 J Clin Aesthet Dermatol. 2020;13(4):45–50
J Clin Aesthet Dermatol. 2020;13(4):45–50
by Pooja Bains, MBBS, MD and Simplepreet Kaur, MBBS, MD
Drs. Bains and Kaur are with the Department of Dermatology, Venereology, and Leprosy at Sri Guru Ram Das Institute of Medical Sciences and Research in Amritsar, India.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. To evaluate the dermoscopic features of alopecia areata (AA) and correlate these features with patterns and severity.
Design and Setting. The present study was performed over a period of six months, from September 2018 to February 2019, in a tertiary care hospital where clinically diagnosed patients with AA were enrolled. A thorough clinical examination followed by dermoscopy was performed. The results were tabulated and then analyzed statistically.
Participants. The study included 52 patients with AA of either sex and all age groups. Patients whose diagnosis was unclear and those who received treatment for their AA in the month prior to the study were excluded.
Results. The mean age of presentation was 22.8±12.1 years. The most common dermoscopic finding was presence of black dots (BD) seen in 82.7 percent of patients; the least common was tulip hairs (TH), seen in 9.6 percent. Significant associations between yellow dots (YD) and broken hair (BH) and severity of alopecia was observed. There was a significant correlation of alopecia areata disease pattern with YD and BH (p<0.05).
Conclusion. This study highlights the potential use of dermoscopy in AA as a means to understand various disease characteristics that can act as predictors of severe disease or poor prognosis.
KEYWORDS: Alopecia areata, dermoscope, patterns, dermoscopic findings
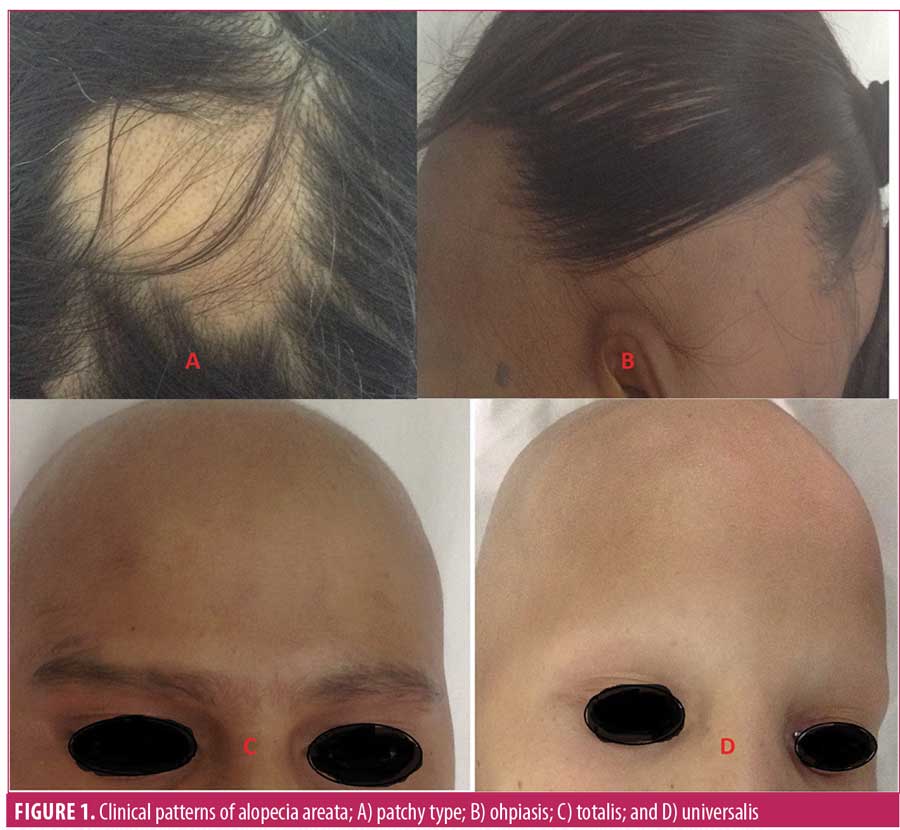
Alopecia areata (AA) is a common form of nonscarring alopecia involving the scalp and/or body, characterized by hair loss without any clinical inflammatory signs. It is considered a hair follicle-specific autoimmune disease, triggered by environmental factors in genetically susceptible individuals.1 It accounts for 25 percent of all the alopecia cases and 0.7 percent of all new dermatology outpatients in India.2 Both male and female patients are equally affected and it can occur at any age, though in most patients the onset is within the first three decades of life.1 Alopecia areata can present clinically as patchy alopecia, diffuse alopecia, reticulate alopecia, ophiasis, ophiasis inversus, alopecia totalis (i.e., loss of all hair on the scalp), or alopecia universalis (i.e., loss of hair all over the body) (Figure 1).3 The clinical diagnosis is based on the presence of well-circumscribed, round, or oval bald patches with a smooth surface and characteristic exclamation mark hair.4
Dermoscopy is a noninvasive tool which aids the diagnosis in uncertain cases of alopecia areata. It allows a magnified view of the subtle patterns of skin lesions which are not visible to the unaided eye.5 Characteristic dermoscopic features in AA include black dots (BDs), yellow dots (YDs), exclamation mark hairs (EHs), and broken hairs (BHs). There is a variation in dermoscopy findings depending on the stage, site, and severity of the disease.6 BDs and EHs are the most specific findings in AA and correlate well with disease activity, whereas YDs are seen in all the stages of the disease and correlate with disease severity.7 The aim of our study was to evaluate the dermoscopic features of AA and correlate these features with disease patterns and severity.
Methods
This observational study was performed over a period of six months, from September 2018 to February 2019, after obtaining clearance from an ethical committee of institution. It included patients with AA of either sex and all age groups who presented to the outpatient department of dermatology in a tertiary care center. Diagnosis and categorization of AA was made on the basis of clinical examination. Patients whose diagnosis was uncertain and those who received treatment for their AA in the month prior to the study were excluded. After obtaining informed consent from all patients, their demographic details, duration of disease, and associated complaints were recorded. A thorough clinical examination to assess number, site, morphology and distribution of alopecia patches, and presence of nail changes was performed, along with photographic documentation. All patients then underwent dermoscopic examination.
