 J Clin Aesthet Dermatol. 2021;14(5):14–21.
J Clin Aesthet Dermatol. 2021;14(5):14–21.
by Thu Q. Nguyen, PhD; Alisar S. Zahr, PhD; Tatiana Kononov, BS, MBA; and Glynis Ablon, MD, FAAD
Dr. Nguyen is with Galderma in Fort Worth, Texas. Dr. Zahr and Ms. Kononov are with Revision Skincare in Irving, Texas. Dr. Ablon is with Ablon Skin Institute & Research Center in Manhattan Beach, California.
FUNDING: The clinical trial described in this manuscript was funded by Revision Skincare.
DISCLOSURES: Drs. Nguyen and Zahr and Ms. Kononov are current or past employees of Revision Skincare. Dr. Ablon was the principle investigator for the clinical trial described in this manuscript and reports no conflicts of interest relevant to the content of this article.
Clinical Trial ID: NCT0454597
ABSTRACT: Background: Mimetic wrinkles, commonly referred to as expression lines, form perpendicular to anatomical regions subjected to repeated facial muscle contraction. Neuromodulating peptides have biological activity and can offer a solution to those concerned with expression lines and facial aging.
Objective: The objective of this randomized, double-blind, placebo-controlled study was to assess the efficacy and tolerability of a line-targeting peptide serum (LTPS) as a stand-alone treatment in improving expression lines and skin health.
Methods: This was an institutional review board-approved study involving healthy subjects. Fifty-five female subjects, 35 to 60 years old, Fitzpatrick Skin Type I to VI, with mild to moderate global face fine lines and wrinkles were recruited. Subjects were randomized to apply LTPS or a placebo serum to their face twice daily for twelve weeks. Short-term efficacy was assessed after fifteen minutes of serum application at baseline. Long-term efficacy and tolerability, self-assessment questionnaire, and VISIA® clinical photography were performed at baseline, Weeks 4, 8, and 12. 3D PRIMOS CR imaging and wrinkle analysis were obtained at baseline and Weeks 8 and 12.
Results: The LTPS significantly improved expression lines at fifteen minutes (short term), Weeks 4, 8, and 12 (long term) when compared to the placebo serum as evaluated by a board-certified dermatologist. The LTPS significantly outperformed the placebo serum in improving skin parameters at all time points. VISIA and PRIMOS CR wrinkle analysis substantiated the LTPS’s efficacy. LTPS was well-perceived and well tolerated by the subjects.
Conclusion: This IRB-approved clinical study demonstrated that LTPS was effective in improving expression lines, wrinkles, and skin health after twelve weeks of application.
Keywords: Expression lines, randomized study, double-blind, placebo-controlled, peptide serum, neuromodulating peptides, prebiotic
Mimetic wrinkles, commonly referred to as expression lines, form perpendicular to anatomical regions subjected to repeated facial muscle contraction.1,2 Repeated movement during activities such as smiling, frowning, and squinting, creates deep dermal creases beneath the skin surface.1–3 Furthermore, the pattern of expression lines predicts the pattern of future persistent wrinkles.4 The anatomical region of expression line formation occurs in the forehead, glabella, crow’s feet, lip, nasolabial folds, and marionette area. Additionally, fine lines and wrinkle formation are further augmented by intrinsic (i.e., natural) and extrinsic (i.e., environmental) factors.3
Peptides, classified as neuromodulating and reparative, have biological activity and can offer a solution to those concerned with expression lines and facial aging.5–8 Neuromodulating peptides have been shown to interfere with pre- and postsynaptic pathways underlying muscle contraction. Specifically, these peptides act as competitive inhibitors with SNAP-25 in the SNARE complex assembly, which obstructs the release of a neurotransmitter, acetylcholine, into the synapse and inhibits muscle contraction.9–12 Reparative peptides specifically target the regeneration of aged extracellular matrix proteins.5 Additionally, peptides modified with long-chain fatty acid moieties, such as palmitoyl or acetyl, are rendered lipophilic and more readily accepted by the lipophilic stratum corneum, thus improving bioavailability.7
Our currently marketed peptide-based treatment serum (Revox™ Line Relaxer; Revision Skincare®, Dallas, Texas) containing neuromodulating and reparative bioavailable peptides, was clinically shown to have statistical significance in reducing the appearance of facial lines, eye lines, and eye wrinkles at maximum smile and at facial rest relative to baseline.13 This serum was subsequently enhanced with additional neuromodulating and reparative bioavailable peptides, botanical extracts, antioxidants, hyaluronic acid, and a prebiotic. This enhanced peptide-based serum combines five neuromodulating peptides, which target pre- and postsynaptic pathways at the neuromuscular junction to induce muscle relaxation and attenuate facial expression, thus minimizing the formation of expression lines. Specifically, these peptides compete with SNAP-25 and Munc-18 in binding to proteins associated in the SNARE complex, destabilizing the complex and ultimately preventing the release of acetylcholine into the neuromuscular junction.9–12 The combination of three reparative peptides re-establishes the connection between the intracellular nucleus and the extracellular matrix to restore the tissue homeostasis, rebuild the protein network in the dermis, and regulate proinflammatory interleukins.14,15 GABA provides immediate relaxation to the look of expression lines and is important in maintaining the skin barrier in the long term.16,17 The botanical extracts, Apium graveolens callus extract and Anigozanthos flavidus extract, which contain polyphenols and flavonoids, provide antioxidant benefits while inducing collagen production, improving skin elasticity, and inhibiting matrix metalloproteinases.18–20 Inulin, a prebiotic ingredient derived from chicory root and agave pinas, assists in rebalancing the skin microbiome and provides long-term hydration in synergy with hyaluronic acid.21
It was hypothesized that this enhanced peptide serum would improve expression lines as well as overall skin health with statistical significance when compared with a placebo serum. The goal of this randomized, double-blind, placebo-controlled clinical study was to assess the efficacy and tolerability of the enhanced peptide serum as a stand-alone treatment in patients with mild-to-moderate facial expression lines and wrinkles.
