J Clin Aesthet Dermatol. 2021;14(12 Suppl 1):S39–S48
J Clin Aesthet Dermatol. 2021;14(12 Suppl 1):S39–S48
by Astra Farmer, RN, NMP; Gillian Murray, MPharm, PG Clin Pharm INP; Brittony Croasdell, NP, ARPN, CANS; Emma Davies, RN, INP; Cormac Convery, MB ChB, MSc; and LEE WALKER, BDS, MFDS, RCS
Ms. Farmer is with Devon and Cornwall Aesthetics Academy in Exmouth, England. Ms. Murray is with the Clinical Academic Kings College, London in London, England. Ms. Croasdell is Clinical Director at The Fitz® in Chicago, Illinois, United States. Ms. Davies is Clinical Director of Save Face UK in Bath, England. Dr. Convery is with the Ever Clinic Glasgow in Glasgow, Scotland. Dr. Walker is with B City Clinic in Liverpool, England. All authors are founding board members of the Complications in Medical Aesthetics Collaborative (CMAC).
FUNDING: No funding was provided for the preparation of this article.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: The Complications in Medical Aesthetics Collaborative (CMAC) is a nonprofit organization established to promote best patient outcomes through educating clinicians in the prevention, diagnosis, and management of complications that can arise following nonsurgical cosmetic procedures. The organization is a global community sharing information, learning, experience, and data to promote best practices. This article explores how dermal filler vascular events can cause tissue ischaemia leading to a facial wound. Ideally, vascular events will be diagnosed early and amenable to reversal with hyaluronidase if caused by a cross-linked hyaluronic acid. If there is significant and extensive hypoxia to the area, there is delayed diagnosis, or the injected product cannot be reversed, the management should center around optimizing wound care. Both simple and complex wounds will benefit from good care. Patients seek aesthetic treatments to improve how they look and can be vulnerable to poor outcomes. Wounds, if not treated appropriately, can result in permanent scarring or other sequalae, such as post-inflammatory hyperpigmentation. There are many publications addressing vascular events in aesthetic practice, but providing optimal care for facial wounds is not addressed.
KEYWORDS: Vascular occlusion, vascular ischaemia, vascular event, facial ischaemia, facial wound, wound, dermal filler, hyaluronic acid, aesthetic complication
Injection of dermal filler for soft tissue augmentation is a rapidly expanding practice. According to Belezney et al,1 the number of soft tissue augmentation procedures in the United States increased 300 percent from the year 2000 to the year 2017.1 The indications for treatment with dermal fillers include correction of acne scars, volume replacement following traumatic injuries, human immunodeficiency virus (HIV)-related lipodystrophy, age-related volume loss, and body contouring.2 In addition to their increased frequency, tissue augmentation procedures are becoming more complex, with greater volumes being injected as product indications and outcome goals evolve. The contribution of these factors will inevitably result in more vascular events causing facial ischemia.
Common products used for soft tissue augmentation include fat, calcium hydroxyapatite, polymethylmethacrylate microspheres, poly-L-lactic-acid, and cross-linked hyaluronic acid.2 While hyaluronic acid-based fillers (HA) can be readily dissolved by the enzyme hyaluronidase, it can be challenging to remove permanent products. As such, the use of non-HA products pose higher risk for ischemic complications, which typically require wound care.3
When considering the incidence of vascular events, a study by Alam et al surveyed 370 dermatologists,2 the results of which indicated an incidence of “vascular occlusion” correlating to one in 6,410 if a needle was used and one in 40,882 with a cannula. However, statistics in relation to these events vary from study to study. One study found that the relative risk of a vascular occlusion was twice as high among practitioners during their first five years of practicing compared to those in practice longer, indicating clinician experience is a factor in vascular occlusion occurrence.2
The purpose of this guideline is to outline the stages of a vascular event; provide guidance on assessing ischemia, identifying necrosis, and managing these conditions; and describe the wound healing process to assist clinicians in effectively supporting wound healing and optimizing patient outcomes.