Severity of AA was evaluated using severity of alopecia tool (SALT).8 Scalp hair loss ranged from S1 to S5 (S0=no hair loss; S1=less than 25 percent hair loss; S2=26%–50% hair loss; S3=51%–75% hair loss; S4=76%–99% hair loss; S5=total scalp hair loss). The loss of body hair ranged from B0 to B2, where B0 represents no loss, B1 some loss, and B2 complete loss. The overall grading included both scalp and body hair loss. Dermoscopic examination was performed using Dermlite DL3N Dermoscope (3Gen, Inc.; San Juan Capistrano, California) with polarized mode of magnification x10. The patches were cleaned and observed through the dermoscope. Features of the lesion and photographs were recorded.
Statistical analysis. The results were tabulated and analyzed statistically using SPSS Software 25.0 version. For calculating frequencies and finding correlation between different variables, Chi square test, Fisher’s exact test, and Spearman rank correlation test were used. Results were considered significant if p<0.05.
Results
The present study included 52 patients with AA. Out of total patients, there were 24 male patients and 28 female patients. The mean age of presentation was 22.8±12.1 years, the youngest being four years and the oldest being 50 years. The mean duration of disease was 9.5±18 months. Table 1 shows detailed demographic data of study population. The clinical patterns observed were localized patchy (single patch) in 17.3 percent, multiple patchy (>1 patch) in 53.9 percent, ophiasis in 9.6 percent, totalis in 7.7 percent, and universalis in 11.5 percent.
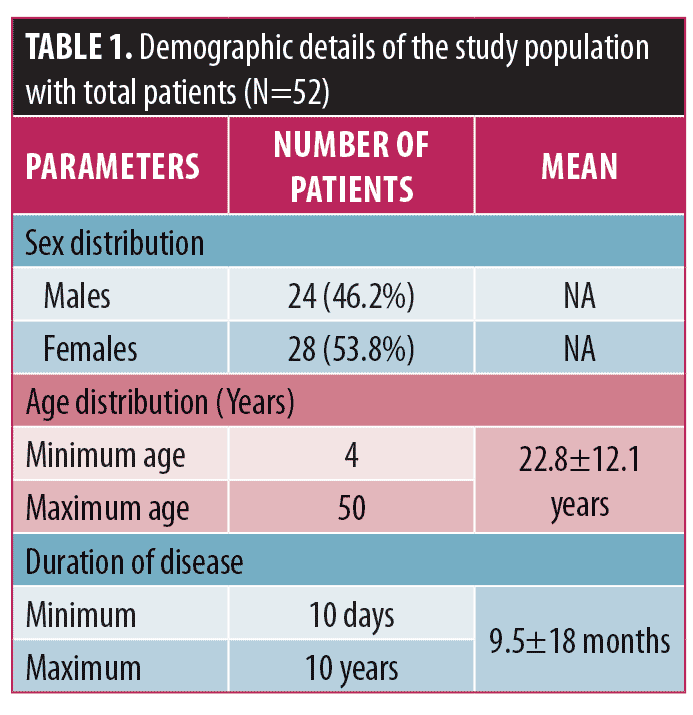
According to the SALT score, 26.9 percent (n=14) of patients scored Grade S1 severity in scalp AA, 21.2 percent (n=11) scored Grade S2,13.5 percent (n=7) scored Grade S3, 11.5 percent (n=6) scored Grade S4, and 11.5 percent (n=6) scored Grade S5. Grading of body hair loss, which included all body parts other than the scalp, scored 57.8 percent (n=30) of patients in Grade B0, 30.7 percent (n=16) in Grade B1, and 11.5 percent (n=6) in Grade B2 (Table 2). The overall grading of scalp and body hair alopecia is shown in Figure 2. Grade S0B1 signifies presence of alopecia over other body areas, such as the beard, eyebrows, or no loss of hair over scalp region. The majority of the patients (21.1%, n=11) belonged to the Grade S1B0 group, and the smallest group was the Grade S3B1 group (1.9%, n=1). Underlying systemic diseases were observed in 13.4 (n=7) percent of total patients. Out these, 7.6 (n=4) percent patients had hypothyroidism, 1.9 (n=1) percent had diabetes mellitus, and 3.8 (n=2) percent had hypertension.
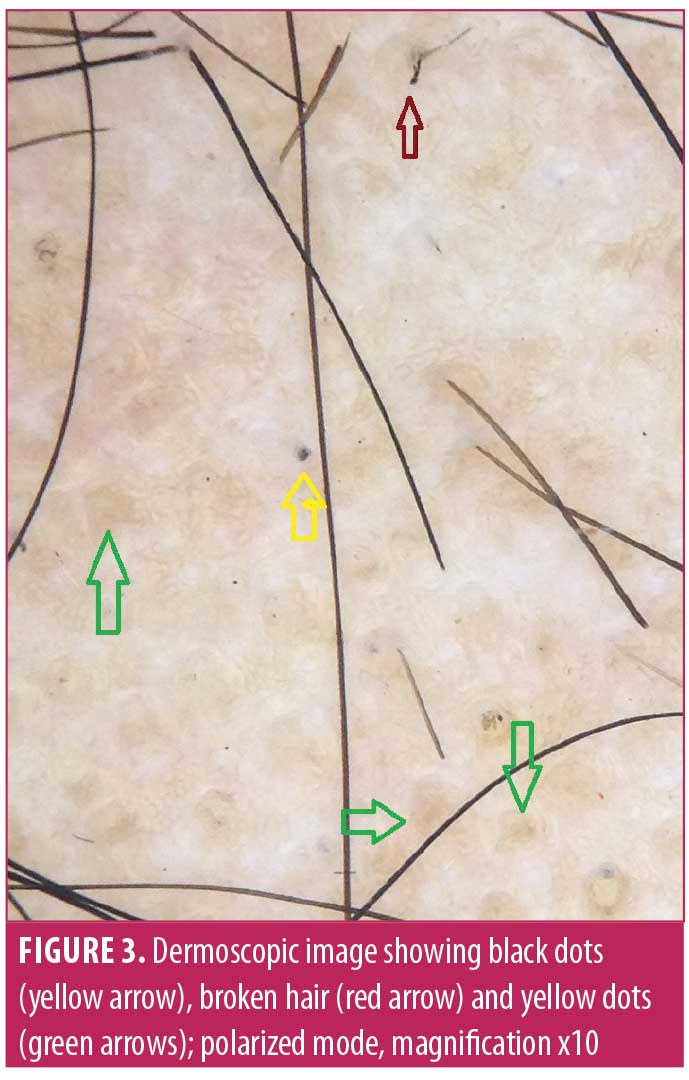
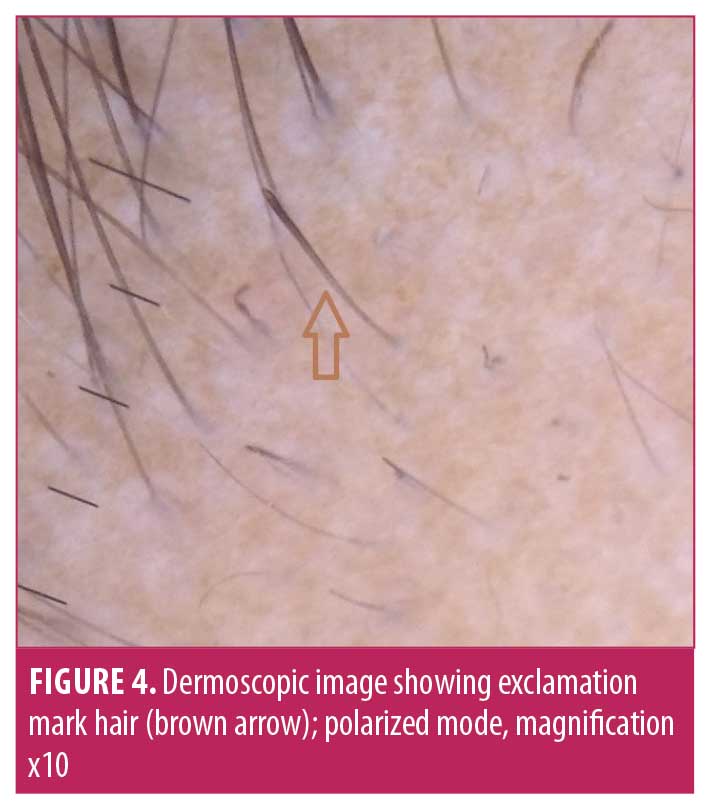
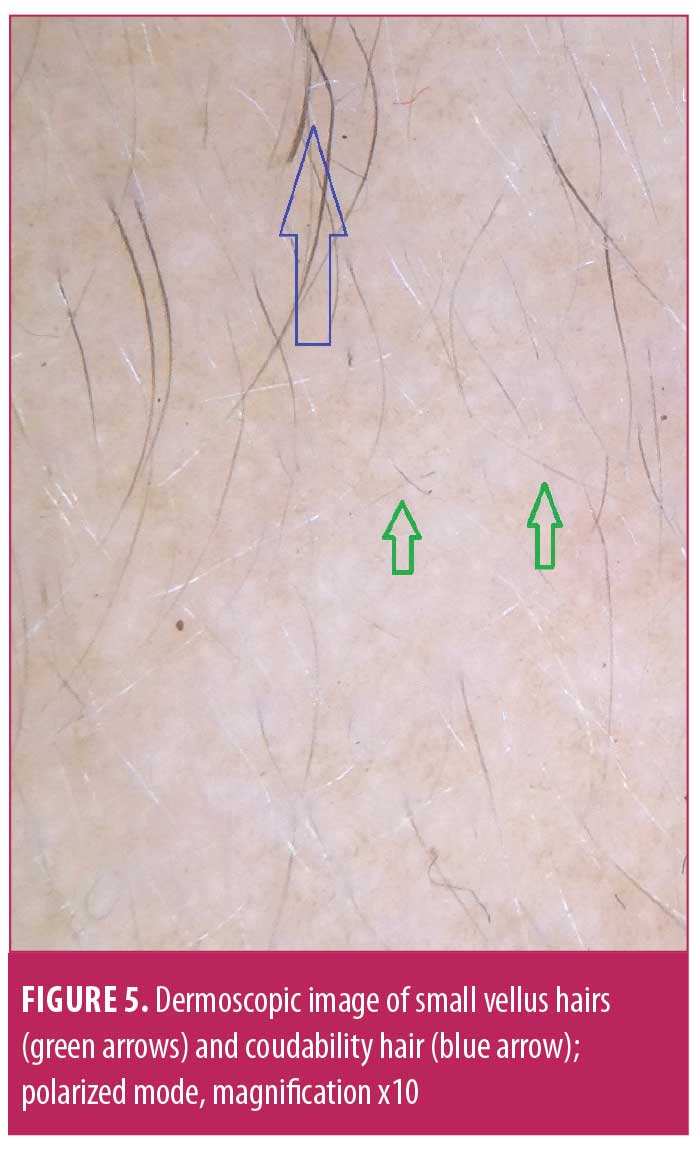
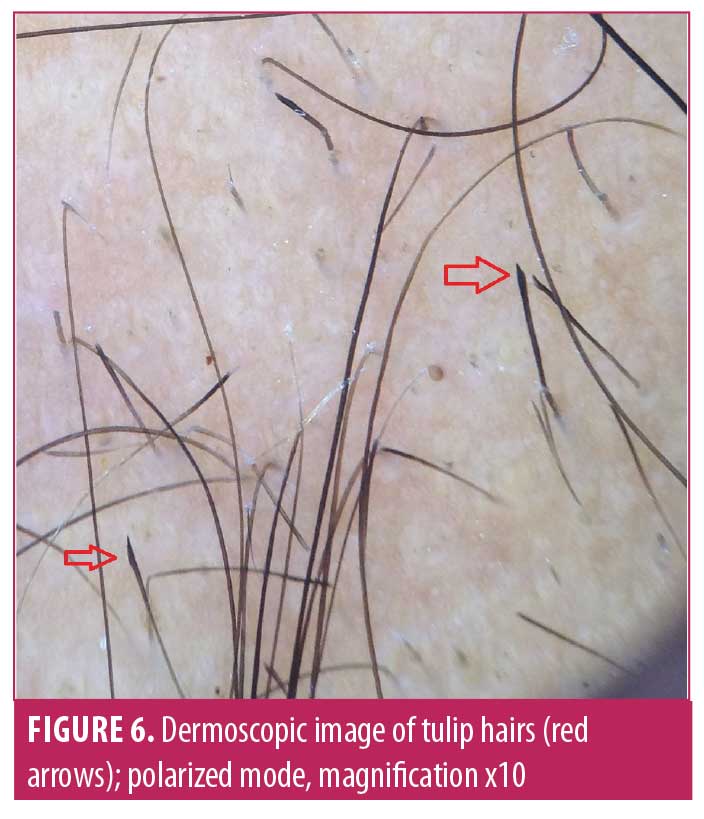
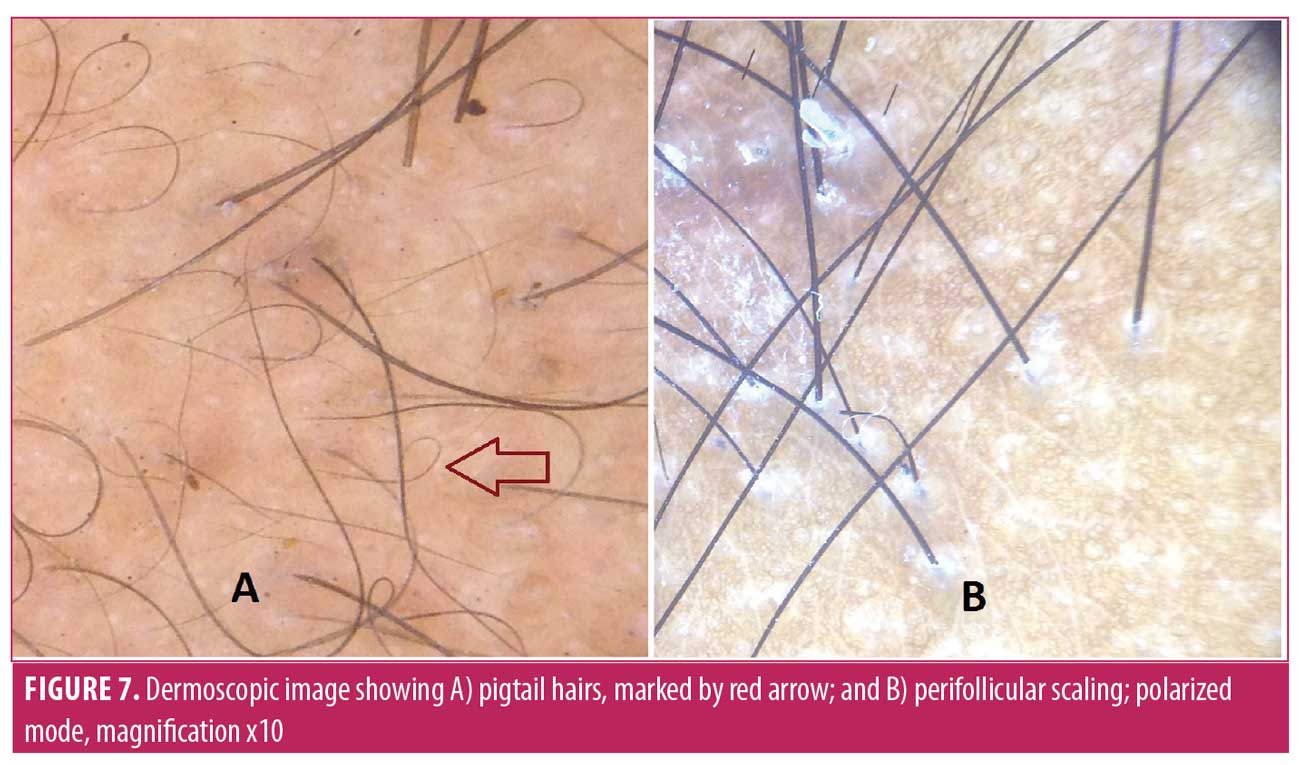
Characteristic dermoscopic findings.Dermoscopic findings detected using Dermlite DL3N dermoscope included YD in 61.5 percent (n=32) of patients, BD in 82.7 percent (n=43), BH in 69.2 percent (n=36), EH in 26.9 percent (n=14), small vellus hair (SVH) in 57.7 percent (n=30), coudability hair (CH) in 36.5 percent (n=19), tulip hair (TH) in 9.6 percent (n=5), pigtail hair (PTH) in 28.8 percent (n=15), perifollicular scaling (PS) in 21.1 percent (n=11), and telangiectasia in 15.3 percent (n=8) (Figures 3–7) (Table 2). The most common finding was BD, seen in 82.7 percent of cases and the least common was TH, in 9.6 percent.