Methods
Study ethics. The conduct of this study followed all applicable guidelines for the protection of human subjects for research as outlined in the United States FDA 21 CFR Part 50, in accordance with the accepted standards for Good Clinical Practices and the standard practices of Ablon Skin Institute and Research Center. The protocol, informed consent form, and all addenda of this study were reviewed and approved by institutional review board.
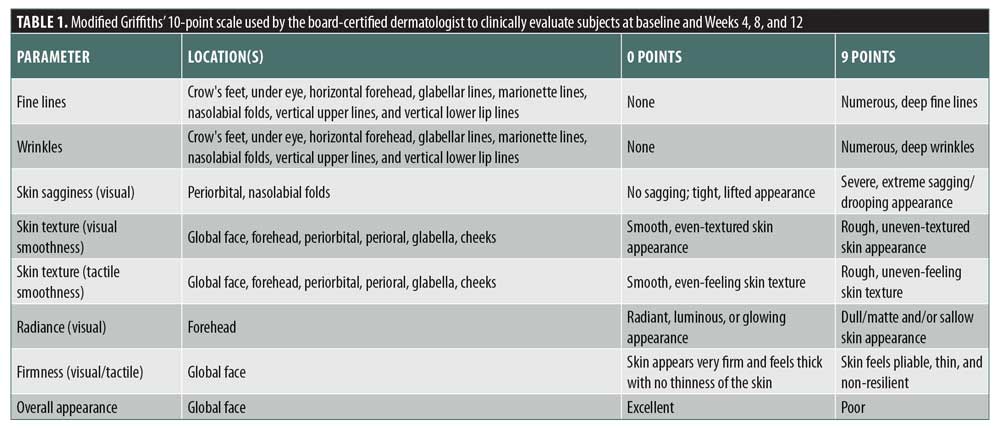
Study subjects. Fifty-six female subjects were recruited for the clinical study. All subjects gave written informed consent and signed a photography release form before entering the study. Eligible subjects were enrolled in the study if they were in good health, aged 35 to 60 years, with Fitzpatrick Skin Types I to VI. Subjects were included in the study having mild-to-moderate global face wrinkles and mild-to-moderate global fine lines based on a modified Griffiths’ 10-point scale.22 Subjects were excluded from the study if they were pregnant, nursing, or planning a pregnancy; had undergone any cosmetic facial procedures administered by a physician or skin care professional within the past six months; or had demonstrated hypersensitivity reaction to skincare products.
Study design. This was a randomized, double-blind, placebo-controlled clinical study in which participants were randomly assigned to the line-targeting peptide serum (LTPS) arm or the placebo serum arm. To ensure continuous participants and quality of the study, participants were instructed to follow a standard skincare regimen that included Gentle Cleansing Lotion (Revision Skincare®, Irving, Texas) to be used morning and evening, followed by LTPS or placebo serum to be applied all over the face in the morning and evening, Neutrogena® Sheer Zinc Dry-touch Broad Spectrum SPF 30 (Johnson & Johnson Consumer Inc., New Brunswick, New Jersey) to be applied in the morning before sun exposure, and a bland facial moisturizer to be applied in the evening. The study was conducted over a 12-week period and consisted of a total of four visits at baseline and Weeks 4, 8, and 12.
Clinical evaluation. Efficacy evaluations for all subjects were performed by a blinded board-certified dermatologist (clinical grader) to assess the short- and long-term efficacy of LTPS and the placebo serum at baseline and Weeks 4, 8, and 12 using a modified Griffiths’ 10-point scale (0 points = none, 1–3 points = mild, 4–6 points = moderate, 7–9 points = severe) to describe the skin condition. For short-term efficacy, at baseline, the clinical grader evaluated wrinkles and fine lines of the global face, focusing on the forehead, crow’s feet, nasolabial folds, and marionette lines of the face after application of LTPS and the placebo serum approximately 15 minutes postapplication. For long-term efficacy, evaluations at Weeks 4, 8, and 12 in comparison with baseline were conducted for the following parameters: fine lines, wrinkles, skin sagginess (visual), skin texture (visual smoothness), skin texture (tactile smoothness), radiance (visual), firmness (visual/tactile), and overall appearance (Table 1).
Clinical photography. VISIA® facial imaging (Canfield Imaging Systems, Fairfield, New Jersey) was performed at baseline and Weeks 4, 8, and 12, with a total of three full-face digital images taken of each study subject’s face (left, right, and center views) under standard light. 3D PRIMOSCR imaging (Canfield Scientific, Parsippany, New Jersey) was performed at baseline and Weeks 8 and 12, with clinical images taken of the bilateral periorbital region of the face of both the left and right sides under standard light and a robust high-pass filter. The 3D PRIMOSCR digital fringe projection technology was used to determine overall skin surface roughness and conduct wrinkle analysis of the periorbital region.
Self-assessment questionnaire. Subjects completed a self-assessment questionnaire to evaluate their facial features, skin appearance, and product satisfaction at baseline and Weeks 4, 8, and 12. In addition, subjects were asked to answer a self-assessment questionnaire on the test products’ aesthetic attributes and to record their testimonials on the test products at Weeks 4, 8, and 12. A five-point scale (1=completely disagree, 2=slightly disagree, 3=neither agree or disagree, 4=slightly agree, 5=completely disagree) was used to assess each question accordingly.
Tolerability evaluation. Objective tolerability evaluation was performed by the clinical grader at baseline and Weeks 4, 8, and 12 to assess the signs of erythema, edema, and dryness. Subjective tolerability assessment was performed by the subjects at baseline and Weeks 4, 8, and 12 to assess the degree of burning, itching, and stinging on their face. A four-point scale (0=none, 1=mild, 2=moderate, 3=severe) was used to assess each parameter accordingly.
