16) were excluded. Demographics are reported in Table 1. 94.1 percent (160/170) of residents recognized that COCs are an effective treatment for acne.
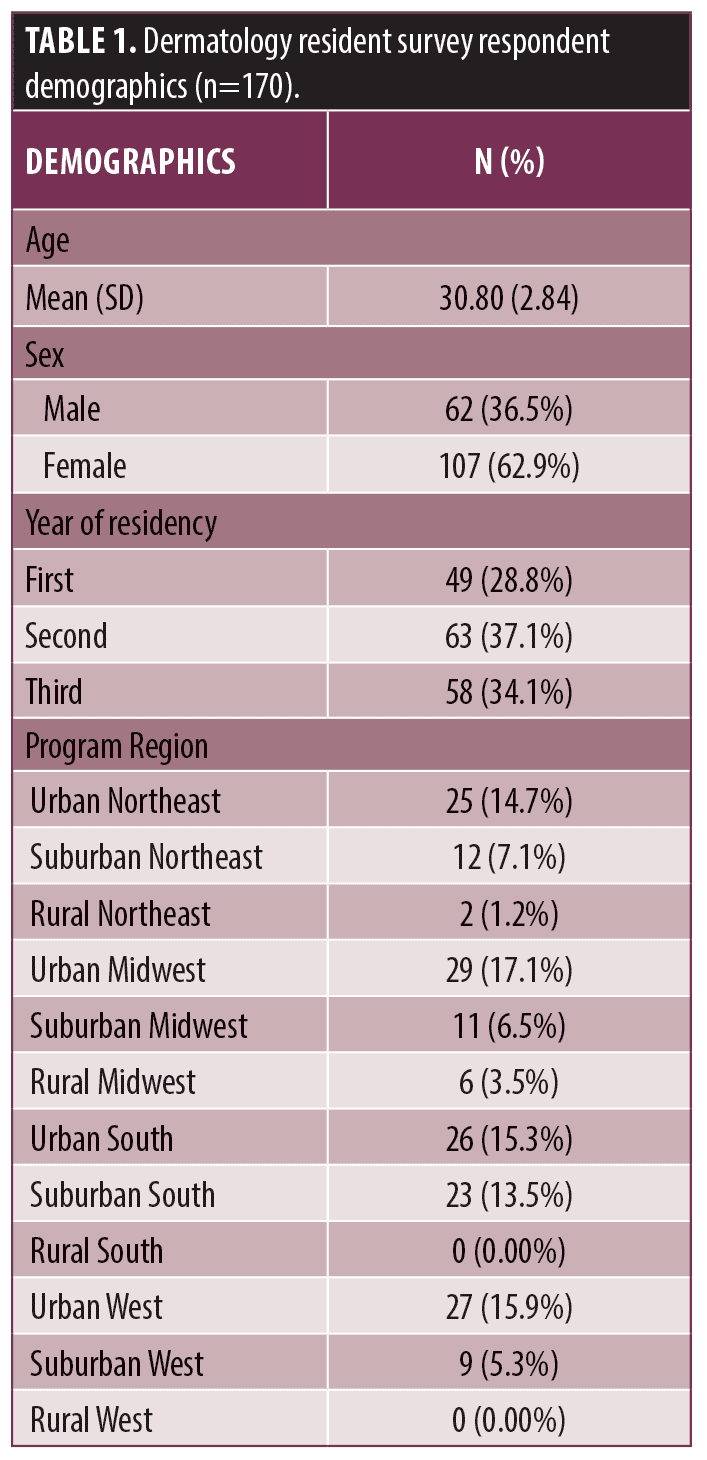
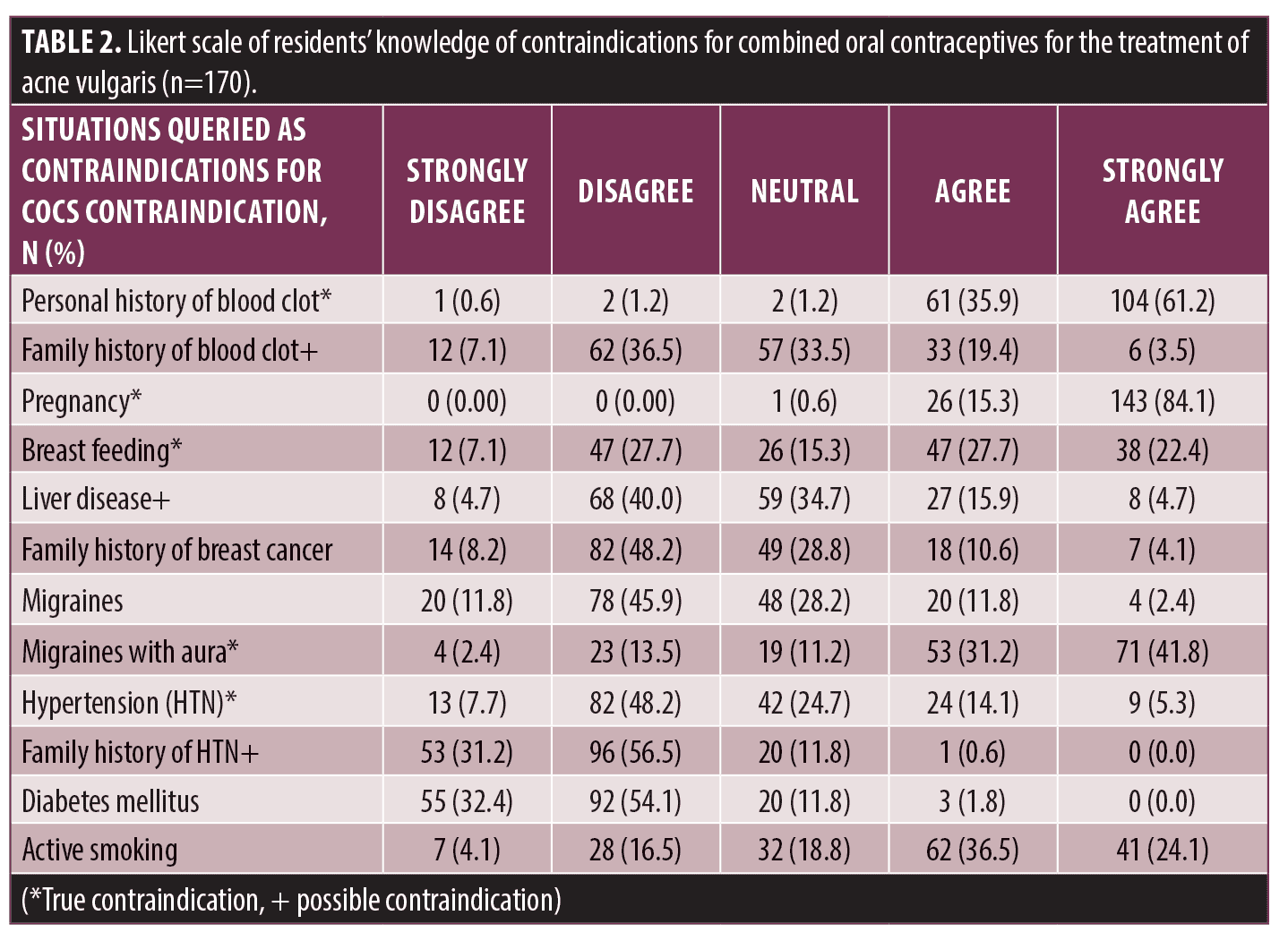
Residents’ knowledge of contraindications for COCs was queried (Table 2), with personal history of blood clots (165/170, 97.1%), pregnancy (169/170, 99.4%), breast feeding (85/170, 50.0%), and migraines with aura (124/170, 72.9%) identified appropriately. Of scenarios in which there was a potential contraindication, 77.1 percent (131/170) of residents did not identify a family history of blood clots, 79.4 percent (135/170) did not identify liver disease, and 99.4 percent (169/170) did not identify family history of hypertension as contraindications. 80.6 percent (137/170) of residents did not identify hypertension as a contraindication. 60.6 percent (103/170) of residents identified active smoking as a contraindication. The remaining queried scenarios were not true contraindications and were correctly identified by residents as such including family history of breast cancer (145/170, 85.3%), migraines (146/170, 85.9%), and diabetes mellitus (167/170, 98.2%).