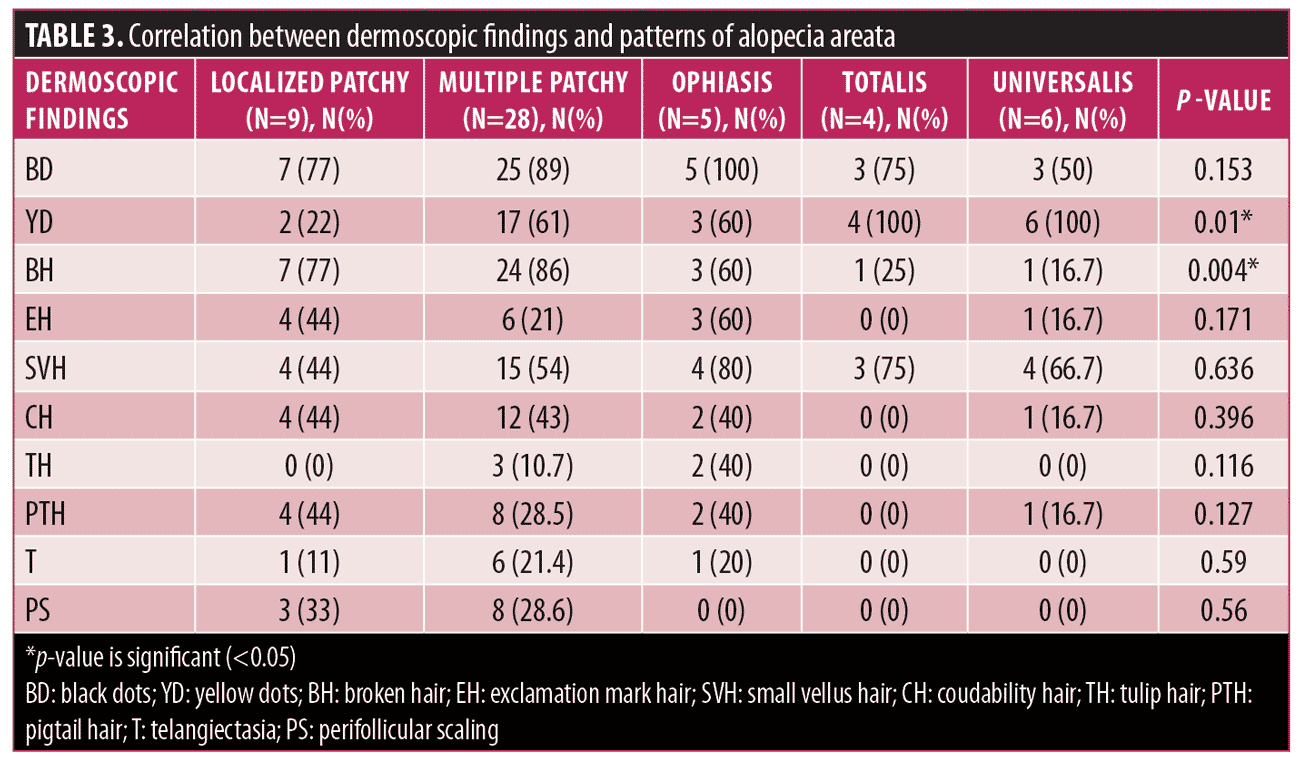
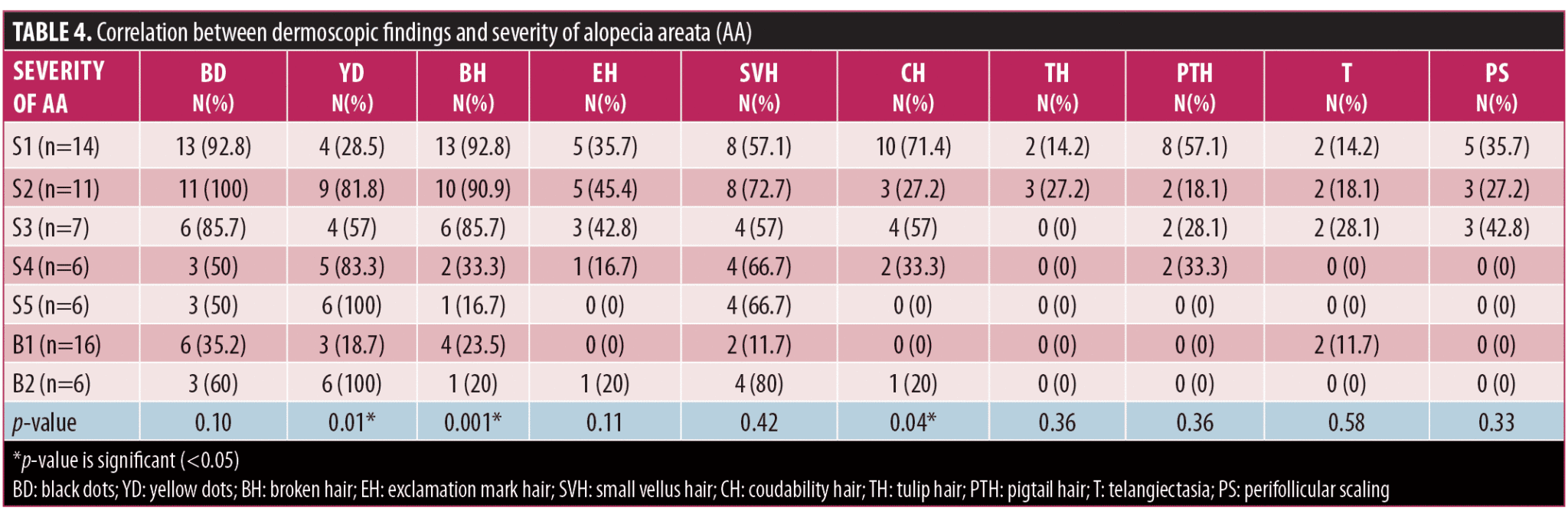
Correlation of dermoscopic findings with patterns of AA and severity of AA. There was a significant correlation of patterns of AA with YD and BH (p<0.05). YD were seen in all patients with alopecia universalis and totalis (Table 3). We also calculated correlation between severity of scalp and body hair alopecia and dermoscopic findings. Significant association was found between severity and YD (p=0.01), BH (p=0.001), and CH (p=0.04), as depicted by Table 4 using spearman rank correlation test.