Stages of Vascular Occlusion
In the event of vascular compromise following injection with a dermal filler, skin color and gradual deterioration are important markers of issue ischemia severity and how much time has passed since the event. If the vascular flow is disrupted, there are progressive changes and, if caught early, a wound can be averted. Although efficient and swift management is important, there is time to escalate and seek collaborative management if required.3
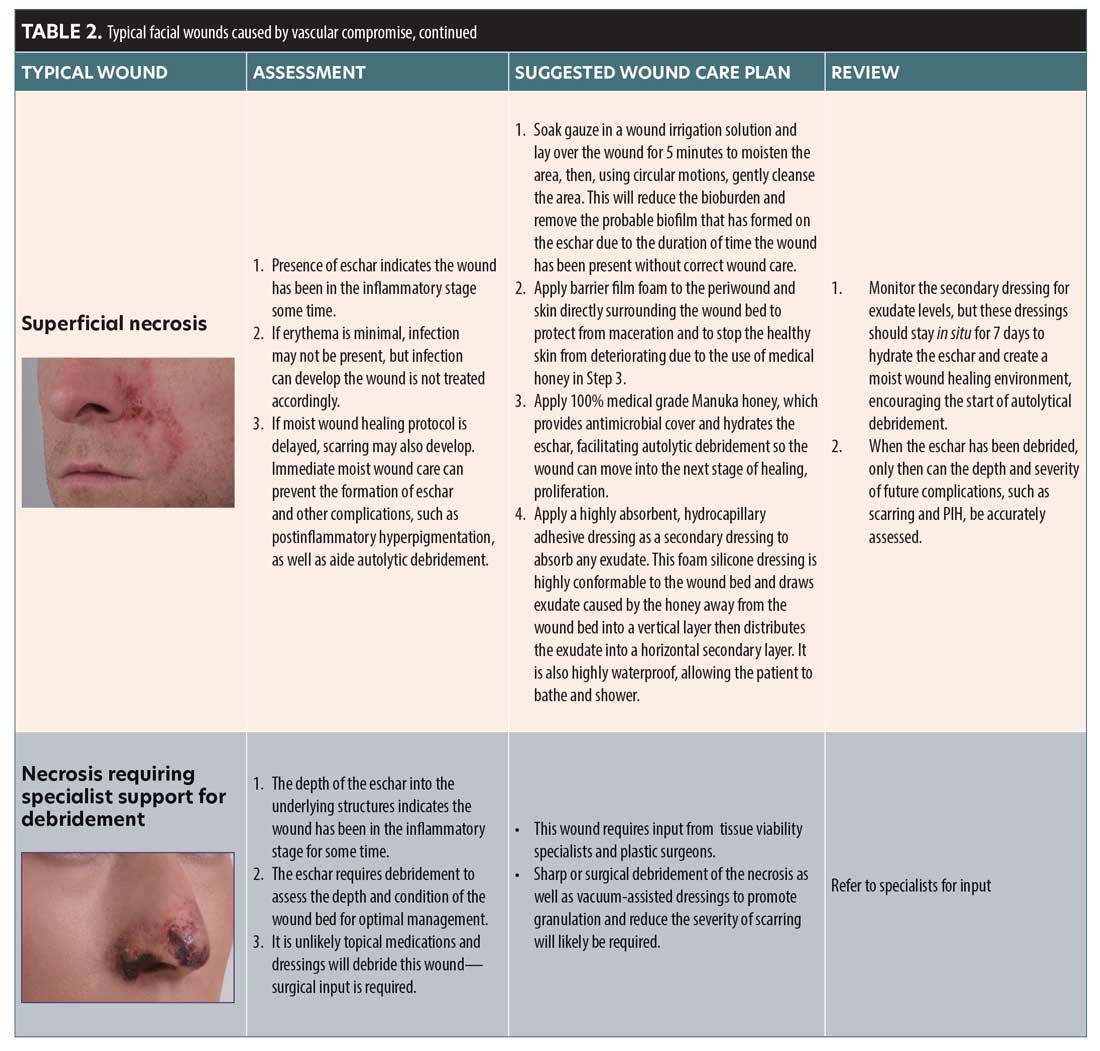
In the CMAC Guideline for the Management of Hyaluronic Acid Filler-induced Vascular Occlusion, stages of vascular occlusion were described to help aid in the understanding of ischemic severity and the likelihood of requiring ongoing wound care (Table 1).3
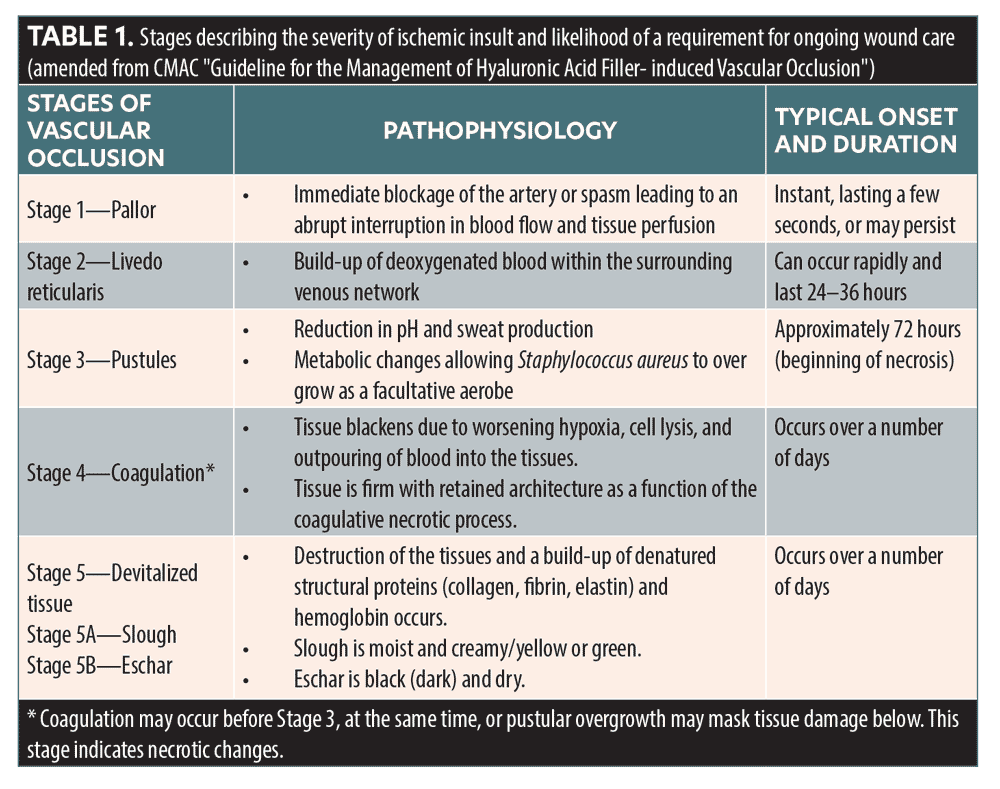
It is important to highlight that pallor and a reticulated appearance (described as Stage 1 or 2) does not necessarily indicate that necrosis will occur.4 If the vascular obstruction is removed at this point, and flow is restored, it is likely that the tissue will resolve to baseline uneventfully. If the vascular occlusion progresses to Stage 3 or beyond, supportive wound care may be required.