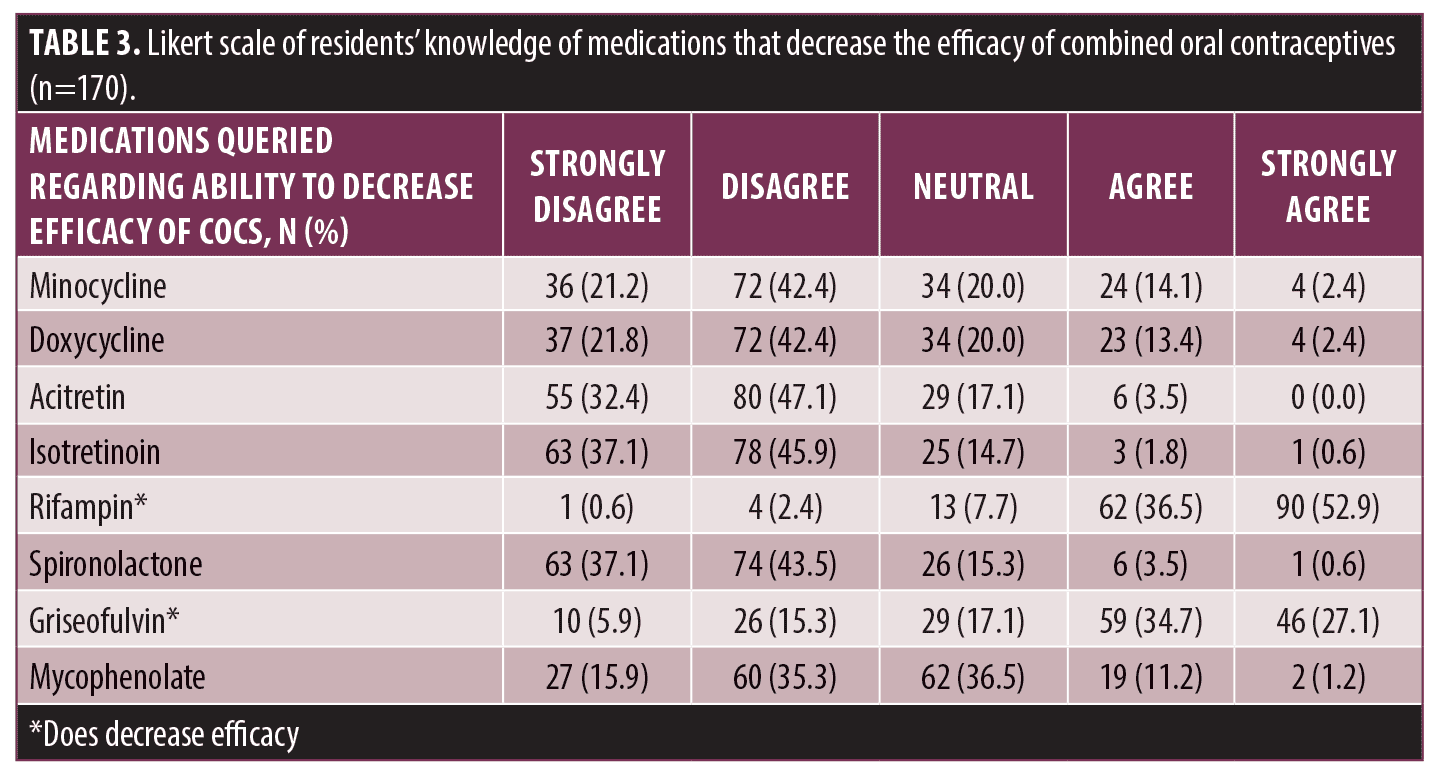
Familiarity with medications that decrease COC efficacy were also assessed (Table 3). 89.4 percent (152/170) of residents correctly identified rifampin and 61.8 percent (105/170) noted griseofulvin as medications that decrease COC efficacy. Additionally, medications that do not decrease efficacy of COCs were appropriately identified including minocycline (142/170, 83.5%), doxycycline (143/170, 84.1%), acitretin (164/170, 96.5%), isotretinoin (166/170, 97.6%), spironolactone (163/170, 95.9%), and mycophenolate (149/170, 87.6%).