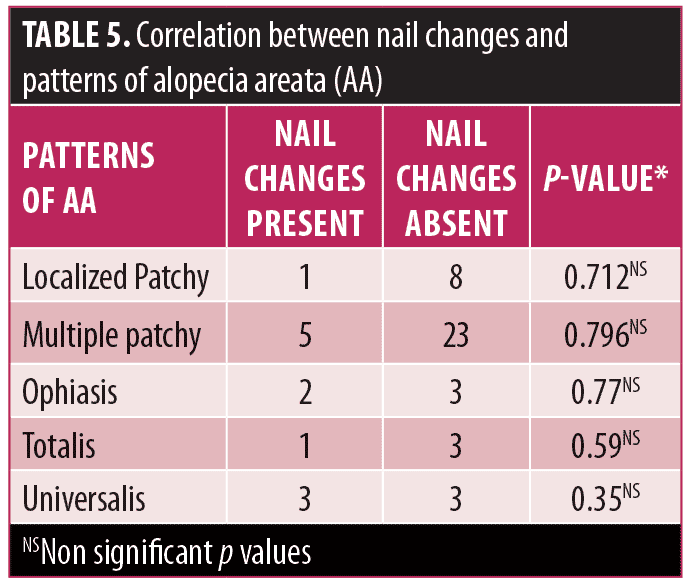
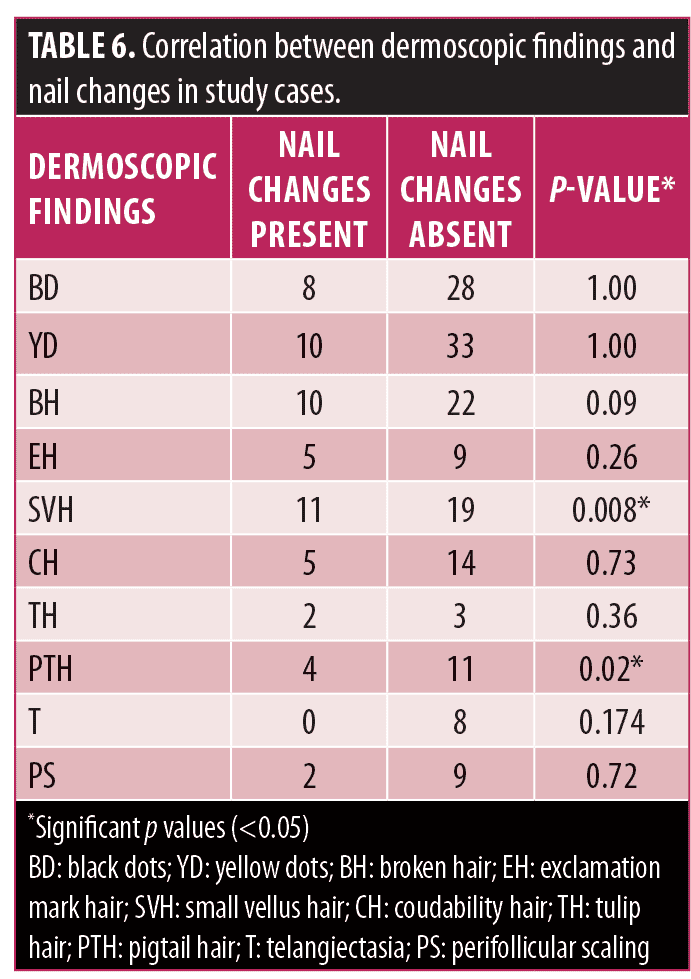
Correlation of dermoscopic findings with nail changes in AA. Nail changes were observed in 23 percent (n=12) of total patients. Changes were observed in the form of nail pitting (15.4%), trachyonychia (1.9%), nail plate thinning (3.8%), and leuconychia (1.9%). Association was calculated between nail changes and patterns of AA and results revealed no significant association (Table 5). Further, nail changes did not show any significant association with severity of alopecia. However, there was significant association of nail changes with SVH and PTH with p<0.05 (Table 6).
Discussion
Dermoscopy has been established as a useful noninvasive diagnostic tool for AA. Common dermoscopic findings of AA are YD, BD, SVH, EH, and BH. The most common pattern of AA in our study was patchy alopecia (localized and multiple) seen in 71.2 percent of patients. Patchy alopecia is the most common pattern seen in various dermoscopic studies of patients with alopecia areata.4,9,10
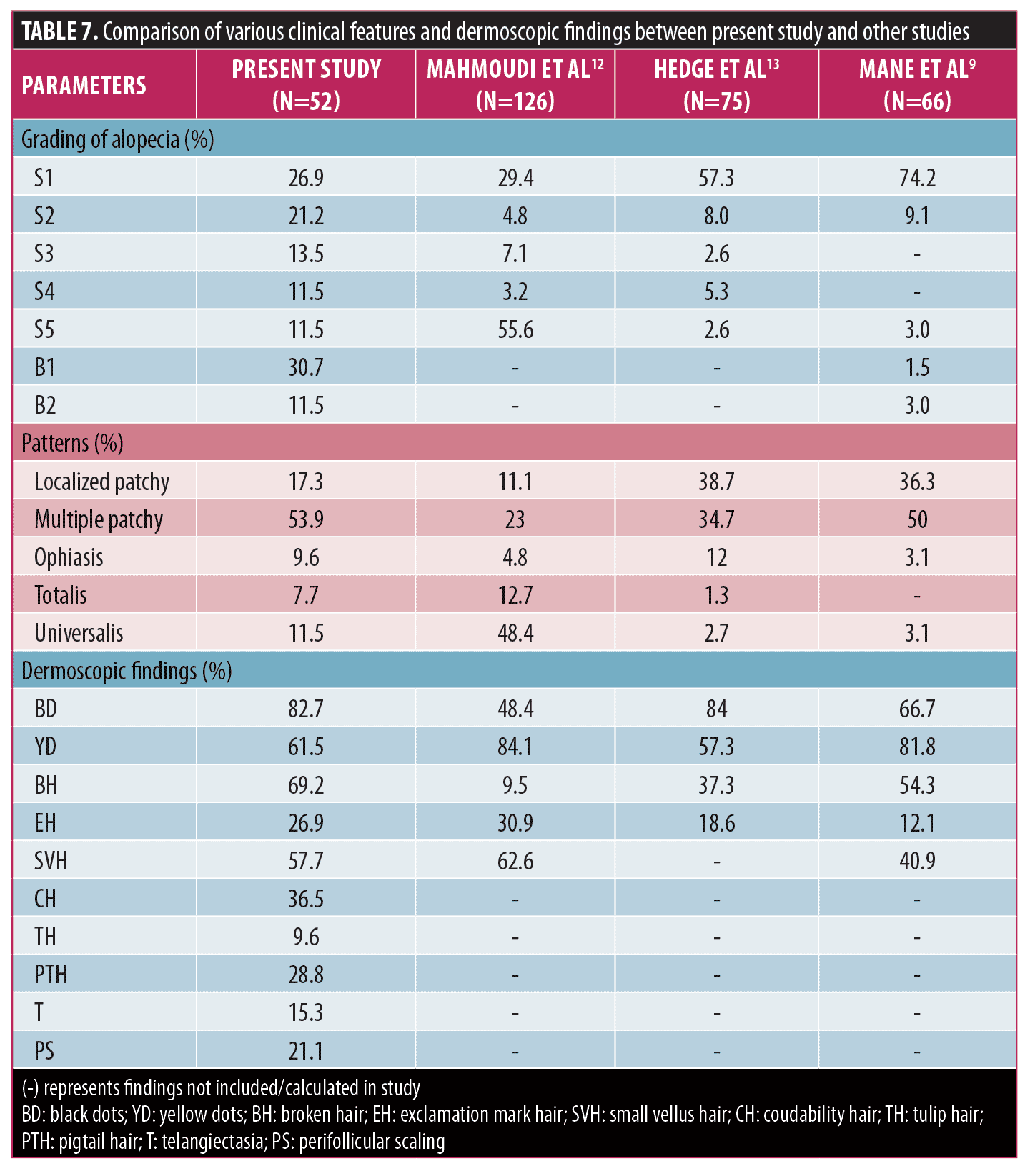
Grades S1 and B1 severity was found in 26.9 percent and 30.7 percent of patients, respectively, in the study population as determined by SALT score, followed by Grade S2 in 21.2 percent of patients. In study by Bapu et al,11 the most common severity was S1 (72.4%). Mahmoudi et al12 observed 53.3 percent of patients with S5 severity; many of their patients were selected from the diphenylcyclopropenone clinic.12
In our study, YD were observed in 61.5 percent of cases, BD in 82.7 percent, BH in 69.2 percent, EH in 26.9 percent, SVH in 57.7 percent, CH in 36.5 percent, TH in 9.6 percent, PTH in 28.8 percent, PS in 21.1 percent, and telangiectasia in 15.3 percent. A significant finding in our study was the correlation of different dermoscopic findings with different patterns of AA and severity of AA. It was demonstrated that all patients with alopecia universalis and totalis pattern had YD. As YDs are related with scalp severity, this significant association between YD and universalis and totalis pattern is rational. In study by Mahmoudi et al, YD and vellus hair findings were most common in patients with universalis pattern, while in the study by Bapu et al’s study, YDs were most common in patients with universalis and ophiasis patterns. Inui et al10 and Mane et al9 could not find any association between dermoscopic findings and pattern of AA. In our study, BH were mostly observed in patchy multiple pattern, which is in agreement with the study by Mahmoudi et al. In regard to scalp severity, YD and BH related positively to scalp severity. Mahmoudi et al observed that YDs related positively, while vellus hairs, BHs, and EHs related negatively to scalp severity.12 Bapu et al also observed significant association between YDs and scalp severity. In the study by Inui et al, there was a positive correlation between BDs and YDs with scalp severity, but a negative correlation between vellus hair and scalp severity.10 Our results reiterate that YDs are useful severity markers of AA. Since severity of AA is an important prognostic factor, determination of severity markers by dermoscopy is helpful in deciding the optimal treatment protocol for patients with AA.
BDs are destroyed hair in the hair follicle opening and are regarded as specific markers of alopecia disease activity and severity.10 In our study, BDs were the most common dermoscopic feature, seen in 82.7 percent of patients. Inui et al observed BDs in 44.3 percent (133/300) of patients with AA. In addition, 67.7 percent (44/66) of patients studied by Mane et al had BDs, while 84 percent of patients showed BDs in study by Hegde et al.13