A current limitation to the vascular occlusion staging system identified in the CMAC guideline is that depth of ischemic injury is not addressed. The authors found that Stages 4 and 5 can be further complicated depending on which artery has been affected, its anatomical variations/anastomotic connections, and/or formation of sufficient collaterals. Anatomical variations are commonly discussed in the literature.5 Therefore, injury may be limited to the dermis, or it may also progress through deeper tissue structures, such as fat and muscle. Severe ischemic injuries may require escalation to secondary care for surgical management.
An ischemic wound might be covered in necrotic nonviable tissue, which can occur as slough (usually yellow or green) or eschar (dry, hard black tissue).6 Necrosis is usually visible if reperfusion does not occur and the tissue is unable to gain oxygen via sufficient collateral formation. This is usually evident in Stages 3 to 5 if perfusion of the area isn’t sufficient. In a case series of ischemic injuries, Hong et al7 reported that treating ischemic injuries within the first three days is best for optimal intervention and healing the wound without scarring.7 Khalil et al8 observed that whether an ischemic area develops to Stage 3 or beyond depends on individual variation, extent of blockage, vascular anatomic connections, extent of spasm in adjacent arteries, and development of prompt and adequate collateral vessels.8 Less severe superficial sequelae following an ischemic insult may heal uneventfully, even after Day 3, and wound care should only be deployed if required.
