 J Clin Aesthet Dermatol. 2021;14(9):33–40.
J Clin Aesthet Dermatol. 2021;14(9):33–40.
by Natalia M. K. Spierings, Bsc (Hons), MBBS, MRCP (UK), MRCP (Derm), MBA, Msc
Dr. Spierings is with London Medical in London, England and Kings College Hospital London in Dubai, United Arab Emirates.
FUNDING: No funding was provided for this article.
DISCLOSURES: The author reports no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Skincare retailers sell a plethora of retinol-containing products, ranging from serums and moisturisers to masks and eye creams.
Objective. The purpose of this review is to critically appraise the randomized, double-blind, vehicle-controlled trials of the use of over-the-counter retinol products in the treatment of facial skin aging in order to assess evidence regarding their efficacy.
Methods. A PubMed search was conducted for relevant clinical trial publications, using the terms “retinoid,” “tretinoin,” “retinol,” “retinal,” “retinaldehyde,” and “skin.”
Results. Nine randomized, double-blind, vehicle-controlled clinical trials were found. Four of these trials reported no statistically significant differences between the retinol-containing treatment and vehicle. The remaining five trials provide weak evidence for retinol potentially having a mild ameliorating effect on fine facial skin wrinkle lines only. However, these five trials showed major methodological flaws, which were critically analyzed in this review, calling into question the validity of any positive results.
Conclusion. It can be suggested that, in the case of retinols, the “positive” trials should not inform clinical decision-making but rather may serve as tools for advertising. Until at least one high-quality clinical trial of retinol-containing products in the treatment of (photo-)aged skin is published, there is very little, if any, trustworthy evidence available to support the use of over-the-counter cosmetic retinol-containing products to improve the appearance of aged skin.
Keywords: Tretinoin, facial skin, aging, photo-aging, anti-aging, skincare, retinol, wrinkles, vitamin A
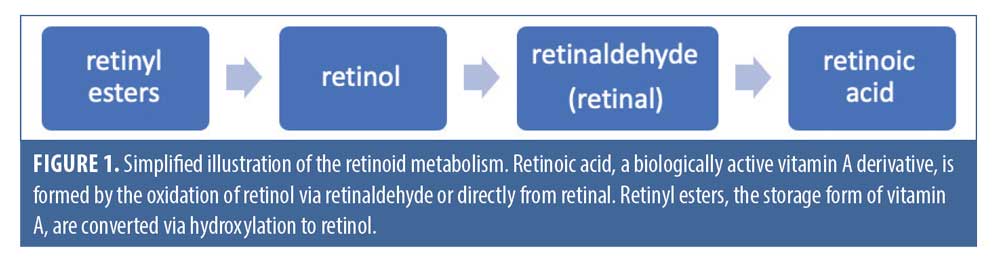
The term “retinoid” encompasses a family of chemical compounds that are natural or synthetic derivatives of vitamin A, sharing structural and functional similarities with this vitamin. In the body, retinol is the main circulating form of vitamin A, retinoic acid is its main active metabolite, and the vitamin is stored in the liver in a variety of retinyl ester forms. The structural differences between the compounds are accounted for by the polar end-group, which is hydroxyl in retinols, aldehyde in retinals, carboxylic acid in retinoic acid, and a variety of esters in the retinyl esters, respectively.1 Retinoic acid, the biologically active form of vitamin A, is converted from retinol and retinyl esters through a number of enzymatic steps, involving oxidation and hydroxylation.2 A simplified illustration of retinoid metabolism is shown in Figure 1.
More than 2,000 derivatives of vitamin A have been synthesized since 1955, classified into three generations.1 The first generation includes the naturally occurring, nonaromatic retinoids, which include retinol, retinal, isotretinoin, and tretinoin. The second-generation retinoids are the mono-aromatic compounds, including etretinate and acitretin. Finally, the third-generation retinoids are formed by cyclization of the polyene side chain and include adapalene and tazarotene; these molecules have a more rigid structure than the first- and second-generation retinoids, which makes them bind to a narrower set of receptors.1

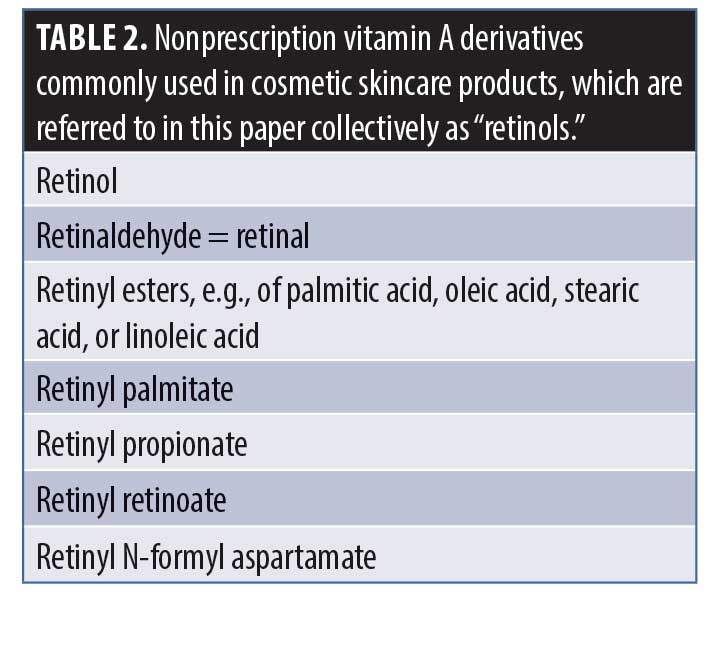
Tretinoin is a synthetic retinoic acid and is the retinoid most extensively investigated in the treatment of facial skin (photo-)aging.3 For the sake of clarity, in this paper, the term “tretinoin” will be used to refer to the biologically active retinoid approved by the United States Food and Drug Administration (FDA), while “retinol” will be the term used here for nonprescription vitamin A derivatives, commonly found in cosmetic skincare products, with the ones most often used listed in Table 2. The purpose of the many chemical alterations of the retinol molecule concerns mostly attempts to increase the stability of the compound.