Statistical analysis. Statistical analysis for the clinical grading of efficacy and tolerability (subjective and objective) parameters, self-assessment questionnaires, and bio-instrumental measurements was performed using the Wilcoxon signed-rank test at each visit relative to baseline and between the LTPS group and the placebo serum group at each time point. To calculate statistical significance for the percentage of subject improvement, a number was assigned to each of the three changes (1=improved, -1=worsened, and 0=no change). The data were tabulated and compared between the LTPS and placebo serum groups using Wilcoxon–Mann–Whitney test. Data at all time points for both the active and placebo groups were not normally distributed by the Shapiro–Wilk test, a standard test for normality. Therefore, the calculation of average percent change was replaced with the calculation of median percent change for each clinical parameter and time point. Statistical significance was achieved with *p<0.05 and **p<0.01, and high statistical significance was achieved with ***p<0.001.
Results
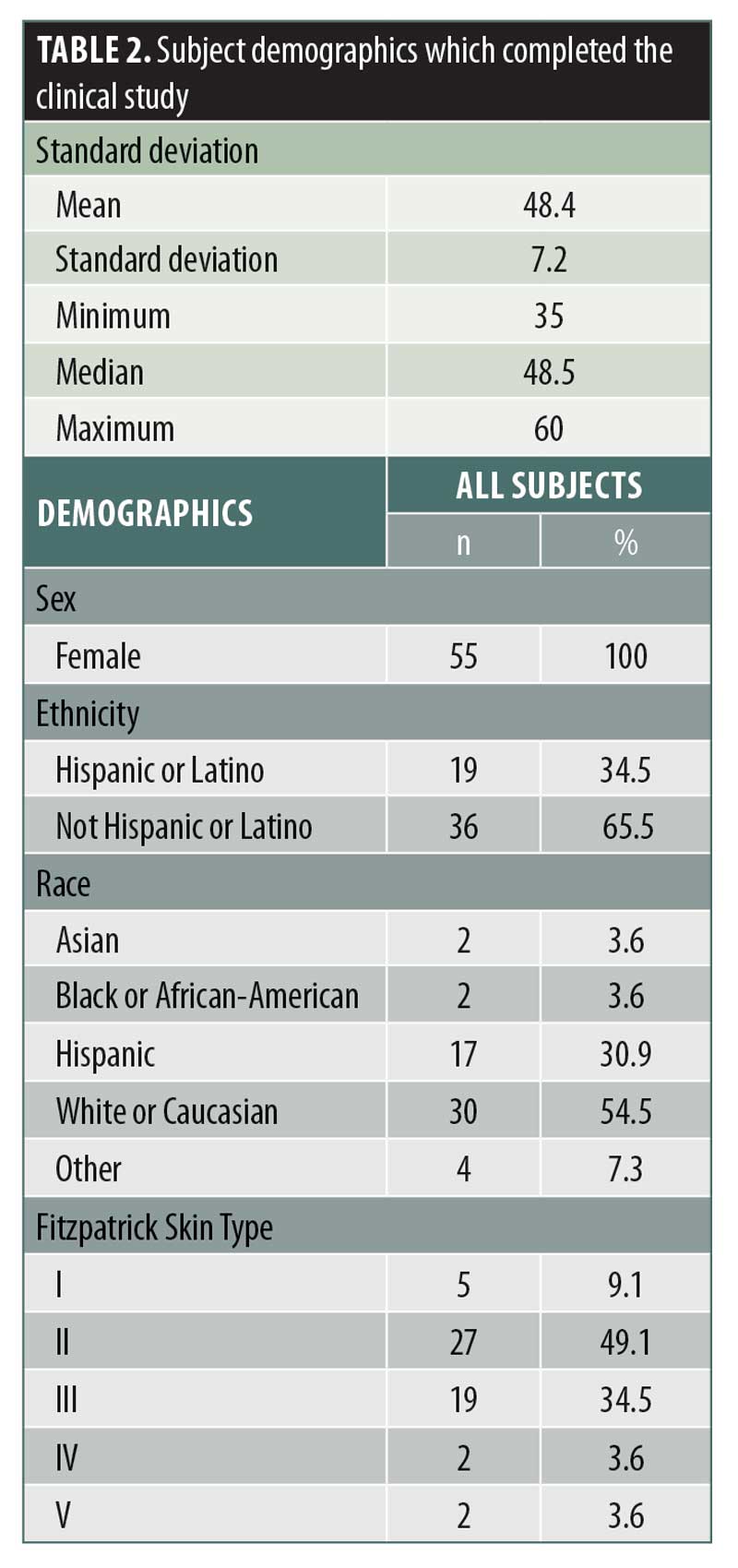
Study subjects. Fifty-five female subjects (33 subjects in the LTPS arm and 23 subjects in the placebo serum arm) with a mean age of 48.4 years (age range of 35–60 years) completed the 12-week study. Female subjects were primarily Caucasian (54.5%) and Hispanic (30.9%), and had Fitzpatrick skin types I through V (Table 2).
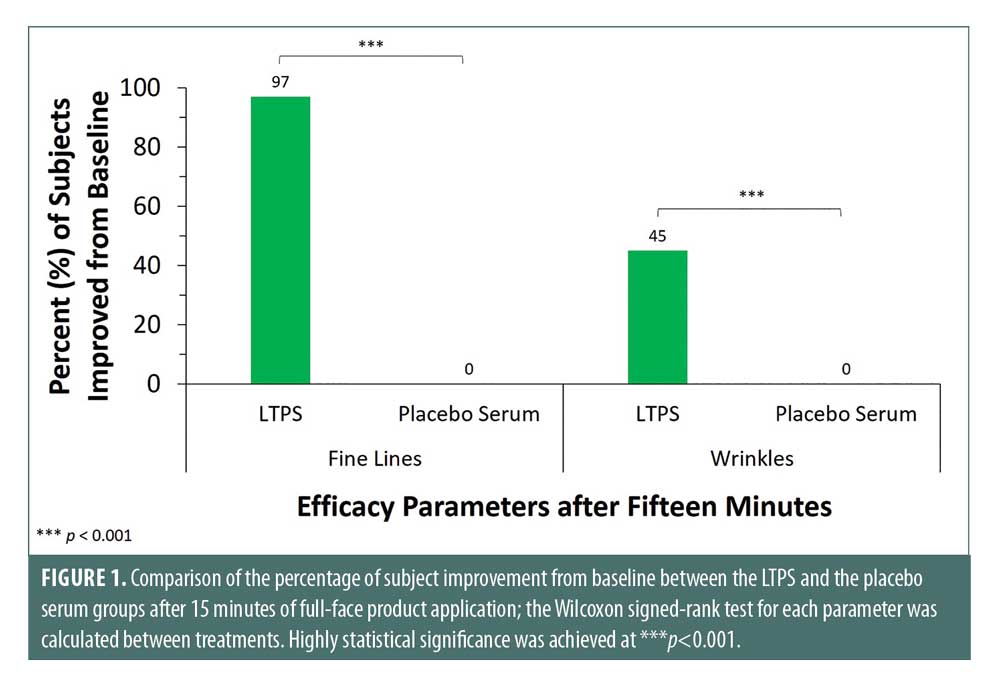
Clinical evaluation. Clinical assessment by a board-certified dermatologist showed improvement in expression lines, fine lines (97% of subjects) and wrinkles (45% of subjects) within 15 minutes of LTPS application. Furthermore, the LTPS significantly exceeded the placebo serum in which none of the subjects in the placebo serum arm had improvements in both parameters, fine lines, and wrinkles (***p<0.001) (Figure 1).
