 J Clin Aesthet Dermatol. 2021;14(4):43–48.
J Clin Aesthet Dermatol. 2021;14(4):43–48.
by Angela Yen Moore, MD; Edward L. Lain, MD; Amy McMichael, MD; Leon Kircik, MD;
Andrea L. Zaenglein, MD; Adelaide A. Hebert, MD; and Ayman Grada, MD
Dr. Moore is with Arlington Center for Dermatology and Arlington Research Center in Arlington, Texas and Baylor University Medical Center in Dallas, Texas. Dr. Lain is with the Austin Institute for Clinical Research in Pflugerville, Texas. Dr. McMichael is with Wake Forest Baptist Health in Winston-Salem, North Carolina. Dr. Kircik is with Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Grada is with Almirall, LLC in Exton, Pennsylvania. Dr. Zaenglein is with Penn State/Hershey Medical Center in Hershey, Pennsylvania. Dr. Hebert is with UTHealth McGovern Medical School in Houston, Texas.
FUNDING: This study was sponsored by Almirall, LLC. Writing and editorial assistance was provided to the authors by Regina Kelly, MA, of Peloton Advantage, LLC, an OPEN Health company (Parsippany, New Jersey, USA) and was funded by Almirall, LLC (Barcelona, Spain).
DISCLOSURES: Drs. Moore, Lain, and Kircik have served as investigator, consultant, advisor, or speaker for Allergan and Almirall. Dr. McMichael has served as an investigator for Allergan, Intendis, Procter & Gamble, Samumed, Cassiopea, Concert, and Aclaris and as a consultant for Johnson & Johnson, Procter & Gamble, Allergan, Galderma, Incyte, Samumed, Aclaris, Anacor, Pfizer, Nutrafol, and Bioniz. Dr. Grada is the Director of R&D and Medical Affairs at Almirall (USA). Dr. Zaenglein has served as an investigator and/or consultant for Allergan, Sun Pharma, Incyte, Verrica Pharmaceuticals, Dermavant Sciences, Cassiopea, and Douglas Pharmaceuticals, and has received the following: Pfizer (honoraria, research grants), Incyte (research grants), Allergan (research grant), Ortho Dermatologics (research grant), AbbVie (research grant), and Dermavant (research grants). Dr. Hebert has received the following: honoraria (Pfizer, Allergan, Almirall, Cassiopea, and Ortho Dermatologics); all research grant funds were paid to the medical school (Allergan, Cassiopea, OrthoDermatologics, Galderma, Pfizer, and Anacor).
ABSTRACT: Clinical Trials id. NCT02959970
Background. Acne vulgaris in patients aged younger than 12 years is increasingly common and primarily noninflammatory (i.e., comedonal). Dapsone 7.5% gel is indicated for the topical treatment of acne vulgaris in patients nine years of age or older.
Objective.We sought to evaluate efficacy, safety, tolerability, and pharmacokinetics (PK) of once-daily topical dapsone 7.5% gel.
Methods. This was a Phase IV, multicenter, open-label study in patients with acne aged 9 to 11 years. Patients applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. Patients in the PK cohort applied dapsone 7.5% gel under maximal-use conditions for eight days and a thin layer for the remaining 11 weeks. Lesion counts and proportions of patients with an Investigator’s Global Assessment score of zero points (clear) or one point (almost clear) were assessed. Plasma concentrations of dapsone and metabolites were evaluated after one week in the PK cohort. Safety and dermal tolerability were evaluated.
Results. After 12 weeks, facial acne was clear or almost clear in about 47 percent of patients. Inflammatory, noninflammatory, and total lesions decreased from baseline, with a greater reduction apparent in noninflammatory lesions. Systemic exposure to dapsone in PK patients was low. The overall rate of adverse events was low, and dermal tolerability scores indicated no or mild stinging/burning, dryness, scaling, and erythema.
Conclusion. Once-daily topical dapsone 7.5% gel used for 12 weeks was safe, effective, and well tolerated in preadolescent patients with acne.