YDs are seen as yellow to yellow-pink, uniformly colored, round or polycyclic dots in varied sizes. They represent distension of affected follicular infundibulum with keratinous material and sebum.14 The incidence of YDs in our study was 61.5 percent, which is similar to observations by Hegde et al (57.3%) and Inui et al (63.7%). In contrast, Mahmoudi et al and Mane et al demonstrated YD in 84.1 percent and 81.8 percent of cases, respectively. The low incidence in our study might be attributed to the skin color of our patients, which might make the YDs difficult to perceive. YDs show great sensitivity and are observed in more than 60 percent of the patients of AA.14
BHs are the dystrophic hairs produced either by the transverse fracture of terminal hair shafts weakened by the inflammatory process or by rapid regrowth of the incompletely destroyed hair shaft.12 These are clinical markers of the disease activity of AA.15 In our study, BHs were seen in 69.2 percent of the cases. Some studies have reported lesser incidence.11,12 In our study, patients with multiple patchy pattern showed the highest number of BH (86%), whereas patients with universalis pattern had the lowest (16.7%). Among severity grades, 92.8 percent of patients who scored Grade S1 had BH and 16.7 percent of those who scored Grade S5 had BH. Based on these findings, we concluded that there was a significant correlation of BH with disease severity and pattern of alopecia.
EHs, also called tapering hairs, are characterized by wider diameter in the distal shaft and thinner diameter in the proximal shaft.13 In our study, EH was seen in 26.9 percent of cases, which is consistent with previous studies.4,10 Mahmaudi et al observed a negative correlation between EH and disease severity, but there was no correlation between incidence of EH and severity of AA in our study.
SVHs are the thin, nonpigmented hairs, which might demonstrate early disease remission. In our study, 57.7 percent of patients had SVH. Mahmoudi et al demonstrated a negative correlation between disease severity and SVH. This negative correlation was not seen in our study, but we noted a significant association between nail changes and SVH, which was not observed in any other study. On the basis of this association, it can be concluded that despite remission in disease activity, nail changes persist. Nail changes are difficult to reverse and represent refractory disease.16
Some noteworthy nail changes observed in AA are regularly distributed multiple pits, punctate leukonychia, trachyonychia (i.e., sandpaper nails), beaus lines, red lunulae, and onychomadesis.17 The incidence of nail changes observed in our study was higher than a similar study, where pitting, thinning and trachyonychia were observed in 6.06 percent, 3 percent, and 1.5 percent of cases, respectively.9
PTHs are thin twisted vellus hairs that grow a circular pattern, which are described as uncommon in patients that undergo an acute and diffuse hair loss, such as alopecia, induced by chemotherapy and alopecia areata.18 A study by Guttikonda et al4 reported PTH in 14 percent of cases, whereas Peter et al19 reported PTH in 17.5 percent. These studies were not consistent with our study, where PTH was observed in 28.8 percent of patients.