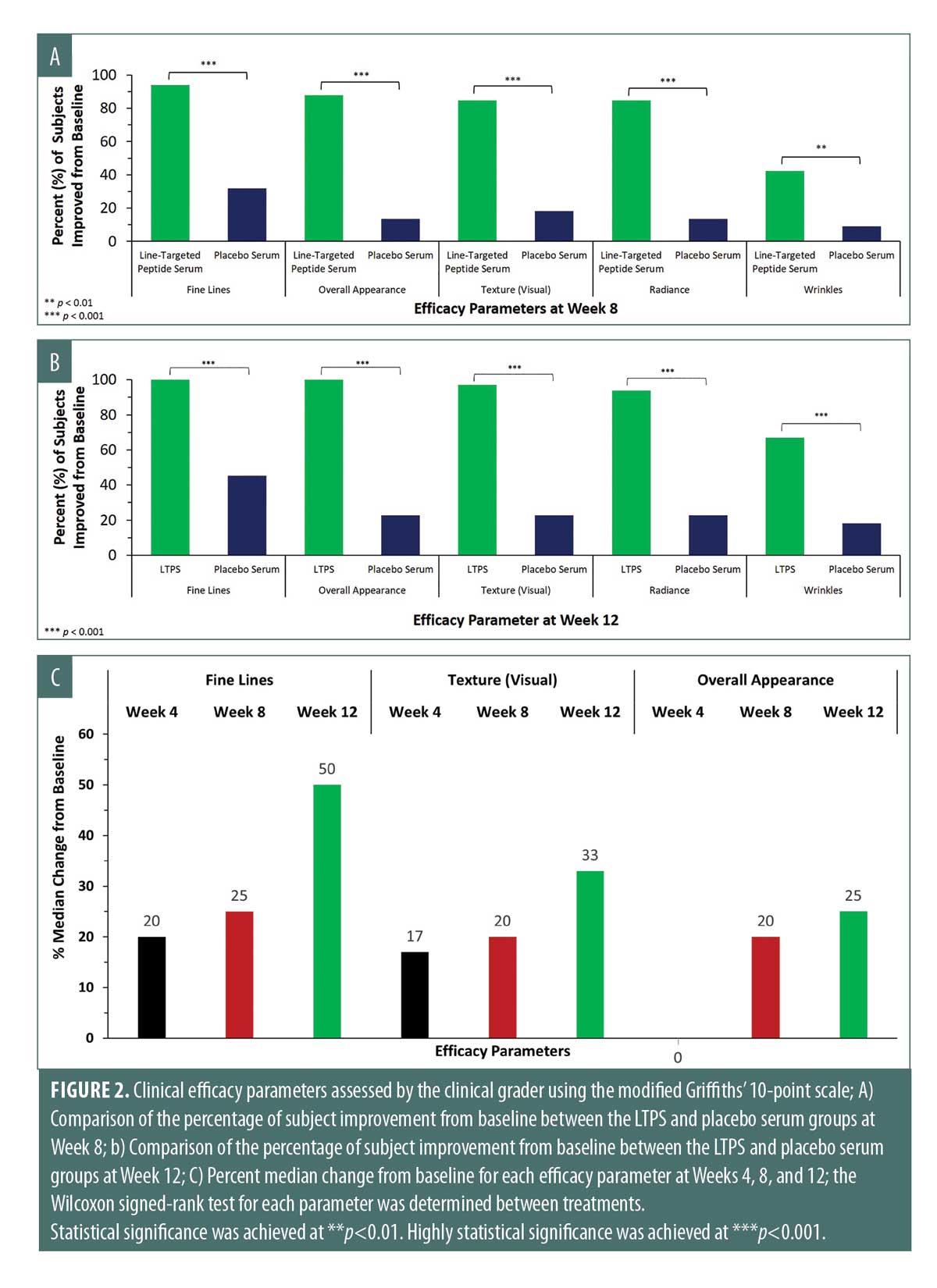
 Long-term efficacy. As early as four weeks, statistically significant improvements were achieved in fine lines, visual texture, radiance, and overall appearance in the LTPS arm as compared with in the placebo serum arm, data not shown. At Week 8, an additional parameter, wrinkles, was found to be significant in the LTPS arm relative to in the placebo serum arm (Figure 2A). Specifically, in the LTPS-treated arm:
Long-term efficacy. As early as four weeks, statistically significant improvements were achieved in fine lines, visual texture, radiance, and overall appearance in the LTPS arm as compared with in the placebo serum arm, data not shown. At Week 8, an additional parameter, wrinkles, was found to be significant in the LTPS arm relative to in the placebo serum arm (Figure 2A). Specifically, in the LTPS-treated arm:
- 94 percent of subjects showed an improvement in fine lines, with a median improvement of 25 percent
- 88 percent of subjects showed an improvement in overall appearance, with a median improvement of 20 percent
- 85 percent of subjects showed improvements in visual texture and radiance, with median improvements of 20 percent and 25 percent, respectively.