 Active retinoic acid is the best-known category of facial skin anti-aging treatment available today. There is high-level scientific evidence, dating back decades, showing the histological effects of tretinoin on collagen synthesis, fibroblast activity, and the inhibition of matrix metalloproteinases. However, there are few high-quality studies providing support for the benefit of these products as anti-aging treatments, despite various retinoic acid formulations being approved by the FDA for the treatment of fine facial wrinkle lines.
Active retinoic acid is the best-known category of facial skin anti-aging treatment available today. There is high-level scientific evidence, dating back decades, showing the histological effects of tretinoin on collagen synthesis, fibroblast activity, and the inhibition of matrix metalloproteinases. However, there are few high-quality studies providing support for the benefit of these products as anti-aging treatments, despite various retinoic acid formulations being approved by the FDA for the treatment of fine facial wrinkle lines.
In 2000, Renova® (tretinoin 0.02%) received FDA approval for the “mitigation of fine facial wrinkles in patients who use comprehensive skincare and sunlight avoidance programmes.” The FDA approved labeling states that “Renova®…does not eliminate wrinkles, repair sun-damaged skin, reverse photoaging, or restore more youthful or younger skin.” Indeed, out of the five clinical trials submitted to the FDA, only two showed a statistically significant improvement in the appearance of fine lines, while none demonstrated efficacy for any other signs of facial skin (photo-)aging.4
More recently conducted clinical trials have also failed to show benefit on signs of facial skin (photo-)aging other than fine wrinkles. For example, Kang et al.5 performed a two-year, multicenter, randomized, double-blind, vehicle-controlled trial of the efficacy of tretinoin 0.05% emollient cream versus vehicle in the treatment of photodamaged facial skin. The sample size was based on a power calculation to ensure that estimates of treatment differences would be clinically meaningful. An intention-to-treat analysis of the results was performed, including all randomized subjects, and the Bonferroni–Holms procedure was used to correct for multiple comparisons. The primary efficacy parameters were fine wrinkling, mottled hyperpigmentation, and tactile roughness of the face, rated using a scoring system of 0 to 9, expressed as the percentage of change from baseline.
In the trial and after two years of treatment, tretinoin 0.05% was statistically significantly superior to vehicle in terms of the percentage of subjects who experienced improvement from baseline in fine wrinkling (76% vs. 55%; p=0.002) and mottled hyperpigmentation (74% vs. 65%; p=0.002) but not tactile roughness (76% vs. 65%; p=0.645). It is important to note that, in the placebo group, the percentage of subjects with improvement from baseline for all three primary efficacy parameters was well over 50%, with the caveat that the definition of improvement was not provided.
Retinols are not classified as medicines due to a lack of safety concerns and are commonly found in over-the-counter cosmetic skincare products. They require conversion to active retinoic acid in the body to demonstrate activity6 and, in vitro, have been shown to be 20 times less potent than retinoic acid.7 Evidence suggests that, once metabolised in the skin, topically applied retinol or retinyl esters are no longer active or are less active than retinol itself.8 Retinyl palmitate is the most commonly used retinol in cosmetic skincare products but is theoretically the least effective because it requires conversion to active retinoic acid by a two-step oxidative process, which is limited in the skin.9
If manufactured under inert atmospheric conditions and stored in aluminium tubes at less than 20°C, retinol can be stable in a formulation for more than six months.8 However, cosmetic skincare products containing retinol are generally not manufactured or stored in this way, therefore making it likely that the retinol in these products has very little, if any, effect when applied to the skin. The impact of the vehicle on penetration and stability of the retinol in a formulation is also crucial in this regard.3
Regulations surrounding the use of retinol in cosmetic skincare products do not require evidence of efficacy because “cosmetics are not medicines.”10 The Cosmetic, Toiletry, and Perfumery Association (CTPA) in the United Kingdom published a guidance document, titled “Confidence in Cosmetic Claims.” Its use is intended for industry, explaining that “claims can be substantiated by using either: formula, experimental studies, consumer perception tests and/or published information or a combination of these.” Therefore, companies can make statements about their retinol-containing products like “this intensive treatment minimises visibility of fine lines and dark spots…promoting skin renewal and boosting collagen production”11 without providing evidence of efficacy, presumably basing such claims on the what is known about tretinoin in general.