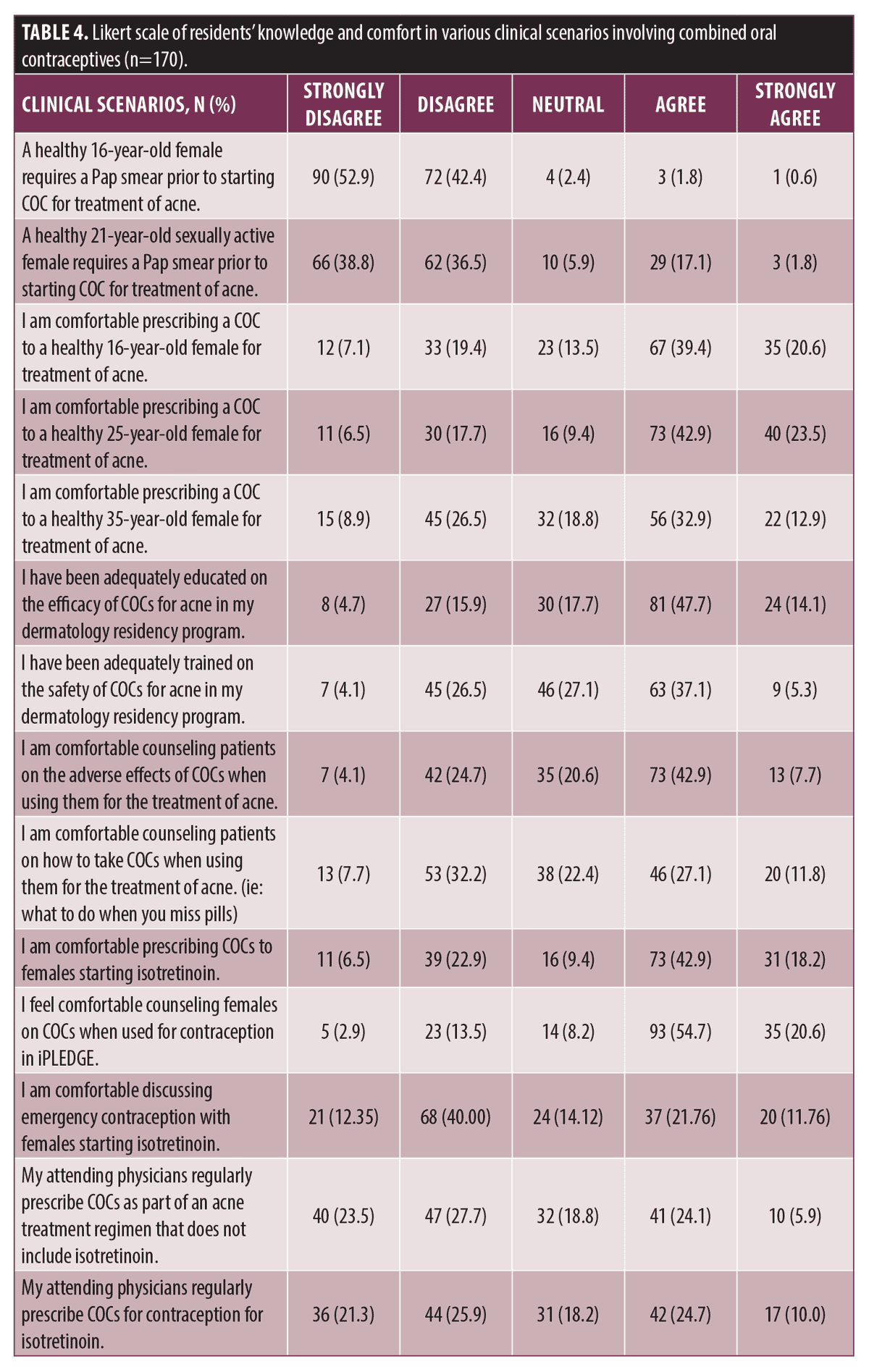
Finally, residents were questioned on their comfort in various clinical scenarios (Table 4). 61.8 percent (105/170) felt adequately trained on the efficacy of COCs while 42.4 percent (72/170) felt adequately trained on the safety of COCs. Approximately half of respondents were comfortable counseling patients on the adverse effects of COCs (86/170, 50.6%) while fewer were comfortable counseling on how to properly take COCs, i.e. what to do if a dose is missed (66/170, 38.8%). 60.0 percent (102/170) of residents were comfortable prescribing COCs to a healthy 16-year-old female to treat acne and 66.5 percent (113/170) were comfortable prescribing to a healthy 25-year-old female. 45.9 percent (78/170) were comfortable prescribing COCs to treat acne in a healthy 35-year-old female. 97.6 percent (166/170) of residents correctly indicated that a Pap smear is not required prior to starting COCs in a 16-year-old female. Similarly, 81.2 percent (138/170) of residents correctly indicated that a Pap smear is not required prior to starting COCs in a 21-year-old female.
30.0 percent (51/170) of residents reported that their attending physicians regularly prescribe COCs for acne while 34.7 percent (59/170) prescribe COCs for isotretinoin contraception. 61.2 percent (104/170) of residents were comfortable prescribing COCs when it is being used as contraception for patients taking isotretinoin. 75.3 percent (128/170) of residents were comfortable counseling patients about COCs when discussing contraception for iPLEDGE while 33.5 percent (57/170) of residents were comfortable counseling patients about emergency contraception when starting isotretinoin.