LTPS showed efficacious superiority by significantly outperforming the placebo serum in improving fine lines, overall appearance, visual texture, radiance, and wrinkles at Week 12 (***p<0.001) (Figure 2B). Furthermore, LTPS provided continuous improvements in fine lines, visual texture, and overall appearance throughout Weeks 4, 8, and 12 (Figure 2C). At Week 12, the following improvements were achieved:
- 100 percent of subjects showed improvements in fine lines and overall appearance, with median improvements of 50 percent and 25 percent, respectively
- 97 percent of subjects showed an improvement in visual texture, with a median improvement of 33 percent
- 94 percent of subjects showed an improvement in radiance, with a median improvement of 33 percent
- 67 percent of subjects showed an improvement in wrinkles, with a median improvement of 20 percent
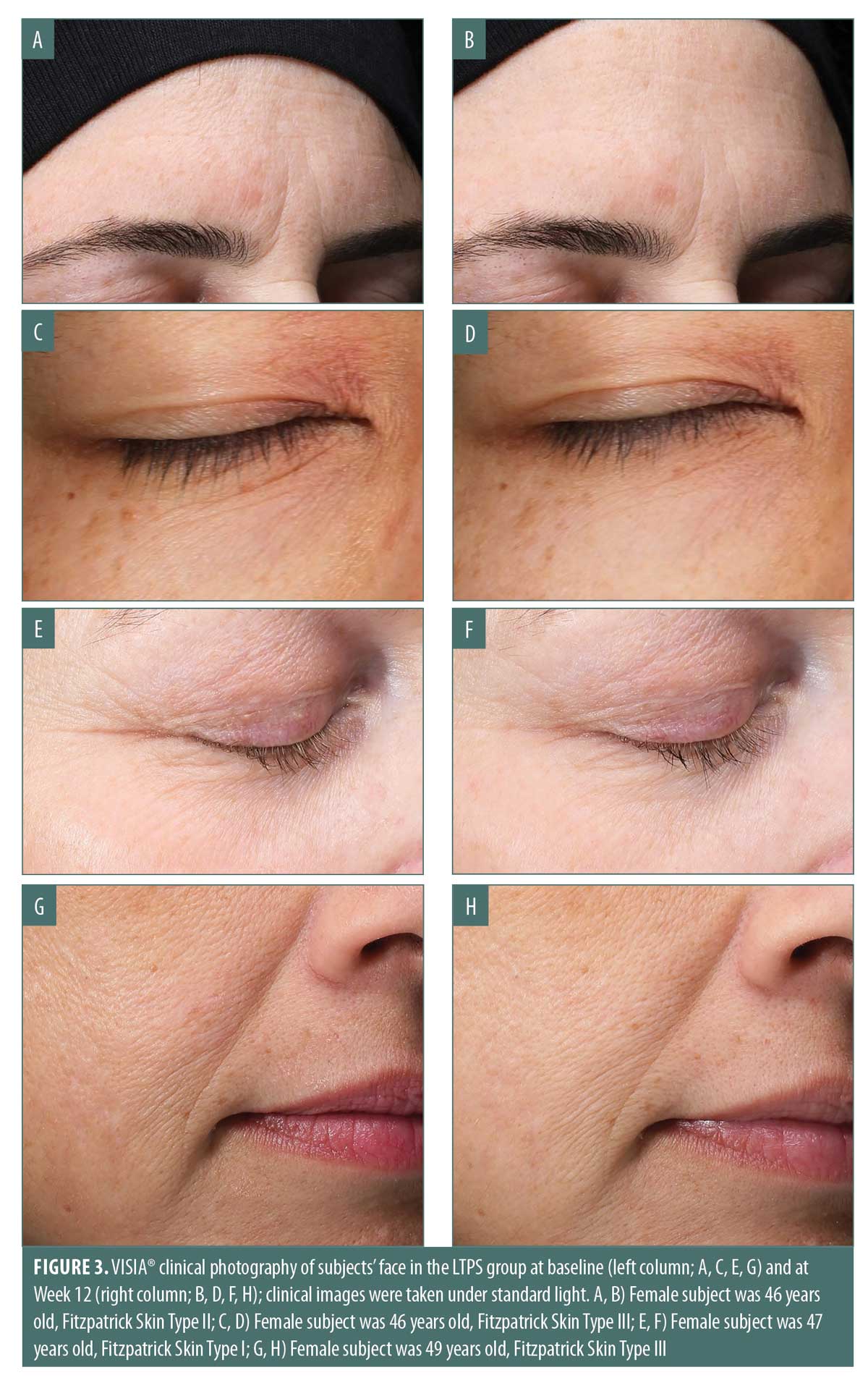
 Clinical photography. Improvements in expression lines and wrinkles in targeted areas of facial muscle movement were evident and demonstrated by VISIA® photography taken under standard light. Specifically, horizontal forehead and glabellar lines (Figures 3A and 3B); under-eye fine lines (Figures 3C and 3F); and nasolabial folds, marionette, and lip lines (Figure 3G and 3H) improved from baseline to Week 12 with twice-daily application of LTPS. Furthermore, facial skin appeared brighter and smoother at Week 12 relative to baseline.
Clinical photography. Improvements in expression lines and wrinkles in targeted areas of facial muscle movement were evident and demonstrated by VISIA® photography taken under standard light. Specifically, horizontal forehead and glabellar lines (Figures 3A and 3B); under-eye fine lines (Figures 3C and 3F); and nasolabial folds, marionette, and lip lines (Figure 3G and 3H) improved from baseline to Week 12 with twice-daily application of LTPS. Furthermore, facial skin appeared brighter and smoother at Week 12 relative to baseline.