Online skincare retailers sell a plethora of retinol-containing products, ranging from serums and moisturizers to masks and eye creams. Performing a search for “retinol” on a popular beauty website generates more than 250 products, ranging in price from £4.20 for a 30-mL serum to £210 for a 50-mL cream. The question that many consumers rightly ask themselves is: are these products at all effective? Considering that many of the products are more expensive than the equivalent amount of prescription tretinoin, sometimes, even when adding in the cost of seeing a dermatologist to get the prescription, is it worth purchasing them instead of obtaining a tretinoin prescription?
The purpose of this review is to critically appraise the existing randomized, double-blind, vehicle-controlled trials of the use of over-the-counter cosmetic retinol products in the treatment of facial skin aging in order to assess the level of evidence regarding their efficacy.
Methods
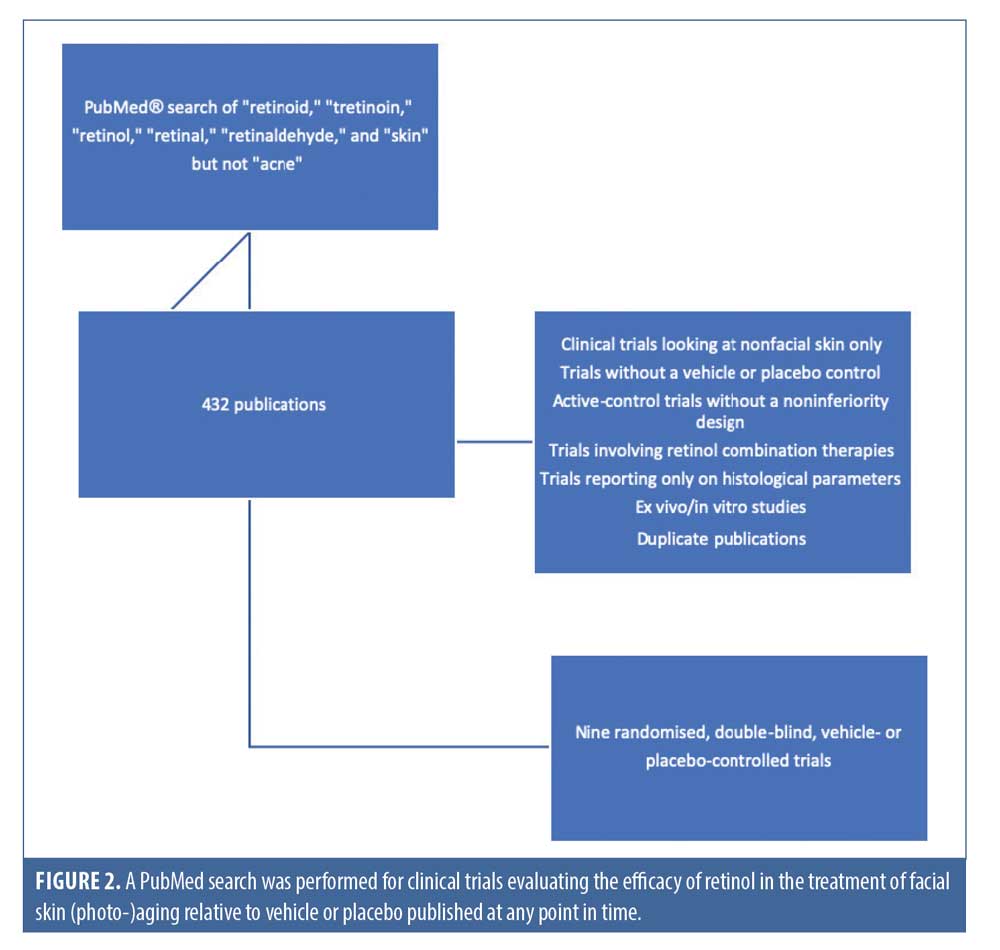
A PubMed® search was conducted for relevant clinical trial publications, using the terms “retinoid,” “tretinoin,” “retinol,” “retinal,” “retinaldehyde,” and “skin“ but not “acne.” The selection of trials was based on the following criterion: clinical trials evaluating the efficacy of retinol in the treatment of facial skin (photo-)aging compared to vehicle or placebo published at any point in time. Exclusion criteria included clinical trials looking at nonfacial skin only; trials without a vehicle or placebo control; active-control trials without a noninferiority design; trials involving retinol combination therapies, such as combined with vitamin C or other “novel” medicinal-type ingredients; and trials reporting only on histological parameters. Ex-vivo and in-vitro studies were excluded as well, and duplicate publications were identified to ensure that trials were only included once.