In the event of a vascular occlusion, the goals are to establish reperfusion if possible (reverse a cross-linked hyaluronic acid and support the wound healing. It is important to note that tissue damage does not stop at the ischemic insult. If significant ischemia persists for days, the wound will start to break down once the vascular supply is re-established following dissolving the hyaluronic acid filler by formation of collateral flow. Oxygen carried by the blood prompts enzymatic reactions that produce harmful mediators including superoxide, hydrogen peroxide, and hydroxyl free radicals. These free radicals cause damage to the endothelial cells, reduce nitric oxide (NO) levels, and impair neutrophil-mediated killing of bacteria. They further induce production of more inflammatory mediators, causing a localized increase in neutrophils. Intervention is therefore time sensitive, with prolonged ischemia resulting in a more significant reperfusion injury. When necrosis occurs, there will already be a wound on presentation, and reperfusion may accelerate the breakdown of the tissues. While optimal time to reperfusion is well understood in other organs (e.g., cardiac tissue), it remains unclearly defined in skin, underscoring the importance of promptly managing ischemic injury.3 Further research is needed to better understand optimal time to reperfusion of ischemic tissue in the skin.
Physiology and Anatomy of The Skin
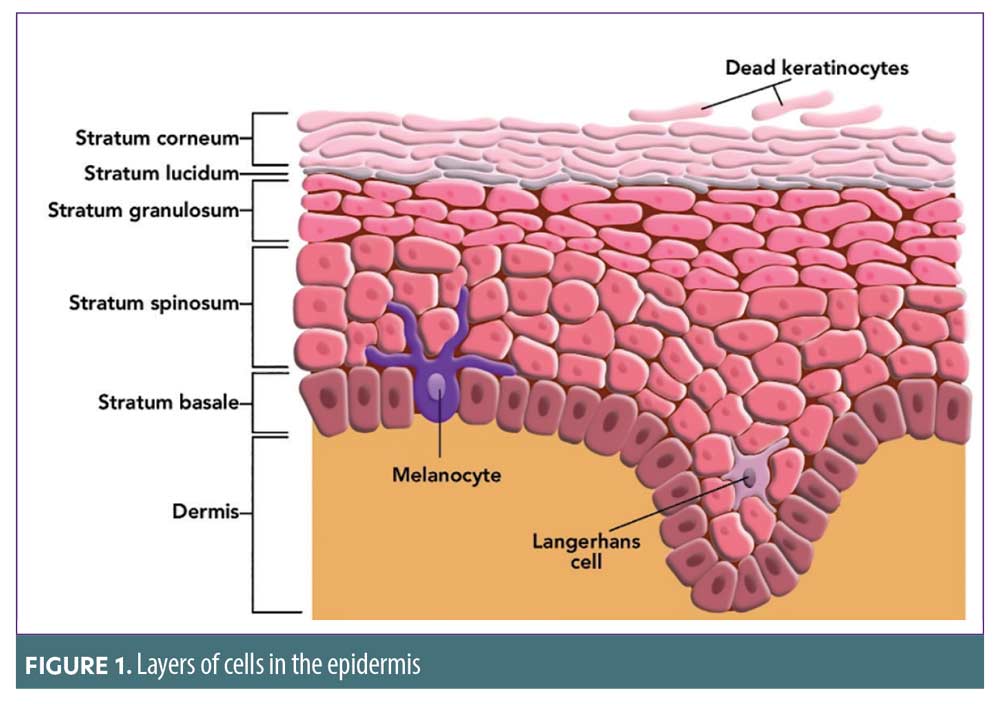
Epidermis. The epidermis, or outer layer, of the skin contains multiple layers of cells, including the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale.10 Figure 1 illustrates the layers of cells in the epidermis. The stratum basale is the deepest layer of the epidermis, and, in healthy uncompromised skin, this layer continually produces keratinocytes, which gradually migrate to the top via all the strata layers. By the time these cells reach the stratum corneum, they have evolved into flat dead skin cells, which are worn away through natural shedding. The stratum basale also contains melanocytes, which produce and contain melanin.10 If there is an over production of melanin