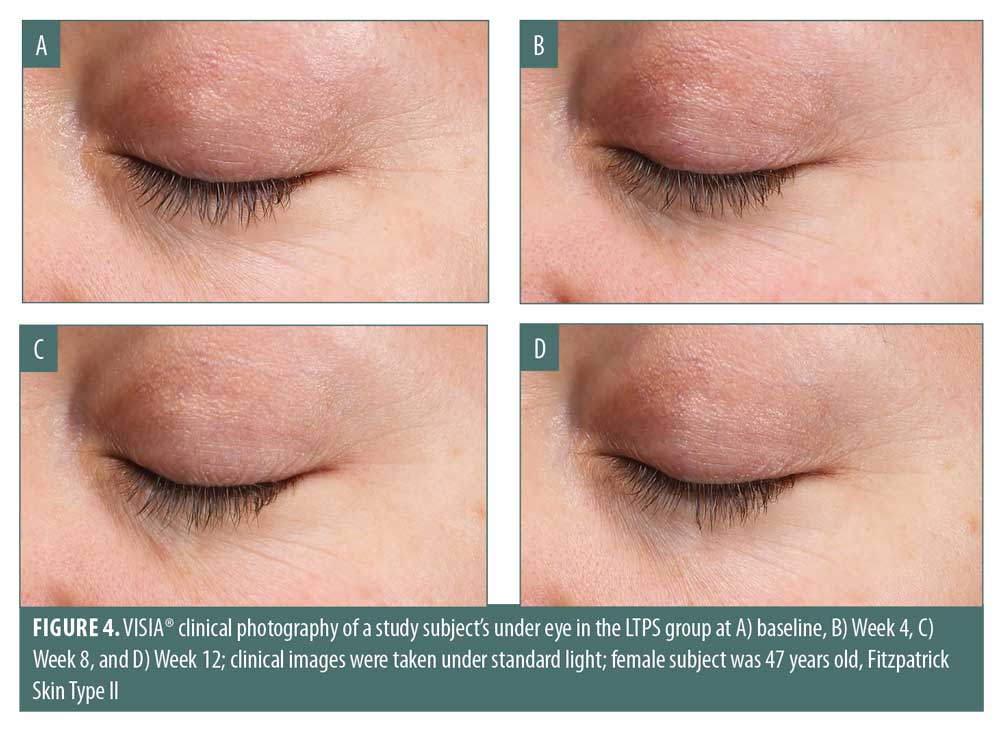
VISIA® photography also exhibited a progressive improvement in periorbital expression lines at Weeks 4, 8, and 12 as compared with at baseline (Figure 4). Fine lines in the subjects using LTPS appeared to be reduced as early as four weeks and were further diminished by the end of the study. Skin texture and overall appearance were also continuously improved throughout the study.
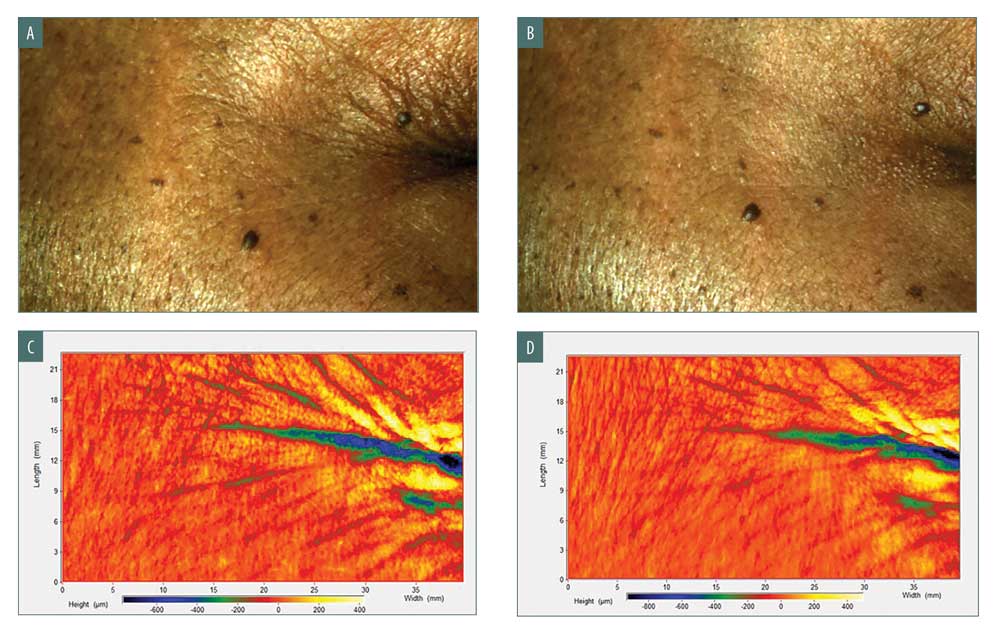
Three-dimensional clinical photography, taken by PRIMOSCR, provided a visualized improvement in the bilateral periorbital region, specifically in the crow’s feet area. High-resolution images showed significant reduction in expression lines and wrinkles at the right crow’s feet at Week 12 compared to baseline (Figures 5A and 5B). The images under robust high-pass filtering captured detailed wrinkle features below the skin surface. Wrinkle volume and depth were reduced at Week 12 relative to baseline as indicated by the decreased intensity of blue and green colors in Figures 5C and 5D.