Key words: Acne vulgaris, topical, pediatric, children, safety, dapsone
Acne vulgaris affects more than five million Americans and is one of the most common dermatologic conditions seen in adolescents and young adults.1,2 Acne was the primary reason for three million outpatient pediatric visits from 2006 to 2010.3 Although mostly associated with adolescence, acne is being diagnosed more frequently in preadolescents.4–6 According to National Ambulatory Medical Care Survey data, children aged 7 to 11 years accounted for nine percent of office visits for acne made by pediatric patients between 1993 and 2009.7 Children under 12 years of age are now an important segment of the population with acne, perhaps owing to the earlier onset of puberty recently observed.4,5,7,8
Acne in children younger than 12 years old may be characterized by facial lesions that are predominantly comedonal, or noninflammatory.2,5,6,9 Topical medications are the most commonly prescribed acne treatments for this age group.7 Preadolescent acne, characterized by significantly more comedonal than inflammatory lesions (i.e., papules and pustules), has been reported in the literature for some time but has been recognized as clinically significant only recently.5,7,10,11
Once-daily topical dapsone 7.5% gel (Aczone gel 7.5%; Almirall, LLC, Exton, Pennsylvania) has been approved by the United States Food and Drug Administration (FDA) for acne vulgaris in patients aged nine years and older.12 It is an aqueous gel formulation of dapsone, a synthetic sulfone that became available more than 70 years ago as an oral antibacterial and anti-inflammatory treatment for various skin diseases.12,13 In 2005, topical dapsone 5% gel was approved by the FDA for twice-daily topical treatment of acne vulgaris in patients aged 12 years and older.14,15 Both topical formulations were developed because an earlier oral formulation of dapsone was associated with systemic side effects, such as increased risk of hemolysis in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency, which limited its usefulness for managing acne. The topical formulations have shown lower systemic drug absorption, reduced potential for hemolytic anemia in G6PD-deficient patients, and enhanced safety and efficacy.13,16,17
The 7.5% concentration of topical dapsone gel was developed to offer once-daily dosing.18,19 Two pivotal, Phase III, multicenter, double-blind, randomized, vehicle-controlled trials supported the safety and efficacy of dapsone 7.5% gel for acne in patients aged 12 years and older and led to its 2016 FDA approval as a once-daily topical acne treatment in this population.2,19–21 The approval required a postmarketing study to assess safety, tolerability, and efficacy in patients aged 9 to 11 years. The postmarketing requirements also included analyzing pharmacokinetics in at least 16 patients aged 9 to 11 years under maximal-use conditions.21 Subsequently, the indication for dapsone 7.5% gel was expanded to include patients aged nine years and older.
This paper presents the results of the postmarketing study with dapsone 7.5% gel once daily for 12 weeks in 9- to 11-year-old patients with acne.
Methods
Study design. This Phase IV, multicenter, open-label, noncomparative study in the United States (ClinicalTrials.gov identifier: NCT02959970) was conducted between October 2016 and March 2018. The study was adherent with Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by an institutional review board. Patients and their legally authorized representatives were informed of study procedures, and authorized representatives provided written informed consent. Patients completed up to seven study visits, which included screening and baseline visits and assessments at Weeks 1, 2, 4, 8, and 12.
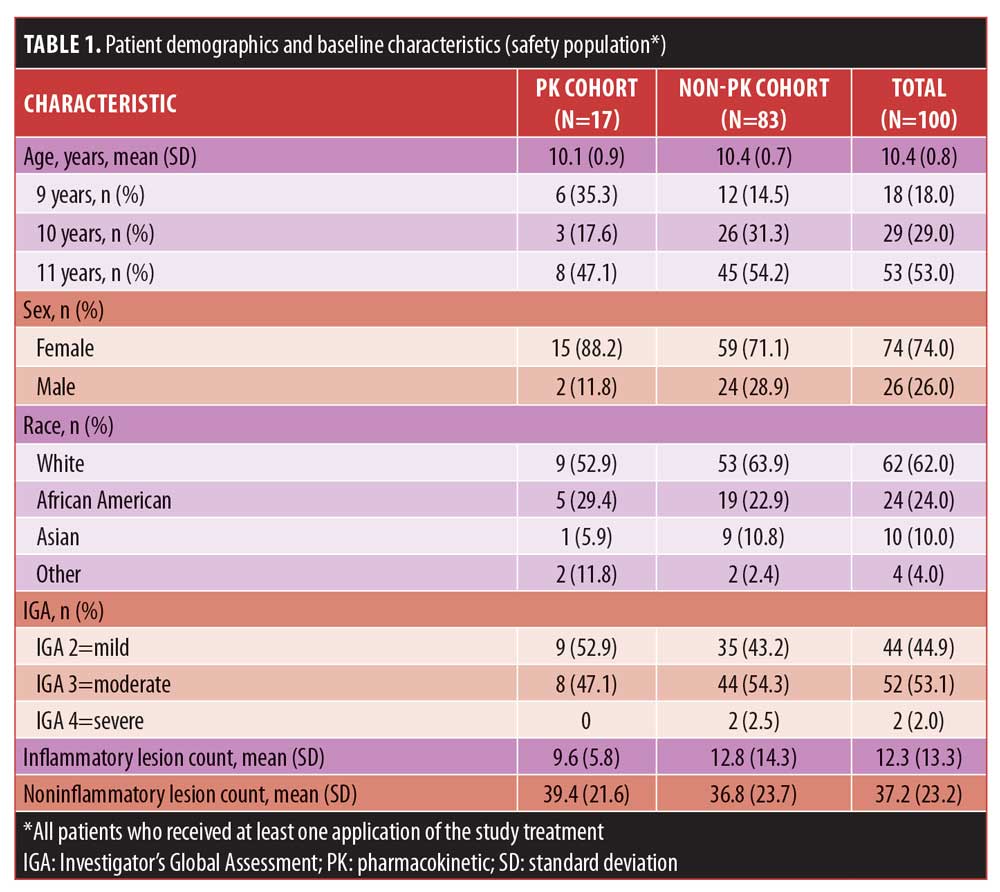