due to ultraviolet (UV) damage, hyperpigmentation occurs. Inflammation can also stimulate the over-production of melanin, leading to post-inflammatory hyperpigmentation (PIH), which can occur in all skin types, but more frequently affects individuals with skin of color, including African Americans, Hispanics/Latinos, Asians, Native Americans, Pacific Islanders, and those of Middle Eastern decent.11 PIH can be minimized if good wound care protocol is facilitated, including moist wound healing principles.11
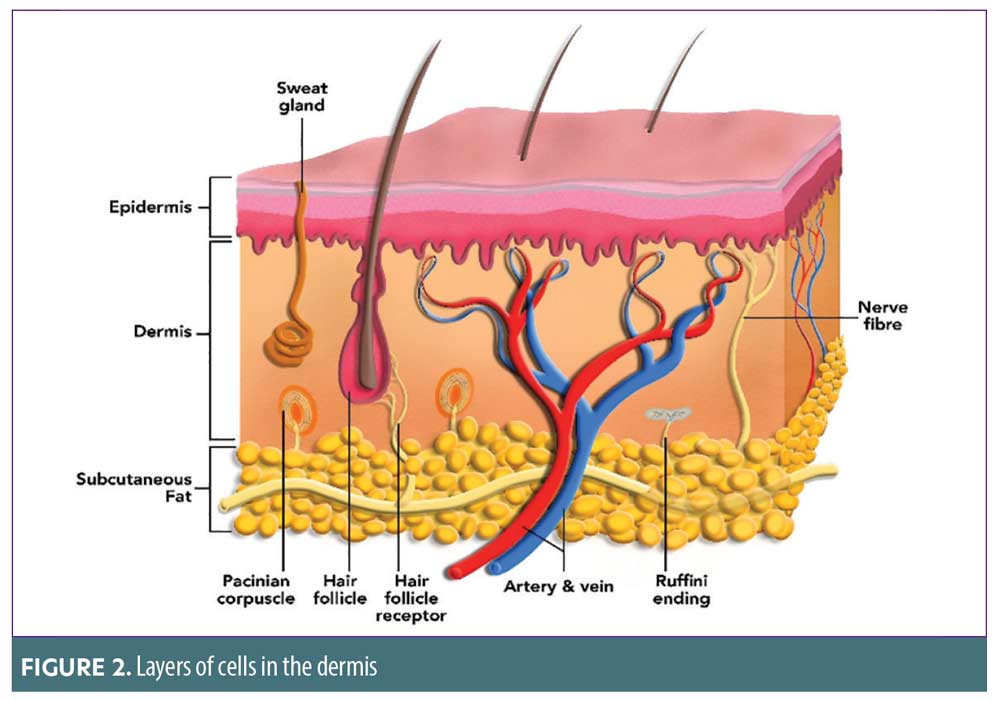
Dermis. The second layer of the skin, the dermis, is composed of connective tissues, collagen, elastin, blood vessels, lymph vessels, nerve endings, sweat, sebaceous glands, and hair follicles (Figure 2). Collagen is a fundamental protein in regard to wound healing by providing structural stability.10 Elastin is intrinsic to giving the skin elasticity and resilience.
Introduction to Ischemic Wounds
There is a lack of research into the breakdown of tissues and formation of wounds following vascular events of the face, and management of these wounds has not been directly addressed in the literature. Ischemic tissue resulting from a dermal filler vascular event can break down quickly (Table 1) and can be painful for the patient. Healing will be delayed if perfusion is not restored. Potential additional trauma to the tissue, due to the following, should also be taken into consideration:
- The initial injecting of the dermal filler
- The hyaluronidase injections, including the quantity and treatment duration
- The firm massage used to try to reperfuse the tissues after hyaluronidase administration
- Any thermal injury that may have occurred through application of heat.
Following a dermal filler-induced vascular event, the longer the ischemia is present with insufficient collateral flow, the more the tissue will be compromised with a risk to deeper structures. Any treatment delay or inability to dissolve the problematic filler may consequently lead to necrosed tissue and a wound. It is paramount to the patient’s recovery that the treating clinician be equipped with a basic knowledge of wound healing.