A total of 432 publications were identified and their the titles and abstracts were reviewed for relevants based on the outcome measures most important to patients and clinicians. Subsequently, a total of nine randomized, double-blind, vehicle- or placebo-controlled clinical trials were found based on the above selection criteria (Figure 2), whose quality was assessed using the Jadad criteria.12
Results
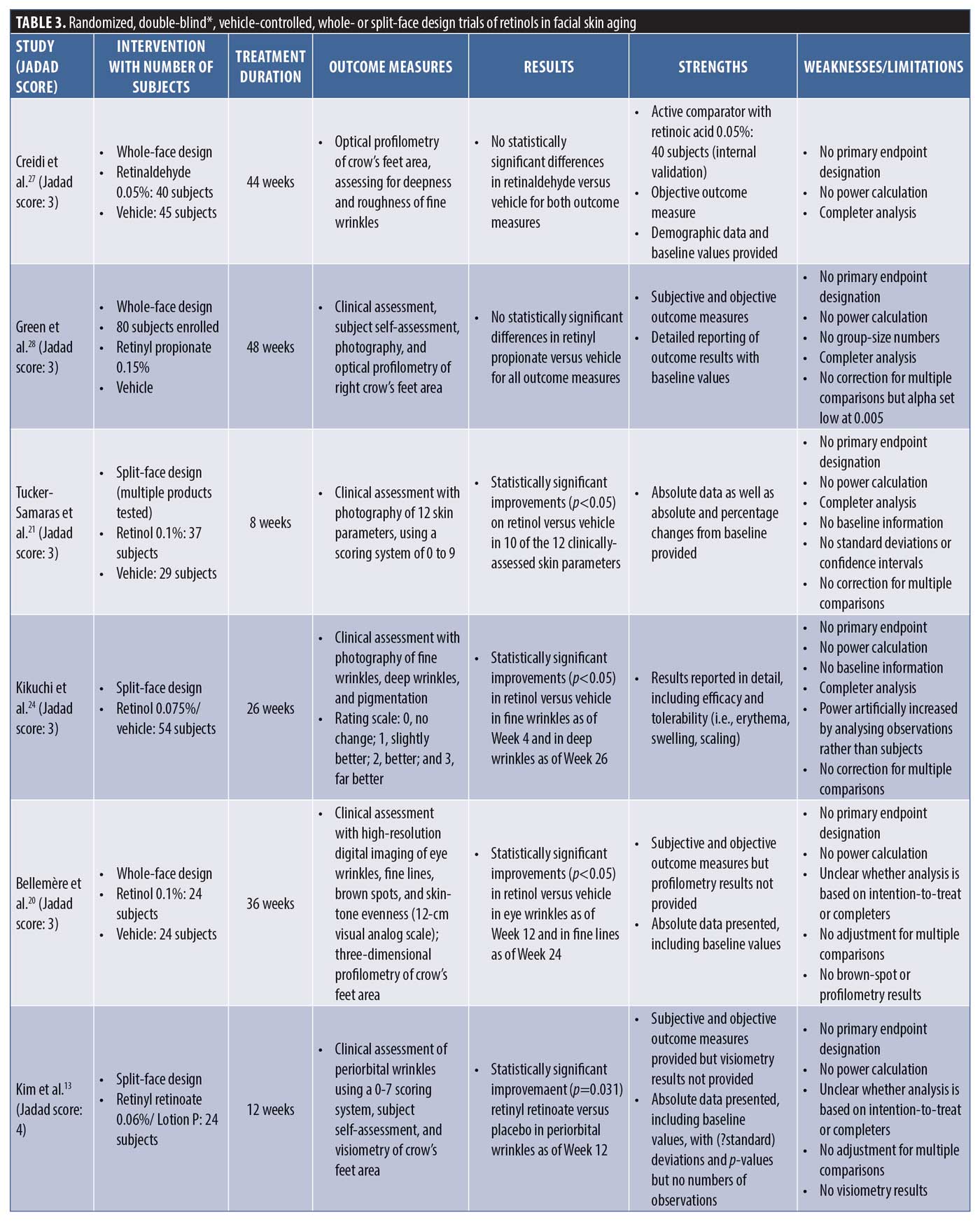
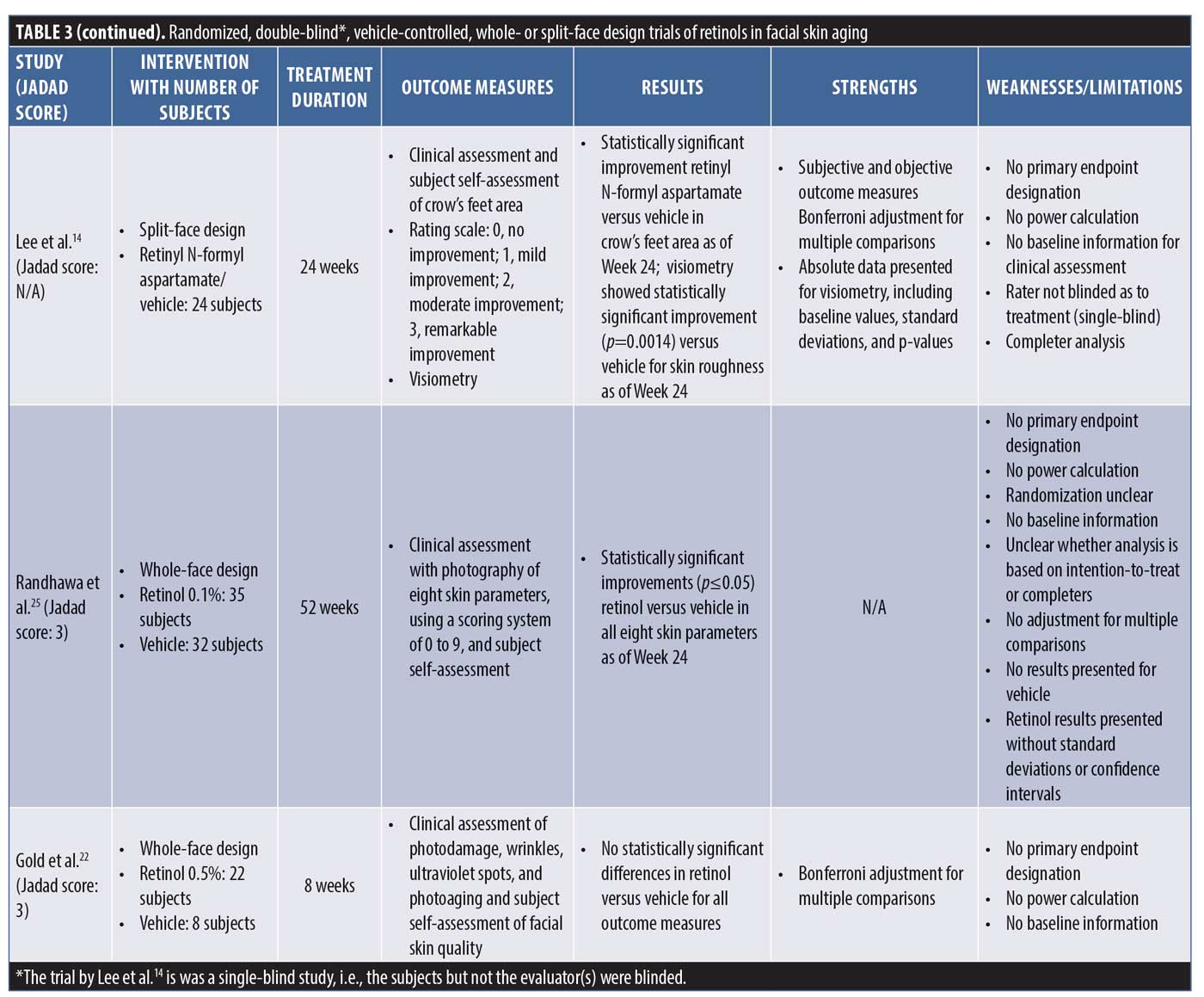
The nine clinical trials generated by the PubMed search and selected based on the above eligibility and exclusion criteria are summarized in no particular order in Table 3 in terms of intervention, with number of subjects, treatment duration, outcome measures, results, strengths, and weaknesses/limitations reported.
Discussion
Clinical trial methodology is well established in terms of design, execution, analysis, and reporting. Trials should follow the established methodology to allow for accurate and generalizable conclusions, which is important from scientific and clinical perspectives alike. If trials do not follow the established methodology, it becomes difficult to draw meaningful conclusions from them or conclusions that are valid. Some of the key aspects of clinical trial methodology that are relevant here are briefly defined and explained in Table 4.
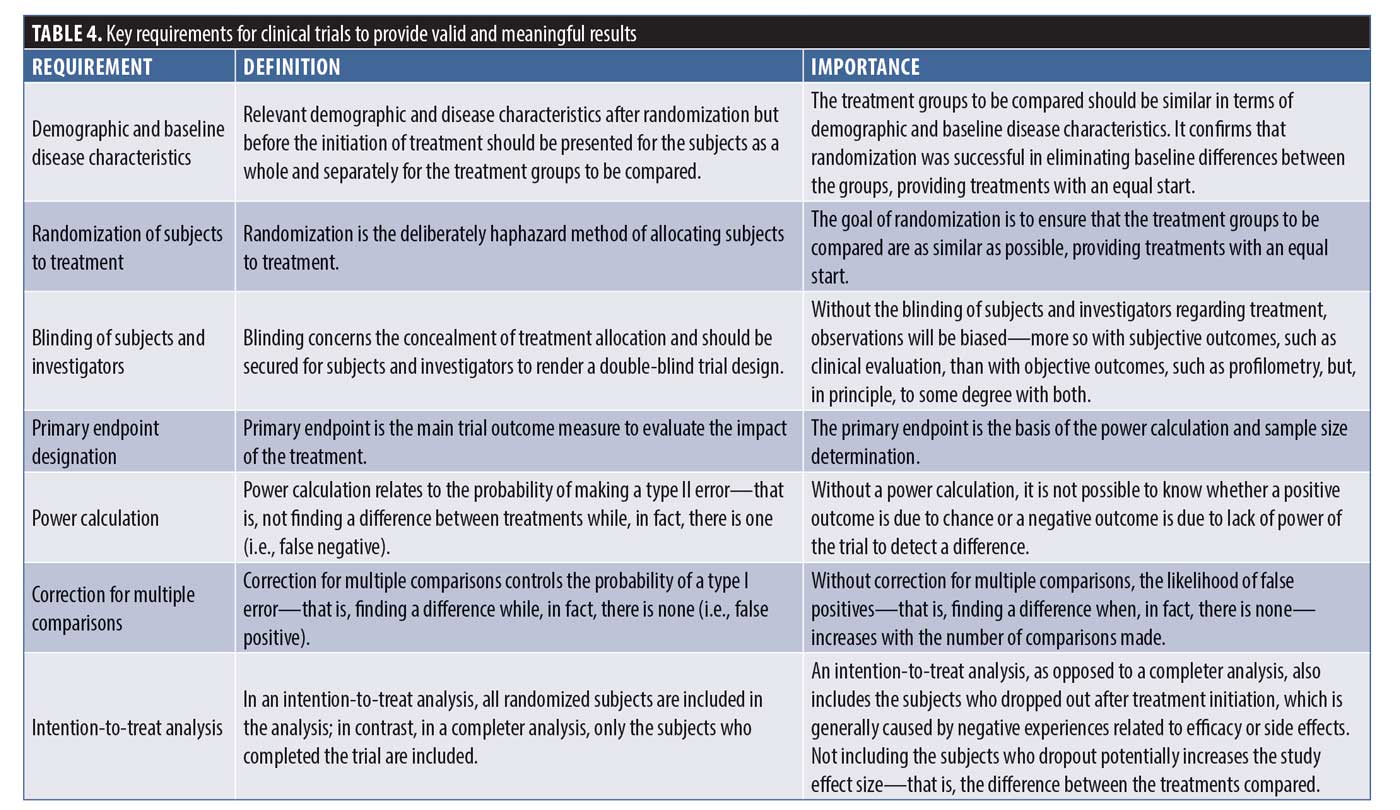
All nine trials that the search yielded suffer from similar methodological problems. None of the trials had an endpoint designation and, therefore, none had a power calculation as part of the statistical analysis to establish how many subjects would be required for valid conclusions to be drawn. In