Study population. Eligible patients were aged 9 to 11 years, in good health, with Grade 2 (mild), 3 (moderate), or 4 (severe) acne as assessed by the Investigator’s Global Assessment (IGA) scale, with 20 to 100 total facial inflammatory or noninflammatory lesions. Patients with severe cystic acne, acne conglobata, acne fulminans, secondary acne, or any condition or physical characteristics that could interfere with study assessments were excluded. Patients were not allowed to use the following: hormonal contraceptives; topical dapsone within one month or oral dapsone within two months before screening; systemic immunosuppressive drugs within four weeks before screening or during the study; systemic antibiotics within four weeks before the baseline visit (except penicillins used for two weeks or less); systemic acne treatments within six months before baseline; topical anti-inflammatory drugs, corticosteroids, benzoyl peroxide-containing products, retinoids, or other topical acne treatments within two weeks before baseline; or topical antibacterials, salicylic acid, sulfur, sodium sulfacetamide, energy-based or phototherapy devices, adhesive cleansing strips, or cosmetic procedures within one week before baseline. Sixteen patients consented to additional evaluation of plasma drug concentrations under maximal-use conditions. All 100 patients were evaluated for efficacy, safety, and tolerability.
Study treatment. All patients received dapsone 7.5% gel to apply once daily in the morning to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. Patients in the PK cohort applied dapsone 7.5% gel to the entire face, neck, upper chest, upper back, and shoulders starting on Day 1 under maximal-use conditions (~2 grams/day) for eight consecutive days, then a thin layer to the face and acne-affected areas on the upper chest, upper back, and shoulders for the remaining 11 weeks.
Efficacy analysis. Facial IGA scores and facial lesion counts of inflammatory (papules, pustules, and nodules), noninflammatory (open and closed comedones), and total lesions (inflammatory and noninflammatory) were evaluated at baseline and each study visit. Neither measurement was designated as primary or secondary. The exploratory efficacy assessments were proportions of patients who achieved an IGA score of zero (clear) or one (almost clear) and proportions of patients who achieved both an IGA score of zero or one and at least a two-grade improvement from baseline in their IGA score. Mean reductions and mean percent reductions in facial lesion counts from baseline for inflammatory, noninflammatory, and total lesion counts were measured.
All efficacy analyses were analyzed using observed data without data imputation. Efficacy analyses were based on the modified intent-to-treat (mITT) population, which included all enrolled patients who had a baseline assessment and at least one postbaseline assessment; pooled data from the PK and non-PK cohorts were part of these analyses. No subgroup analyses or inferential statistical tests were conducted.
Pharmacokinetics. On Day 8 (+2 days), blood samples from the PK cohort were collected prior to dosing and at approximately 10 hours postdose (+3 hours) to determine the trough and peak plasma concentrations, respectively, of dapsone and its metabolites, N-acetyl dapsone (NAD) and dapsone hydroxylamine (DHA), using validated liquid chromatography with tandem mass spectrometry. The 10-hour postdose time point was selected to align with the anticipated time to reach the maximum concentration (Tmax) based on a previous study.22 Evaluable PK patients were those who had blood samples for PK analysis, were receiving treatment under maximal-use conditions, and were without major protocol deviations. The lower levels of quantification for the PK analysis were 0.05ng/mL for dapsone and NAD and 0.1ng/mL for DHA. Plasma concentrations of dapsone, NAD, and DHA were reported using descriptive statistics.