For optimum patient recovery, administration of hyaluronidase and prescribing of drug-based adjuvants (e.g., antibiotics, steroids, nitrate preparation or phosphodiesterase inhibitors) is often recommended19 However, consideration is not often given to the presence of a wound. Drug adjuvants alone are not sufficient for optimal wound healing.12 The wound should not be allowed to become dry, as this will increase the risk of secondary complications and scarring.17 For the patient to make a full psychological and physical recovery, the tissue must be treated with the correct topical agents for the best possible outcome. Topical agents and dressings that facilitate the restoration of collagen, elastin, and support the extracellular matrix are essential to good wound healing.12 For example, moist wound healing, the benefits of which have been well-documented over the last 40 years,13-18 has been shown to increase the rate of healing and reduce the risk of infection and scarring.
Stages of wound healing (WHS) and optimal management
A wound is a disruption to the epidermis or both the epidermis and the dermis. If an aesthetic clinician is faced with a wound, it is important to understand the stages of wound healing and to identify and assess what, if any, management is required.20 If there is no skin breakdown, there is no wound.
The stages of wound healing, once being described as three stages and evolving to four, differ in their description within the literature. However, the series of events that culminate into the healing of a wound, as we understand them now, are represented the various models described. The classification model chosen to represent wound healing in the context of an ischemic wound does not refer to the hemostasis phase, a phase (or stage) that is commonly included in other wound healing classification models. This is because wounds encountered after dermal filler ischemic events are not inflicted by trauma and present mainly at the inflammation stage of the wound process.21 Thus, in the context of this article, the four stages of wound healing are denoted as inflammation, proliferation, epithelialization, and remodeling.20
A wound that is likely to present acutely after a vascular event, or if the wound has not been managed correctly, will be stuck in Phase 1—the inflammatory stage—and will not progress into healing.12,13
Wound Healing Stage (WHS) 1: Inflammation. The inflammatory stage is the first stage that occurs when the skin integrity is compromised due to the epithelial cells being disturbed. This will occur during Stages 3 to 5 of a vascular occlusion.
Edema, pain and erythema. Cells require oxygen to maintain aerobic metabolism and retain cellular function. As cells become hypoxic, oxidative phosphorylation ceases. Adenosine triphosphate (ATP) stores are depleted, and energy-dependent functions stop. Ion transport is affected, with loss of intracellular k+ and increased intracellular Na+. Glycolysis is initially accelerated, and cells produce large quantities of lactic acid. These variations in ion balance and lactic acid production contribute to cellular edema.23 On assessment, the inflammatory stage will also present as erythematous, painful, and edematous. Pain, in response to skin injury, is referred to as cutaneous neurogenic inflammation. Skin damage activates receptor Types TPRV1 and TPRA1, which are present on primary sensory neurones, keratinocytes, mast cells, dendrites, and endothelial cells. Activation of these receptors stimulates a nociceptive response by cascading the production of substance P and calcitonin gene-related peptide. These neuropeptides have three main targets: smooth muscle of blood vessels (increasing blood flow and causing vasodilation); vascular endothelium (causing an increase in vascular permeability, edema, and recruitment of inflammatory leukocytes); and stimulation of mast cell degranulation (with release of histamine, serotonin, proteases, and other mediators). This cascade of events promote microvascular permeability of blood vessels surrounding the wound (redness and heat) and cause an influx of inflammatory mediators into the area, causing swelling.20,23,24 The blood vessel dilation and series of events also allow essential cells—antibodies, white blood cells, platelets, growth factors, enzymes, and nutrients—to reach the wounded area, which assists in fighting any bacteria present and formation of a platelets plug to protect the wound. This is the body’s natural response to tissue trauma.20
Pustules. In many vascular occlusions, visible white pustules form during the inflammatory phase. This is commonly seen on Day 3 of a vascular occlusion if collateral supply is insufficient or individual anatomy has not presented anastomotic connections with other arteries. The pustule formation around Day 3 is not fully understood, as it only seems to occur on the face. During ischemia, anaerobic metabolism in the tissue is dominant and there is dysfunction of the sweat glands and a reduction of sweat production, increasing the pH and reducing the amount of salt on the skin.3 These factors, combined with the breakdown of normal cellular architecture, allow bacteria to overgrow. Resident bacterial flora penetrate deeper into the dermis, which provides a warm, moist, oxygen-deficient, and nutrient-rich environment where they can thrive.25 Staphylococcus, a normal skin pathogen that is usually harmless if the skin barrier in intact, is facultative, can thrive in oxygen-reduced environments, and is the immediate cause of bacterial overgrowth. The authors consider that the density of pilosebaceous units on the face may explain this phenomenon.3 Pilosebaceous units, in addition to being colonized by Staphylococcus aureus (S. aureus), also contain Cutibacterium acnes (C. acnes), which is a gram-positive anaerobic organism. More research is needed to fully understand the pathology that occurs as part of dermal filler ischemic events.26 If the artery remains compromised, this is when the tissue will begin breaking down further.