addition, only one trial (Kim et al.13) described the method used to achieve randomization. In two of the trials described as double-blind studies, it was not specifically stated that the investigator was blinded. None of the trials described how, in fact, blinding was achieved and one trial was actually only subject-blinded (Lee et al.14), allowing for evaluator bias. An intention-to-treat analysis was not performed in any of the trials, thus potentially overestimating the treatment benefit by eliminating those subjects who dropped out after randomization and treatment initiation. Subjects generally drop out after the initiation of treatment because of negative experiences related to efficacy or side effects, unduly affecting the treatment outcome in a favorable way (“completer analysis”). In terms of statistical analysis, five trials performed multiple comparisons but only two corrected for them, increasing the likelihood of false-positive findings. Only three of the nine trials provided both baseline data and the results of treatment with p-values, while the others provided only one or the other or neither.
Four of the trials used a split-face design (side-to-side comparison), with no details provided as to how the risk of cross-contamination would be controlled. The use of a split-face design to compare topical treatments is efficient from a subject perspective but comes with the risk of subjects mixing up treatments, invalidating the results.15
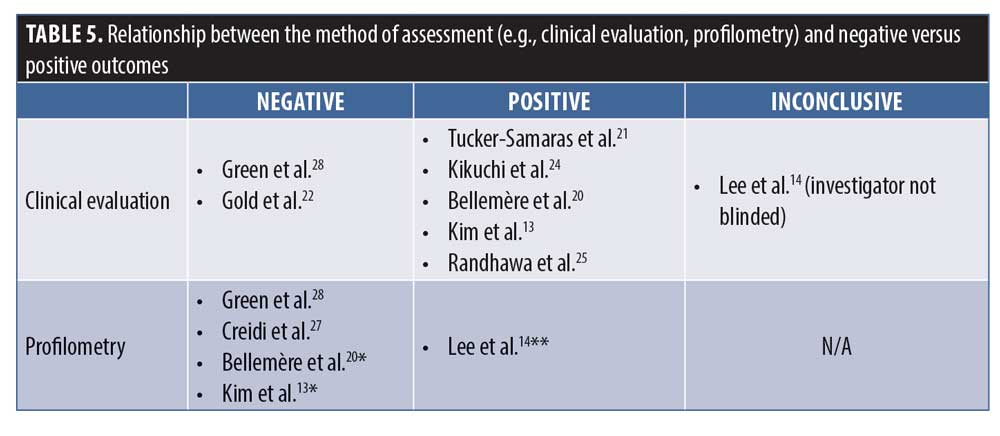