Safety and tolerability. Investigators and sponsor medical safety physicians monitored safety throughout the trial. Adverse events (AEs) were recorded and coded using preferred terminology and primary system organ class from the Medical Dictionary for Drug Regulatory Activities (MedDRA). Safety analyses were performed in the safety population (patients who received at least one application of study treatment), without data imputation.
Measurements of local dermal tolerability included investigator or trained evaluator ratings of dryness, scaling, and erythema and the patient rating of stinging/burning on the face, using a rating scale of 0 to 3 (where 0=none, 1=mild, 2=moderate, and 3=severe). These assessments were summarized using descriptive statistics by tolerability parameters and visits.
Results
Patient disposition. The screened and enrolled populations included 105 and 101 patients, respectively. The safety population consisted of 100 patients (PK cohort, n=17; non-PK cohort, n=83). The mITT population had 98 patients, with 16 in the PK cohort. Among the nine (9%) patients who discontinued the study prematurely, the most frequent reason for discontinuation was loss to follow-up (6%).
Demographics and baseline characteristics. Demographic and baseline characteristics of the PK and non-PK cohorts were comparable (Table 1). Most of the population (n=100) was female (74%), White (62%), and non-Hispanic/Latino (74%), although African-American and Asian patients were also well represented (24% and 10%, respectively). The mean age was 10.4 years. Most patients had noninflammatory lesions and a baseline IGA score indicating moderate severity (53.1%).
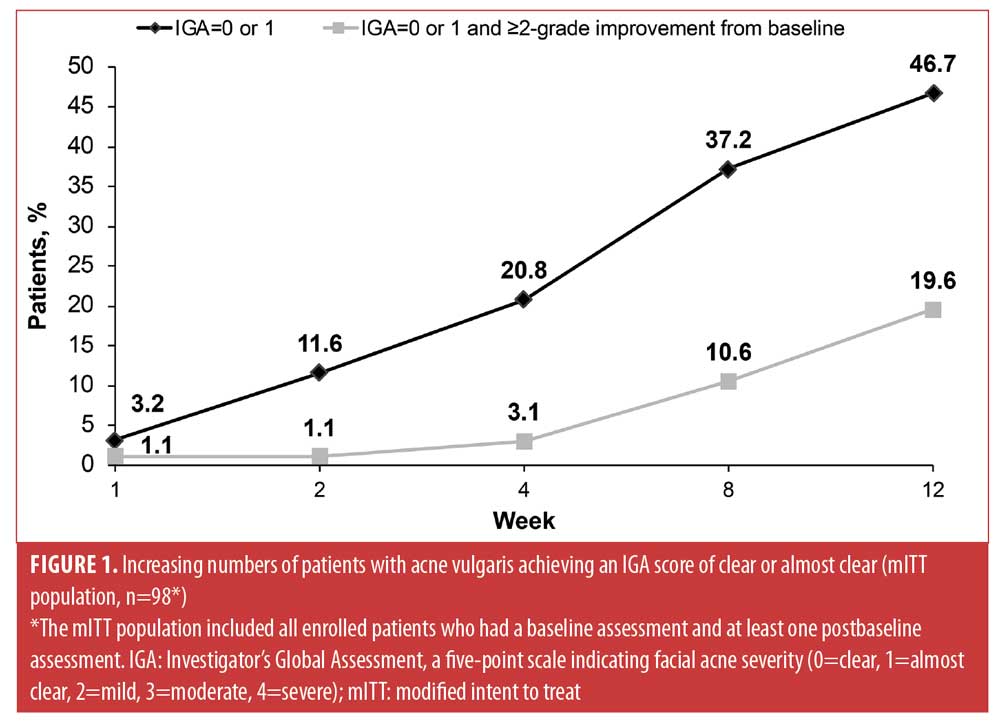
Efficacy. From Week 1 through Week 12, increasing proportions of patients in the mITT population achieved IGA scores of zero (clear) or one (almost clear) on the face (Figure 1). After 12 weeks, 47 percent of patients had faces that were clear or almost clear of facial acne and 20 percent of patients achieved the more rigorous endpoint of both an IGA score of zero or one and at least a two-grade improvement in IGA score from baseline.