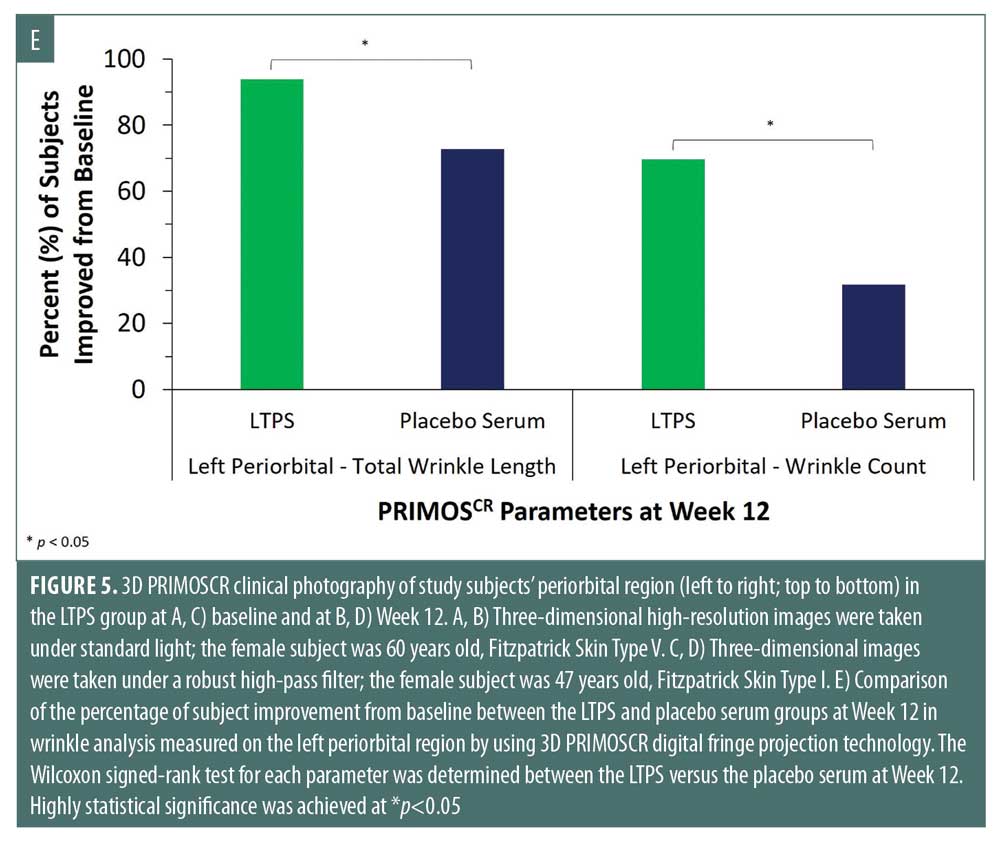
In addition to 3D photography, PRIMOSCR analysis showed that total wrinkle length improvement was achieved in 94 percent (left periorbital region) and 91 percent (right periorbital region) of subjects, wrinkle count improvement was found in 70 percent (left periorbital) and 67 percent (right periorbital) of subjects, and total wrinkle area improvement was observed in 85 percent of subjects on both sides of the periorbital regions (data not shown). The percent of subject improvement in the LTPS group significantly outperformed the one in the placebo serum group in total wrinkle length and wrinkle count on the left periorbital area at Week 12 (*p<0.05) (Figure 5E). Furthermore, the percent median change for total wrinkle length and wrinkle count in the LTPS group outperformed the placebo serum group at Week 12 (*p<0.05) (data not shown).
Self-assessment questionnaire. Subjects were ask to assess the appearance of their facial features at baseline and Weeks 4, 8, and 12. At Week 12, the majority of subjects in the LTPS group agreed that their fine lines and wrinkles appeared minimized around their eyes and cheeks (**p<0.01) when compared with at baseline (statistically significant), around their mouth, on their forehead, and between their eyebrows (***p<0.001). Also, subjects agreed that their skin appeared plump, radiant, firm, smooth, and youthful (***p<0.001).
When asked to evaluate the aesthetic attributes of LTPS after 12 weeks of twice-daily use, all subjects agreed that the product was easy to apply. Additionally, nearly all subjects agreed that the product was mild and did not irritate their skin and their eyes.
Tolerability. Following each visit, objective and subjective tolerability were evaluated. Results indicated no statistically significant increase in all tolerability parameters (objective and subjective) erythema, edema, burning, itching, or stinging at all time points relative to baseline for both LTPS and the placebo serum. There was a statistically significant improvement in objective tolerability evaluation for dryness at Weeks 4, 8, and 12 as compared with at baseline for both study groups. No adverse reactions were reported during the study.
Discussion
Expression lines formed by repeated skin flexure during facial expression lead to line and wrinkle formation. The pattern of expression lines predicts the pattern of future wrinkles.4 The LTPS investigated in this clinical study was formulated to repair and prevent fine lines and wrinkles, primarily those resulting facial expression at specific anatomical regions. The objective of this clinical study was to assess the efficacy and tolerability of the LTPS as a stand-alone treatment when used twice daily for 12 weeks by female subjects aged 35 to 60 years with mild-to-moderate fine lines and wrinkles as compared with using a placebo serum. Rapid improvement seen shortly after application is desired and can improve consumer compliance and, eventually, long-term clinical outcomes.
Results from the clinical study indicated that the LTPS provided both short- and long-term improvements in the appearance of fine lines and wrinkles in the forehead, periorbital, and perioral regions. Short-term efficacy was observed after approximately 15 minutes of LTPS application, which also significantly outperformed the placebo serum. The exact mechanism of action was unknown but potentially attributed to the combination of GABA and short-term activity of the neuromodulating peptides.16,17 These ingredients synergistically promoted an immediate reduction in the appearance of expression lines by manipulating facial muscle movement to smooth and relax the skin. Long-term efficacy was attributed to the blend of bioavailable peptides, botanical extracts, and antioxidants. In regards to tolerability, both LTPS and the placebo serum were well tolerated throughout the study.
Improvement in fine lines and wrinkles in the areas of expression was observed throughout the 12-week clinical study with a linear progression from baseline to Week 12. The combination of five neuromodulating peptides in LTPS helped interfere with signaling in the pre- and postsynapse pathways to inhibit muscle contraction, further attenuating facial expression. Fine lines and wrinkles are also a result of a loss in the integrity of collagen and elastin fibers in the extracellular matrix due both intrinsic and extrinsic factors.23,24 Three reparative peptides in LTPS assisted in the regeneration of the extracellular matrix by stimulating the production of collagen and elastin while inhibiting matrix metalloproteinases and pro-inflammatory factors. Botanical extracts (Apium graveolens callus extract and Anigozanthos flavidus extract) provided antioxidant benefits by scavenging free radicals. Together, this novel blend of ingredients supported the repair of the extracellular matrix induced repeated muscle movement and intrinsic and extrinsic skin-aging factors.