Endpoint assessment in the trials was done by clinical evaluation, profilometry, or both. Five trials performed profilometry and only one (Lee et al.14) reported a statistically significant improvement versus vehicle in signs of (photo-)aging. Two of the trials did not provide the results of the profilometry that was described as part of the trials in the methods section. Using profilometry, Lee et al.14 observed a statistically significant improvement versus vehicle in skin roughness only, which is interesting considering the fact that skin roughness improvement is not commonly documented in clinical trials of tretinoin.5
It is well established that, even under optimal conditions of subject preparation and lighting, real-time or photographic clinical evaluation can be difficult because even a seemingly minor change in facial expression or lighting can dramatically alter the appearance of facial skin lines and wrinkes.16 Profilometry, on the other hand, offers an objective and quantitative method to evaluate the effects of topical treatments on the signs of (photo-)aging. In profilometry, the surface topography of the skin is replicated using a silicone rubber impression, after which digital image processing is used to quantify the “microtopographic” features of the skin. The results are commonly reported as Rz, a measure of deeper wrinkles, and Ra, a measure of fine wrinkles and overall skin topography, respectively.16 Probably the most reliable way to assess skin changes in clinical trials is a combination of blinded clinical evaluation and profilometry. Only five of the nine identified trials performed both, with two of them not reporting the profilometry results probably because no statistically significant differences versus vehicle were observed. The association between clinical evaluation and profilometry versus negative and positive outcomes is shown in Table 4.