Inflammatory, noninflammatory, and total lesions on the face progressively decreased from baseline through Week 12, with improvement observed as early as Week 1 and with a greater reduction in noninflammatory than inflammatory absolute lesion counts (Figure 2). After 12 weeks of treatment, inflammatory, noninflammatory, and total lesion counts decreased by 6.2, 17.8, and 24.0, with respective mean percentage reductions in inflammatory, noninflammatory, and total lesion counts of 56.4 percent, 46.5 percent, and 51.9 percent.
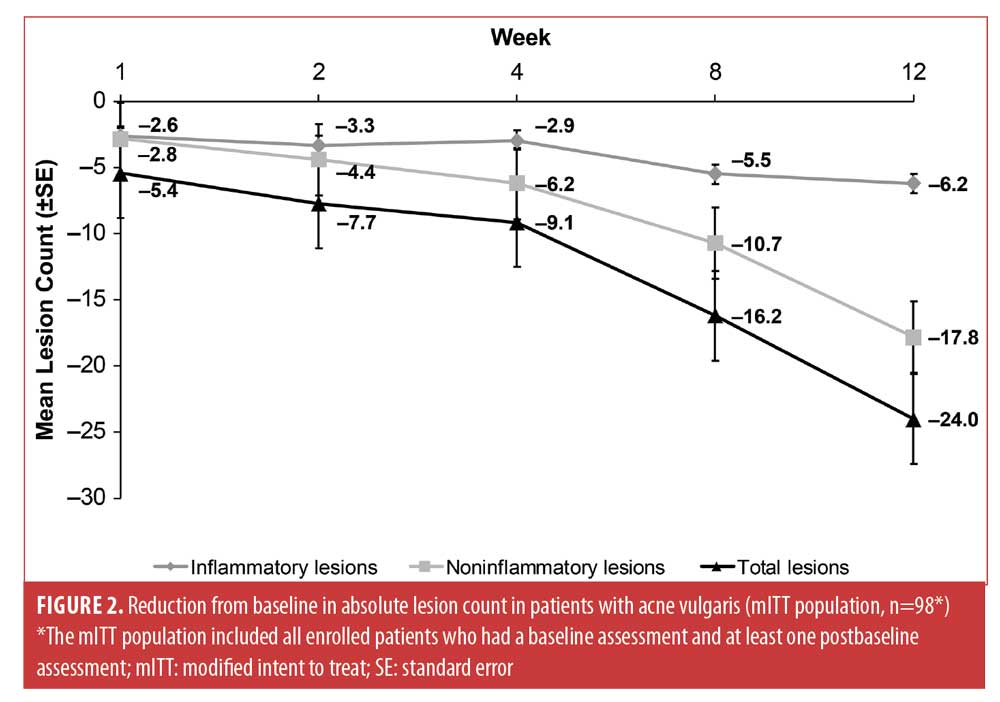
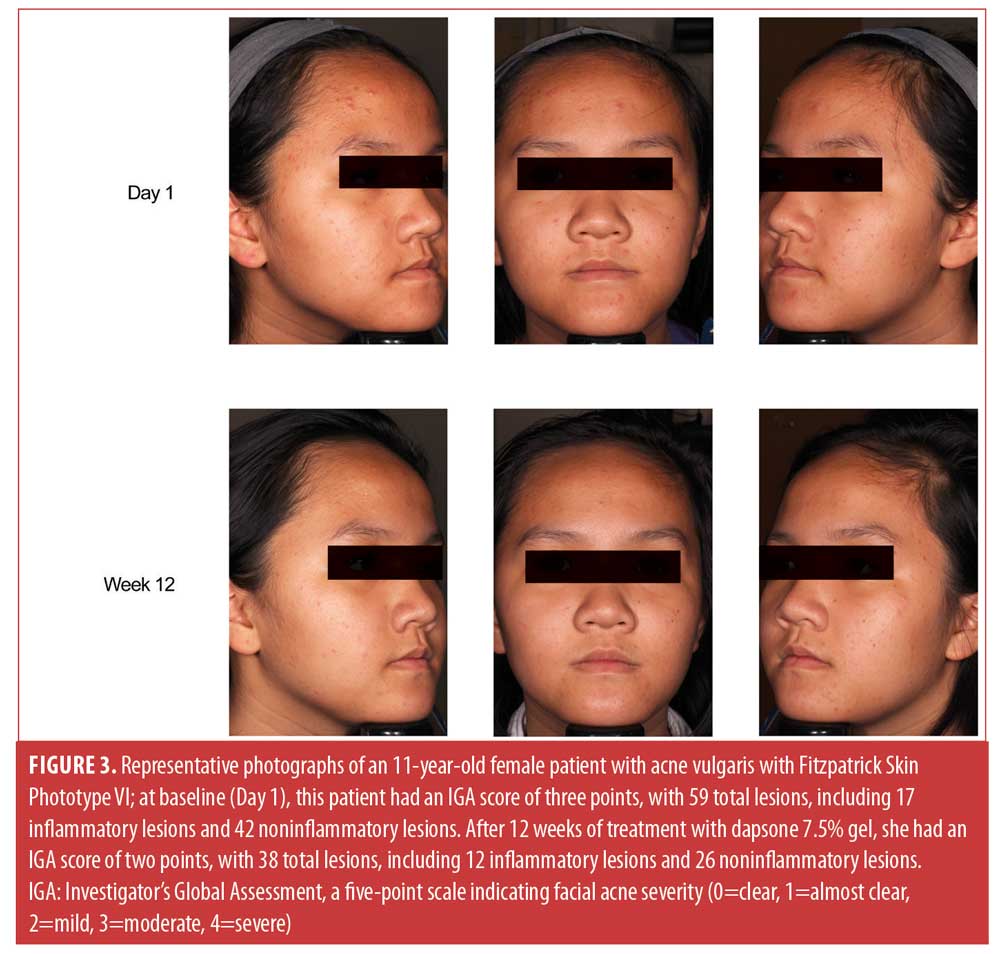
Figure 3 shows representative improvements in acne experienced by an 11-year-old patient treated with dapsone 7.5% gel for 12 weeks.
Pharmacokinetics. Overall plasma concentrations of dapsone and its metabolites were low at the end of Week 1 of maximal dosing in the PK cohort. The approximate mean (standard deviation) trough (predose) and plasma peak (10 hours postdose) concentrations of dapsone and its metabolites, DHA, and NAD, were comparable as follows: 17.2 (14.0) ng/mL and 20.0 (12.5) ng/mL for dapsone, 1.05 (0.979) ng/mL and 1.40 (1.10) ng/mL for DHA, and 7.02 (5.28) ng/mL and 9.01 (7.37) ng/mL for NAD, respectively.