Exudate. Exudate is a generic term to describe the liquid produced from a wound. Exudate is generated as part of the inflammatory response and is essential to healing. It is usual to see a small amount of exudation on the apposed edges of the skin, providing a seal to bacteria and debris. A narrow border of erythema around the edge of a wound and some dry surface exudate are normal findings in a wound that is healing well. Exudate is high in protein and nutrients, providing a moist environment in the absence of a protective epidermis. It also contains electrolytes and a number of inflammatory components and supports the ingress of white blood cells.20,27,28
A wound starts producing exudate in the inflammatory stage. The level of exudate produced will depend on the depth and size of the wound and may change over the different stages of the wound. Exudate can differ in composition and characteristics, and can indicate to the status of the wound (i.e., if infection is present). Moderately to highly exuding wounds can affect the periwound (the area surrounding the wound). The periwound can become compromised and macerated, which will impair the healing process. It is important that exudate is managed to create an optimum wound healing environment without disturbing the periwound integrity (Table 2).27
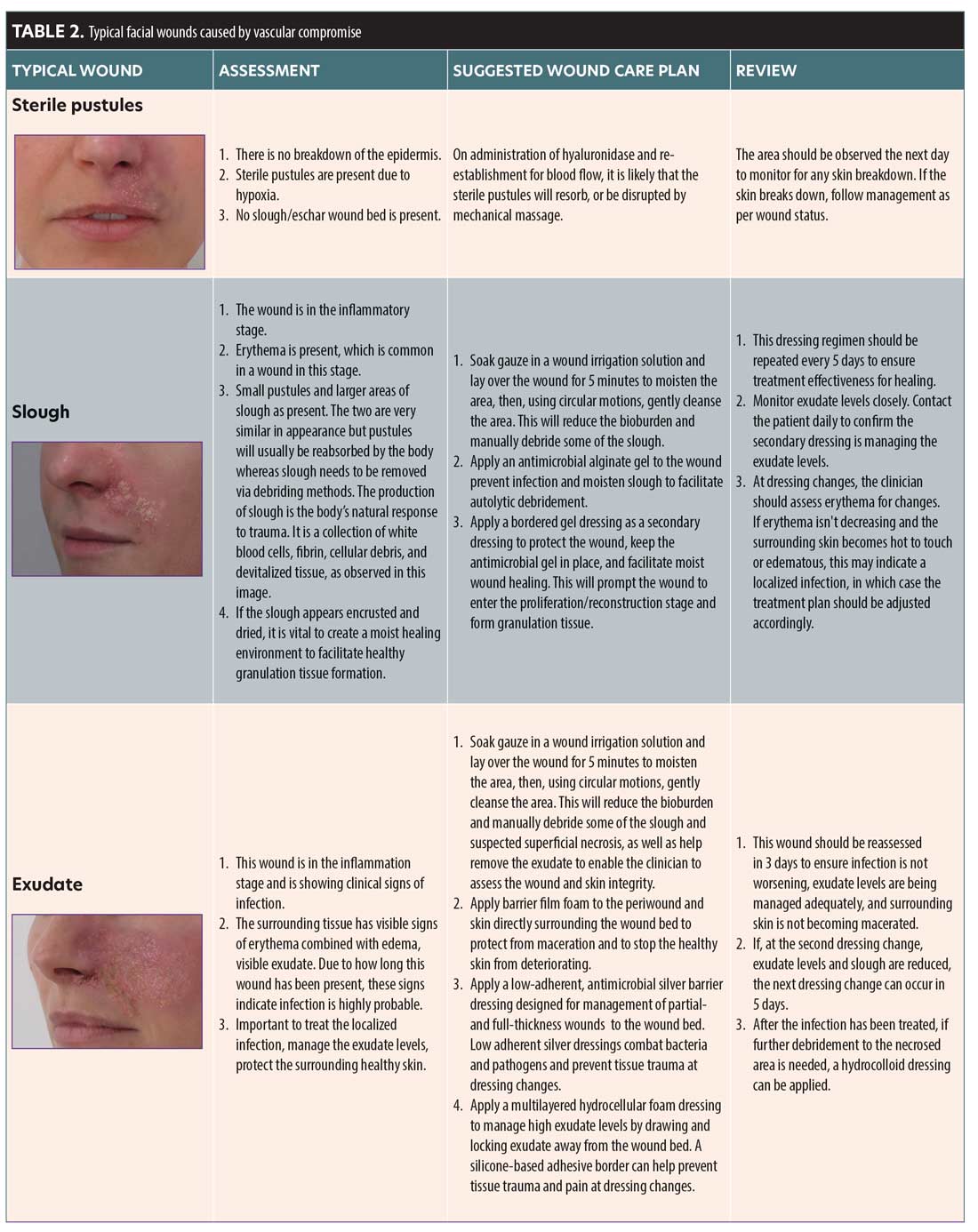
Slough and eschar. Astute observation and prompt action will allow an HA filler occlusion to be reversed, but, in the event of a permanent filler product, slough and/or eschar may be a possibility. Wounds in WHS 1 may remain in this stage of constant inflammation if not properly debrided.28
Slough is a form of nonviable tissue.28 The presence of nonviable tissue is a barrier to effective healing, and removing it reduces the risk of bacterial attachment and biofilm formation. Slough may be recurrent in a wound, so removal may be repeatedly needed. Slough is typically light in color and can be yellow/green if the wound is infected. Slough is loosely attached and contains white blood cells mixed with exudate. It has viscoelastic properties, similar to gel or slime.28