Coudability hairs show a tendency to bend, producing an obvious kink. These hairs are not pathognomonic of AA, but they are a useful clinical feature and marker of disease activity.20 In our study, a significant association was found between CH and severity of AA. CH were observed in 36.5 percent of cases, which was greater than the study by Guttikonda et al, in which CH was observed in only 14 percent of patients. In our study group, telangiectasia was seen in 15.3 percent of patients, which can be attributed to past topical treatments applied over affected areas. Though presence of telangiectasia in the form of tortous and arborizing blood vessels is a characteristic feature of discoid lupus erythematosus, differentiation between these two entities can be made based on the absence of scarring, presence of hair follicle openings with black dots, and upgrowing short vellus hair in former disease.21
In 9.6 percent of cases, we observed short hairs with darker ends and diagonal hair shaft fractures, known as tulip hairs (THs). THs are seen more in cases of trichotillomania but have also been observed in 10 percent cases of AA.21
Perifollicular scaling was observed in 21.1 percent of patients in our study. As per the literature, perifollicular scaling is encountered more frequently in infectious causes of alopecia, such as tinea capitis or cicatricial alopecias like frontal fibrosing alopecia.22 Upon review of the literature, we could not find any other study of AA with which to compare our perifollicular scaling data.
On the basis of the findings in our study, YDs, BHs, and CHs can be used to assess disease severity. In addition, YDs and BHs show significant association with patterns of AA. Table 7 shows comparisons between our study and the few other dermoscopic studies done in patients with AA. Our study has taken into consideration more dermoscopic features compared to previous studies.
Limitations. One limitation of our study was the small cohort size. More studies need to be done in this field using larger sample sizes. Another limitation of our study was the unequal distribution of patients according to clinical types and SALT score. Similarly, sample size for comparison of certain variables, such as tapering hairs, grey hairs, and coiled hairs, was comparatively small.
Conclusion
Our study demonstrates the usefulness of dermoscopy to assess severity of AA. Based on our results, YDs might be useful severity markers of AA, while CH and BH reflect less severe disease. Another finding in our study was the correlation of different dermoscopic findings with different patterns of AA. Elucidation of dermoscopic features that are highly correlated with severe disease could help in developing dermoscopic predictors of severe disease or poor prognosis. Alopecia universalis and totalis had statistically significant associations with YD, while patchy multiple alopecia showed an association with BH. It is important to necessitate the frequent use of dermoscope in all patients of alopecia areata in order to delineate more findings and to understand their relationship with severity, activity of alopecia, and response to treatment. Nail changes were significantly associated with SVH and PTHs.
To summarize, positive correlation has been noted between various patterns of AA with YD and BH. Significant association was found between severity and YD, BH, and CH. Dermoscopic findings can help in counselling patients regarding disease severity, treatment protocol, and avoidance of superfluous scalp biopsies.
References
- Paus R, Oslen EA, Messenger AG. Hair growth disorders. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine, Seventh Edition. New York: McGraw Hill; 2008. p. 753–777.
- Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in Northern India. Int J Dermatol. 1996;35: 22–27.
- Seetharam KA. Alopecia areata: an update. Indian J Dermatol, Venereol, and Leprol. 2013 Sep 1;79(5): 563–575.
- Guttikonda AS, Aruna C, Ramamurthy D, et al. Evaluation of clinical significance of dermoscopy in alopecia areata. Indian J Dermatol. 2016;61: 628–633.
- Khopkar U. Dermoscopy and Trichoscopy in Diseases of the Brown Skin: Atlas and Short Text, First Edition. India: Jaypee Brothers Medical Publishers; 2012.
- Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: diagnosis simplified. Int J Trichol. 2013;5:170–178.
- Ankad B, Beergouder SL, Panchkattimath VS, Sheik Ahmed M. Trichoscopy of alopecia areata: a diagnostic aide. Hair Ther Transplant. 2014; 4:126.
- Olsen EA, Hordinsky MK, Price VH, et al. Alopecia areata investigational assessment guidelines–part II. National Alopecia Areata Foundation. J Am Acad Dermatol. 2004;51:440–447.
- Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol. 2011;56: 407–411.
- Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: Analysis of 300 cases. Int J Dermatol. 2008;47: 688–693.
- Bapu NG, Chandrashekar L, Munisamy M, et al. Dermoscopic findings of alopecia areata in dark skinned individuals: an analysis of 116 cases. Int J Trichol. 2014;6:156–159.
- Mahmoudi H, Salehi M, Moghadas S, et al. Dermoscopic findings in 126 patients with alopecia areata: a cross sectional study. Int J Trichol. 2018;10:118–123.
- Hegde SP, Naveen KN, Athanikar SB, Reshme P. Clinical and dermatoscopic patterns of alopecia areata: a tertiary care centre experience. Int J Trichol. 2013;5:132–136.
- Lima CS, Lemes LR, Melo DF. Yellow dots in trichoscopy: relevance, clinical significance and peculiarities. An Bras Dermatol. 2017; 92(5): 724–726.
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040-8.
- Kasumagic-Halilovic E, Prohic A. Nail changes in alopecia areata: frequency and clinical presentation. J Eur Acad Dermatol Venereol. 2009;23:240–241.
- Dotz WI, Leiber CD, Wogt PJ. Leuconychia punctata and pitted nails in alopecia areata. Arch Dermatol. 1985;121:1452–1454.
- Esteves Ana LV, Serafini NB, Lemes LR, Melo DF. Circular hairs: nomenclature and meanings. An Bras Dermatologia. 2017. 92(6), 874–876.
- Peter D, George L, Pulimood SA. Trichoscopic features of various types of alopecia areata in India: application of a hand-held dermoscope. Australas J Dermatol. 2013;54:198–200.
- Pirmez R, Piñeiro-Maceira J, Sodré CT. Exclamation marks and other trichoscopic signs of chemotherapy-induced alopecia. Australas J Dermatol. 2013;54: 129–132.
- Rakowska A, Slowinska M, Olszewska M, Rudnicka L. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303–336.
- Khopkar U, Doshi B, Jain N. Trichoscopy in Alopecias: Diagnosis Simplified. Int J Trichology. 2013;5(4):170–158.