Safety and tolerability. Patients aged 9 to 11 years had an overall low rate of AEs with once-daily use of dapsone 7.5% gel over 12 weeks, including those in the PK cohort treated under maximal-use conditions for the first week (Table 2). Treatment-emergent AEs (TEAEs) were reported by 21 percent of patients. All TEAEs were mild or moderate in severity, and most were reported in a single patient and resolved without sequelae during the study.
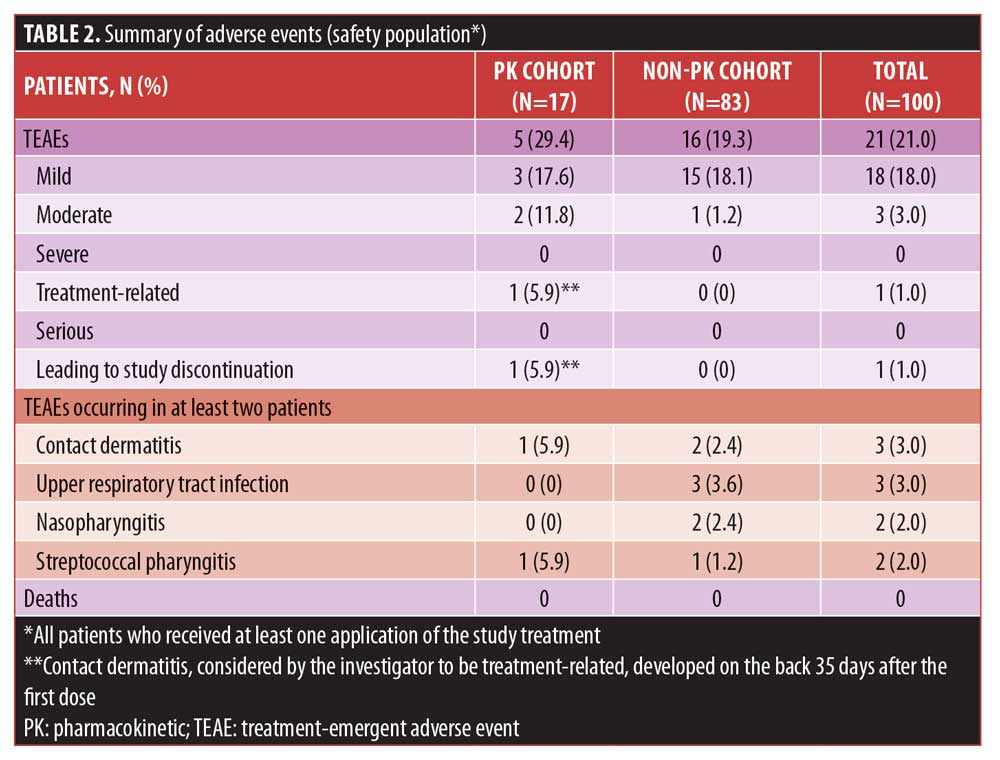
TEAEs that occurred in two or more patients included contact dermatitis (n=3), upper respiratory tract infections (n=3), nasopharyngitis (n=2), and streptococcal pharyngitis (n=2). Of the three cases of contact dermatitis, two were mild and did not lead to study discontinuation, whereas one case was moderate and led to study discontinuation. In this case, the dermatitis developed on the back of a patient in the PK cohort 35 days after the first dose of dapsone 7.5% gel and was considered to be treatment-related. The last dose was applied on Day 36, when the study drug was discontinued and the contact dermatitis was treated with tacrolimus. This TEAE resolved on Day 54, the day that the patient discontinued the study. This was the only TEAE in the study that was moderate in severity and led to study discontinuation. No serious TEAEs or deaths occurred.