The effect of vehicle or placebo in clinical trials is well-recognized and can be relatively high with topical treatments, similar to what is observed in analgesic or antidepressant trials.17 It must be remembered that trials of topical treatments can only be vehicle-controlled; they cannot be placebo-controlled.18 The vehicles used are almost certainly going to have some occlusive or emollient properties and, hence, are “moisturizers” known to have temporary as well as potentially longer-term physiological effects on the skin.19 In the study by Kim et al.,13 the vehicle response was virtually zero over a treatment period of 12 weeks and, in the study by Bellemère et al.,20 the vehicle also showed virtually no change from baseline over the 36 weeks of the trial. In the study by Tucker-Samaras et al.,21 of the 10 outcome measures assessed, there was no response to vehicle in six of them at Week 4 and five of them at Week 8, while all but one outcome measure (skin sagging) improved significantly relative to baseline in the treatment group. A placebo effect of zero with the use of a moisturizing vehicle over 8 to 36 weeks of treatment is not congruent with what would be expected and, hence, calls into question the reliability of the assessments or methods thereof.
The trials by Tucker-Samaras et al.21 and Gold et al.22 had the shortest treatment periods of eight weeks only. Although Gold et al.22 used a retinol 0.5%–containing product and Tucker-Samaras et al.21 used a retinol 0.1%–containing product, Gold et al. found no statistically significant differences from vehicle, while Tucker-Samaras et al. did. Aside from the obvious difference in retinol strengths, trial evidence suggests that topical tretinoin treatment improves the clinical appearance of (photo-)aged skin only after three to six months of treatment,23 with many showing most of the clinically noticeable improvements to occur after six months.3 Eight weeks of treatment as was adopted in the Tucker-Samaras et al. trial would be a surprisingly short period of time to allow for actual quantifiable improvement to be seen with a much less potent topical.
Finally and regarding a potential conflict of interest, eight of the nine trials were sponsored by the test-product manufacturer. It is unclear whether the trial published by Lee et al.14 was industry-sponsored. Three (Tucker-Samaras et al.,21 Kikuchi et al.,24 and Randhawa et al.25) of the eight industry-sponsored trials were sponsored by the same global skincare company. Though the test product was not the same in the three trials (retinol 0.1% moisturizer, retinol 0.075% cream, and “stabilized” retinol 0.1%), the trials had the “strongest” positive results compared to the other trials and similar methodological flaws. Whether this is merely coincidence, a true reflection of efficacy, or systematic bias is unclear.
Conclusion
Clinical trials often have results that are more impressive than what is observed in real life because of subjects’ adherence to treatment, which is essential in trials and, therefore, emphasized and monitored.26 In the real world, consumers pay for the products they use, which generally motivates them to use them, especially when they are expensive.26 As demonstrated in numerous vehicle-controlled dermatological trials, the vehicle can facilitate impressive improvements on its own. It stands to reason that, if a patient is compliant with the treatment and has high expectations of it due to marketing and positive reviews, it is bound to “work” and meet the consumer’s expectations, regardless of whether the active ingredient is indeed “active” or not. It is certainly possible that the improvements seen with cosmetic skincare products containing “active” ingredients are due to the vehicle alone.
An analysis of the nine clinical trials of retinol versus vehicle demonstrates this phenomenon. Four of the nine trials reported no statistically significant differences between the retinol-containing treatment and vehicle. The remaining five trials have major methodological flaws affecting design and analysis, which calls into question any positive results obtained. If cosmetic manufacturers are keen to show true efficacy for their retinol-containing products, they would be wise to adhere to the generally accepted guidelines for randomized, double-blind, vehicle-controlled trials and the publication thereof. This includes primary endpoint designation; power calculation; sample size determination; intention-to-treat analysis; well-described randomization and double-blinding processes; reporting of subject demographic and baseline disease characteristics; reporting of all primary, secondary, and exploratory outcomes as absolute values or absolute or percentage changes from baseline, along with standard deviations or confidence intervals; and performing appropriate statistical analyses with correction for multiple comparisons, if applicable. Until at least one—although preferably more than one for confirmatory purposes—high-quality clinical trials of retinol-containing products in the treatment of (photo-)aged skin are published, there is very little, if any, trustworthy evidence available to support the use of retinol-containing products to improve the appearance of (photo-)aged skin.
The existing positive trials do provide weak evidence for retinol potentially having a mild ameliorating effect on fine facial skin wrinkle lines only. One might ask, is this enough evidence to justify choosing a retinol over a prescription tretinoin or viewing retinols as “equivalent” to tretinoin? However, equivalence can only be established through active-control trials with a noninferiority design and, to date, no such trials for retinols have been performed. In medicine in general with treatments impacting morbidity, quality of life, and mortality, basing treatment recommendations on poor-quality clinical trials would be considered negligent practice. It can be suggested that, in the case of retinols, the “positive” trials discussed in this review are not appropriate to inform clinical decision-making but rather serve as tools for advertising and marketing. No one’s life is at risk if these trials do not provide valid data and, because they constitute over-the-counter products, no physician-based clinical decision-making is involved. Hence, skincare companies are not motivated to invest the time, money, or effort required to design, execute, and analyse high-level clinical trials of their cosmetic skincare products. Instead, they rely on the relative ignorance of the consumer, using carefully worded and often misleading statements based on poor-quality trials to sell their products. It is thus imperative for physicians as well as other health care professionals involved in evidence-based dermatology practice to regularly appraise such trials critically, allowing them to guide consumers in their choice of products and treatments.