LTPS was formulated to have a positive impact on long-term skin health by supporting the skin barrier and skin microbial ecosystem, in part due to the formula’s skin-neutral pH and inclusion of a prebiotic. This was reflected by significant improvement in clinical efficacy parameters, including visual texture, radiance, and overall appearance. Thus, twice-daily application of LTPS was able to restore and strengthen the structural integrity of the skin layers as well as the skin barrier and was reflected outwardly in the improvement of overall appearance. In addition, the prebiotic, inulin, assisted in rebalancing the skin microbiome as well as adding hydration to the skin. A balanced and diverse skin microbiome is an indication of a harmonious microbial ecosystem, which is often known to push the skin to a healthier state.25
Clinical photography by VISIA® and PRIMOSCR further supported the clinical data by visually showing reductions in wrinkles and fine lines in the forehead, periorbital, and periocular regions. VISIA® clinical photos showed that the subjects’ skin appeared smooth, radiant, and youthful after twice-daily usage of LTPS. In addition, the LTPS efficacy was further confirmed by clinical grading as it significantly outperformed the placebo serum in improving visual texture, radiance, and overall appearance at the end of the clinical study. PRIMOSCR assessment of LTPS confirmed reductionss in wrinkle count and total wrinkle length in the left periorbital region relative to with the placebo serum at Week 12.
LTPS was well-perceived by the subjects regarding their facial skin condition, product efficacy, and product aesthetics attributes. Future research efforts could elucidate whether LTPS could be beneficial in pairing pre- and postneurotoxin injections. The limitation of this clinical was that the study was not designed as split-face study. This type of study would eliminate any genetic bias between the active and placebo treatment arms.
Conclusion
This randomized, double-blind, placebo-controlled clinical study demonstrated that the LTPS, a water-based peptide treatment, was effective and tolerable in statistically significantly improving expression fine lines and wrinkles induced by repeated facial muscular movements as well as improving overall skin health when used twice daily for 12 weeks. Composed of a novel patent-pending technology incorporating a unique blend of bioavailable neuromodulating and reparative peptides, GABA, botanical extracts, antioxidants, and prebiotic innovation, the LTPS proved to be a suitable cosmetic treatment for expression lines and long-term skin health.
Acknowledgments
The authors would like to acknowledge Fred Wilson for conducting the statistical analysis for this clinical study; Haley Wilson and Shelley Joyce RN, BSN, for their assistance in running the clinical study; and Taylor Jensen in providing editorial assistance.
References
- Lemperle G, Holmes RE, Cohen SR, Lemperle SM. A classification of facial wrinkles. Plast Reconstr Surg. 2001;108(6):1735–1750; discussion 1751–1732.
- Piérard GE, Uhoda I, Piérard-Franchimont C. From skin microrelief to wrinkles. An area ripe for investigation. J Cosmet Dermatol. 2003;2(1):21–28.
- Kligman AM, Zheng P, Lavker RM. The anatomy and pathogenesis of wrinkles. Br J Dermatol. 1985;113(1):37–42.
- Hillebrand GG, Liang Z, Yan X, Yoshii T. New wrinkles on wrinkling: an 8-year longitudinal study on the progression of expression lines into persistent wrinkles. Br J Dermatol. 2010;162(6):1233–1241.
- Zhang L, Falla TJ. Cosmeceuticals and peptides. Clin Dermatol. 2009;27(5):485–494.
- Linder J. The science behind peptides. Plast Surg Nurs. 2012;32(2):71–72.
- Lintner K, Peschard O. Biologically active peptides: from a laboratory bench curiosity to a functional skin care product. Int J Cosmet Sci. 2000;22(3):207–218.
- Schagen SK. Topical peptide treatments with effective anti-aging results. Cosmetics. 2017;4(2):16.
- Blanes C, Llobregat M, Gil A, et al. Neuronal exocytosis inhibiting peptides and cosmetic and pharmaceutical compositions containing said peptide. 2000. U.S. patent no. US7015192B12006.
- Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24(5):303–310.
- Carreno Serraima C, Ponsati Obiols B, Van Den et al. Neuronal exocytosis inhibiting peptides. 2017. Canadian patent no. CA2666516C2017.
- Grau-Campistany A, Silvia P, Carulla P, et al. Peptides and compositions for use in cosmetics and medicine. 2019. WIPO patent no. WO2019166347A12019.
- Draelos ZD, Kononov T, Fox T. An open label clinical trial of a peptide treatment serum and supporting regimen designed to improve the appearance of aging facial skin. J Drugs Dermatol. 2016;15(9):1100–1106.
- Lintner K, Inventor; Sederma, SA, assignee. Use of peptides as cosmetics or pharmaceuticals for the regulation of immunological dysfunctions and in cutaneous inflammation. 2000. WIPO patent no. WO2000043417A12000.
- Leroux R, Ringenbach C, Marchand T, et al. A new matrikine-derived peptide up-regulates longevity genes for improving extracellular matrix architecture and connections of dermal cell with its matrix. Int J Cosmet Sci. 2020;42(1):53–59.
- Watanabe M, Maemura K, Kanbara K, et al. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47.
- Denda M, Inoue K, Inomata S, Denda S. gamma-Aminobutyric acid (A) receptor agonists accelerate cutaneous barrier recovery and prevent epidermal hyperplasia induced by barrier disruption. J Invest Dermatol. 2002;119(5):1041–1047.
- Georgiev V, Slavov A, Vasileva I, Pavlov A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng Life Sci. 2018;18(11):779–798.
- Attia JJ, Shortt M, Lacasse I, Loing E. Tensor effect of anigozanthos flavidus flower extract: Modulation of tenascin-X to regenerate the skin. J Investig Dermatol. 2018;138(5):S247.
- Attia J, Shortt M, Daigle P, et al. Remodeling effect of Kangaroo Paw flower extract: modulation of hexabrachion-like protein (TN-X) to regenerate functionalities of the skin. IFSCC Congress; September 18–21, 2018; Munich, Germany.
- Gonry P. A prebiotic fight against preservatives. Int J Probiot Prebiot. 2018: 18–22.
- Griffiths CE, Wang TS, Hamilton TA, et al. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992;128(3):347–351.
- Uitto J, Fazio MJ, Olsen DR. Molecular mechanisms of cutaneous aging. Age-associated connective tissue alterations in the dermis. J Am Acad Dermatol. 1989;21(3 Pt 2):614–622.
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 Suppl):s12–s16.
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244–253.