For patients in the PK cohort at the end of the maximal-use period (Week 1), the majority of local dermal tolerability scores for stinging/burning, dryness, scaling, and erythema were zero (none) or one (mild). At the end of the maximal-use period, 81.3 to 87.5 percent of patients in the PK cohort and 75.6 to 88.5 percent of patients in the non-PK cohort had a score of zero for all parameters. Overall, scores at Week 12 were similar to or better than baseline scores for all four local dermal tolerability parameters. Across all time points, the majority of patients had a severity rating of none for all parameters.
Discussion
This study demonstrated that dapsone 7.5% gel was effective, safe, and well-tolerated for the treatment of acne in pediatric patients aged nine to 11 years. Systemic exposure to dapsone was low in the PK cohort, who were treated under maximal-use conditions for the first week, further supporting the low risk of systemic toxicity reported in previous PK studies with dapsone 7.5% gel.13,22
Efficacy results with dapsone 7.5% gel in patients nine to 11 years of age were consistent with those from randomized, double-blind, vehicle-controlled, Phase III clinical trials in patients aged 12 years and older, which showed a 54.6-percent reduction from baseline in inflammatory lesions and a 45.1-percent reduction from baseline in noninflammatory lesions after 12 weeks of treatment with dapsone 7.5% gel23 and a 48-percent reduction from baseline in inflammatory lesions and a 32-percent reduction from baseline in noninflammatory lesions after 12 weeks of treatment with dapsone 5% gel.16
In this study, noninflammatory lesions, or comedones, were decreased by a mean of 17.8 lesions after 12 weeks of treatment with dapsone 7.5% gel. The enrollment criteria did not specify a minimum number of inflammatory lesions; indeed, most of the patients in this study had primarily noninflammatory lesions at baseline (mean noninflammatory lesion count: 37.2; mean inflammatory lesion count: 12.3). This is consistent with existing data showing that acne in preadolescent patients is primarily comedonal.2,5,6,9–11 A recent study postulated that the microbiome in preadolescent patients with acne differs from that in adolescents, resulting in more comedones and follicular plugging and less susceptibility to dense accumulation of the bacteria responsible for inflammatory lesions.24 Previous studies have indicated that Toll-like receptor activation and secretion of interleukin (IL)-1alpha from keratinocytes may be initiate steps in comedogenesis,25 and that dapsone suppresses the production of IL-1alpha and IL-8 in human epidermal keratinocytes.26 The results of this study support the hypothesis that the effect of dapsone in acne may be through suppression of IL-1alpha production and comedogenesis.
As adherence tends to be poor among preadolescents relative to adolescents,27 once-daily dosing and a lack of irritation is desirable. In the present study, dapsone 7.5% gel yielded favorable dermal tolerability results, even in the PK cohort under maximal-use conditions.