References
- Khalil S, Bardawil T, Stephan C, et al. Retinoids: a journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J Dermatolog Treat. 2017;28(8):684–696.
- Connor MJ, Smit MH. The formation of all-trans-retinoic acid from all-trans-retinol in hairless mouse skin. Biochem Pharmacol. 1987;36(6):919–924.
- Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327–348.
- LLC VPNA. RENOVA ® (TRETINOIN CREAM) 0.02% Package Insert.; 2000.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6(4):245–253.
- Kurlandsky SB, Xiao JH, Duell EA, et al. Biological activity of all-trans retinol requires metabolic conversion to all-trans retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. J Biol Chem. 1994;269(52):32821–32827.
- Kang S, Duell EA, Fisher GJ, et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105(4):549–556.
- Scientific Commmittee on Soncusmer Safety. Final Version of the Opinion on Vitamin A (Retinol, Retinyl Acetate and Retinyl Palmitate) and Corrigendum. Available at: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_199.pdf. Accessed May 17, 2020.
- Diana Draelos Z. Therapeutic moisturizers. Dermatol Clin. 2000;18(4):597–607.
- Cosmetic Toiletry and Perfumery Association (CTPA). Confidence in Cosmetic Claims. Available at: https://www.ctpa.org.uk/file.php?fileid=3330. Accessed May 17, 2020.
- A-Passioni Retinol Cream by Drunk Elephant | Cult Beauty. Available at: https://www.cultbeauty.co.uk/drunk-elephant-a-passioni-retinol-cream.html. Accessed May 17, 2020.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
- Kim H, Kim N, Jung S, et al. Improvement in skin wrinkles from the use of photostable retinyl retinoate: a randomized controlled trial. Br J Dermatol. 2010;162(3):497–502.
- Lee MS, Lee KH, Sin HS, er al. A newly synthesized photostable retinol derivative (retinyl N-formyl aspartamate) for photodamaged skin: profilometric evaluation of 24-week study. J Am Acad Dermatol. 2006;55(2):220–224.
- Samuel M, Brooke R, Hollis S, et al. Interventions for photodamaged skin. Cochrane Database Syst Rev. 2015;2015(6):CD001782.
- Grove GL, Grove MJ, Leyden JJ, et al. Skin replica analysis of photodamaged skin after therapy with tretinoin emollient cream. J Am Acad Dermatol. 1991;25(2):231–237.
- Evers AWM. Using the placebo effect: how expectations and learned immune function can optimize dermatological treatments. Exp Dermatol. 2017;26(1):18–21.
- Feldman SR. Placebo response? Psychosom Med. 2010;72(5):505.
- Draelos ZD. The science behind skin care: moisturizers. J Cosmet Dermatol. 2018;17(2):138–144.
- Bellemère G, Stamatas GN, Bruère V, et al. Antiaging action of retinol: from molecular to clinical. Skin Pharmacol Physiol. 2009;22(4):200–209.
- Tucker-Samaras S, Zedayko T, Cole C, et al. A stabilized 0.1% retinol facial moisturizer improves the appearance of photodamaged skin in an eight-week, double-blind, vehicle-controlled study. J Drugs Dermatol. 2009;8(10):932–936.
- Gold MH, Kircik LH, Bucay VW, et al. Treatment of facial photodamage using a novel retinol formulation. J Drugs Dermatology. 2013;12(5):533–540.
- Bhawan J, Gonzalez Serva A, Nehal K, et al. Effects of tretinoin on photodamaged skin: a histologic study. Arch Dermatol. 1991;127(5):666–672.
- Kikuchi K, Suetake T, Kumasaka N, et al Improvement of photoaged facial skin in middle-aged Japanese females by topical retinol (vitamin A alcohol): a vehicle-controlled, double-blind study. J Dermatolog Treat. 2009;20(5):276–281.
- Randhawa M, Rossetti D, Leyden JJ, et al. One-year topical stabilized retinol treatment improves photodamaged skin in a double-blind, vehicle-controlled trial. J Drugs Dermatology. 2015;14(3):271–276.
- Ali SM, Brodell RT, Balkrishnan R, et al. Poor adherence to treatments: a fundamental principle of dermatology. Arch Dermatol. 2007;143(7):912–915.
- Creidi P, Vienne MP, Ochonisky S, et al. Profilometric evaluation of photodamage after topical retinaldehyde and retinoic acid treatment. J Am Acad Dermatol. 1998;39(6):960–965.
- Green C, Orchard G, Cerio R, et al. A clinicopathological study of the effects of topical retinyl propionate cream in skin photoageing. Clin Exp Dermatol. 1998;23(4):162–167.