Discussion
Our survey data reveals that a majority of U.S. dermatology residents (94.1%) acknowledged that COCs are a safe and effective treatment for acne vulgaris, consistent with prior studies of U.S. board certified dermatologists (95.4%).9 A majority of residents were also able to identify drug interactions with COCs, specifically with rifampin (89.4%) and griseofulvin (61.8%). A smaller proportion of respondents to our survey felt well trained on the safety (42.4%), efficacy (61.8%), and counseling directions (38.8%) for COCs. While most respondents acknowledged the efficacy of COCs to treat acne, fewer were comfortable prescribing them in various appropriate clinical scenarios, ranging from 60 to 66.5 percent. This data suggests a slightly higher proportion of residents were comfortable prescribing COCs than prior studies of U.S. board certified dermatologists (54%).9 While prior studies have not assessed reasons for not prescribing COCs to treat acne, our study identifies several potential explanations for a lack of prescribing, including knowledge gaps regarding safety, adverse effects, efficacy, and contraindications of COCs. Resident knowledge and prescribing frequency appears to mirror the frequency of their attending physician use of COCs for the treatment of acne.
There is room to increase residents’ comfort levels in prescribing COCs by improving these knowledge gaps. A largely identified educational target from our study includes emphasizing known contraindications for COCs. While most residents identified personal history of blood clots and migraine with aura appropriately as contraindications, a majority (80.6%) failed to recognize that hypertension is a contraindication for using COCs. Clarifying clinical situations where contraindications may be appropriate for COCs could also increase resident comfort level with their prescribing. These situations include liver disease, family history of blood clots, and family history of hypertension. While liver tumors are a known contraindication to COCs, reflectively, our use of “liver disease” lacked specificity while inquiring about contraindications, identifying a potential limitation in our study. It is important to clarify hepatic contraindications clearly to dermatology residents. Additionally, family history of blood clots is a critical point of emphasis to make with residents as there are genetic disorders that increase blood clot risk, such as antithrombin III deficiency, protein C and S deficiencies, factor V Leiden, or prothrombin gene mutations.11 However, other reasons to develop blood clots are more circumstantial, including being sedentary after surgery, and therefore a family history is less concerning.
Knowing relative risk of thromboembolism is also important. In our study, more than half of residents (60.6%) indicated that active smoking is a contraindication for COCs. However, smoking is only a contraindication in patients over 35 years of age. A significant point of educational emphasis is caution with prescribing COCs to females older than 35 years. Our data shows that 45.9 percent of residents were comfortable prescribing COCs to healthy 35-year-old, despite this population having an increased risk of venous thromboembolism when taking COCs.13 Emphasizing this increased risk with COCs in patients over 35 years is critical.
Limitations of our study include a small sample size, risk of response bias, inability to calculate an accurate response rate, and failure of inquiry regarding comfort of using COCs to treat acne in various gender identities. Respondents were well representative of geographic location and level of training, and the data represents a larger population size compared to prior studies of U.S. board certified dermatologists prescribing practices of COCs. We elucidated several factors that may lead to decreased comfort when prescribing COCs to treat acne including safety, adverse effects, efficacy, and contraindications. Dermatology training programs should include education on use of COCs, an FDA approved treatment for acne, in their therapeutic curriculum. Ultimately, by increasing residents’ knowledge and comfort level with COCs, patients will have improved safety and increased access to this standard acne treatment.
References
- Harper JC. Use of oral contraceptives for management of acne vulgaris practical considerations in real world practice. Dermatol Clin. 2016;34:159–165.
- Koo EB, Peterson TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450–459.
- Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452–457.
- Heymann WR. Oral contraceptives for the treatment of acne vulgaris. J Amer Acad Dermatol. 2007;56(6):1056–1057.
- James WD. Acne. N Engl J Med. 2005;352:1463.72.
- Lynde CW. Hormonal approach to the treatment of acne: a Canadian perspective. J Cutan Med Surg. 2004;8(Suppl 4):1–2.
- Arowojolu AO, Gallo MF, Grimes DA, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2004;(3):CD004425.
- Zaenglein AL. Acne vulgaris. N Engl J Med. 2018;379(14): 1343–1352.
- Fitzpatrick L. Mauer E. Chen CL. US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195–201.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012 Aug;23(4):272–277.
- Ashorobi D, Ameer MA, Fernandez R. Thrombosis. [Updated 2021 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2021 Jan.
- Levanovich PE, Diaczok A, Rossi NF. Clinical and molecular perspectives of monogenic hypertension. Curr Hypertens Rev. 2020;16(2):91–107.
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215(3):330.e1-330.e3307.