Three patients developed treatment-emergent contact dermatitis in this study. One of these cases was considered treatment-related and led to study discontinuation; the investigator did not determine whether the contact dermatitis was allergic or irritant in origin. Prior studies of dapsone 7.5% gel did not find contact dermatitis as an AE of concern related to treatment. In the pooled analysis of the dapsone 7.5% gel trials,23 only one of 2,161 patients receiving dapsone 7.5% gel discontinued due to application site acne and dermatitis.23 The pooled analysis of the dapsone 5% gel trials contained no AE reports referring to contact dermatitis.16
Limitations. This study’s limitations include its small study population and the absence of a vehicle control group or comparator arm. Nonetheless, the present study fulfills a need for more data on the efficacy, safety, and side-effect profile of topical dapsone treatment for acne in children.28
Conclusion
Dapsone 7.5% gel, applied topically once daily for 12 weeks, was an efficacious, safe, and well-tolerated treatment for acne vulgaris in patients aged 9 to 11 years. Improvements in efficacy endpoints were observed at all visits. Plasma concentrations of dapsone and its metabolites after once-daily application under maximal-use conditions were low. Findings of this study demonstrate improvement in comedonal lesion counts, which are a predominant facet of acne in this preadolescent population.
References
- American Academy of Dermatology. Acne by the numbers. 2018. Available at: https://www.aad.org/about/burden-of-skin-disease/burden-of-skin-disease-briefs. Accessed: June 12, 2019.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: Implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75(1):228–229.
- Goldberg JL, Dabade TS, Davis SA, Feldman SR, Krowchuk DP, Fleischer AB. Changing age of acne vulgaris visits: another sign of earlier puberty? Pediatr Dermatol. 2011;28(6): 645–648.
- Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131 Suppl 3:S163–186.
- Friedlander SF, Baldwin HE, Mancini AJ, Yan AC, Eichenfield LF. The acne continuum: an age-based approach to therapy. Semin Cutan Med Surg. 2011;30(3 Suppl):S6–S11.
- Davis SA, Sandoval LF, Gustafson CJ, Feldman SR, Cordoro KM. Treatment of preadolescent acne in the United States: an analysis of nationally representative data. Pediatr Dermatol. 2013;30(6):689–694.
- Farello G, Altieri C, Cutini M, et al. Review of the literature on current changes in the timing of pubertal development and the incomplete forms of early puberty. Front Pediatr. 2019;7:147.
- Poole CN, McNair V. Infantile acne. StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2019.
- Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls: an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130(3):308–314.
- Lucky AW, Biro FM, Simbartl LA, et al. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr. 1997;130(1): 30–39.
- Aczone Gel, 7.5% [package insert]. Exton, PA: Almirall; 2018.
- Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: a review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol. 2009;10(4):221–227.
- Drug approval package: Aczone Gel, 5% (dapsone gel, 5%). 2005. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021794s000TOC.cfm. Accessed: June 1, 2016.
- Aczone Gel, 5% [package insert]. Dublin, Ireland: Allergan plc; 2015.
- Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56(3):439.e431–439.e410.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6(10):981–987.
- Al-Salama ZT, Deeks ED. Dapsone 7.5% gel: a review in acne vulgaris. Am J Clin Dermatol. 2017;18(1):139-145.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15(8):962–969.
- Stein Gold LF, Jarratt MT, Bucko AD, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: first of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15(5):553–561.
- Drug approval package: Aczone Gel, 7.5% (dapsone gel, 7.5%). 2016. Accessed: June 12, 2019.
- Jarratt MT, Jones TM, Chang-Lin JE, et al. Safety and pharmacokinetics of once-daily dapsone gel, 7.5% in patients with moderate acne vulgaris. J Drugs Dermatol. 2016;15(10):1250–1259.
- Thiboutot DM, Kircik L, McMichael A, et al. Efficacy, safety, and dermal tolerability of dapsone gel, 7.5% in patients with moderate acne vulgaris: a pooled analysis of two phase 3 trials. J Clin Aesthet Dermatol. 2016;9(10): 18–27.
- Ahluwalia J, Borok J, Haddock ES, et al. The microbiome in preadolescent acne: Assessment and prospective analysis of the influence of benzoyl peroxide. Pediatr Dermatol. 2019;36(2):200–206.
- Selway JL, Kurczab T, Kealey T, Langlands K. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
- Geyfman M, Debabov D, Poloso N, Alvandi N. Mechanistic insight into the activity of a sulfone compound dapsone on Propionibacterium (Newly Reclassified as Cutibacterium) Acnes-mediated cytokine production. Exp Dermatol. 2019;28(2): 190–197.
- Hester C, Park C, Chung J, et al. Medication adherence in children and adolescents with acne vulgaris in Medicaid: a retrospective study analysis. Pediatr Dermatol. 2016;33(1):49–55.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5): 945–973.e933.