Eschar is necrotic tissue that is dark brown or black in color. It is firmly attached to the wound bed and is dry, housing no exudate or white cells.28 Eschar is a barrier to effective wound healing and can cause infection. If the wound is covered in this leathery substance, topical agents that promote a moist wound healing environment should be used to encourage autolytic debridement. Severe or neglected cases of ischemic injury may require surgical or sharp debridement.28
Use of antibiotics. The inflammation stage of wound healing may be confused with infection, spurring the clinician to commence antibiotic treatment prematurely. However, antibiotic treatment will not heal the damaged tissue, and oral antibiotics should only be initiated if the patient is systemically indicating signs of infection, such as a pyrexia. Many published studies and clinical papers provide evidence that antibiotics should not be a first line treatment for wounds. After analyzing randomized, controlled trials of patients with non-bite wounds, a study by Cummings and Del Beccaro found no evidence to suggest prophylactic antibiotic treatment prevents localized wound infections.29
Use of topical treatments. During WHS 1, it is vital that the correct topical management of the wound and surrounding tissue is commenced as soon as possible to avoid delayed healing. Failure to initiate the appropriate topical management during WHS 1 may result in complications, including biofilm formation, infection, PIH, and scarring.20,22,24 Primary dressings, which include topical creams, gels and ointments, and nonadhesive impregnated dressings, are applied directly onto a wound. They are frequently used underneath adhesive secondary dressings, which are used to hold the primary dressing in place, absorb exudate, and/or protect the wound from further damage. Secondary dressings are adhered to the healthy skin surrounding the damaged area. Silicone adhesive dressings are very versatile, and are suggested if a secondary dressing is required. Silicone dressings are soft and can be removed without causing tissue trauma. This allows the clinician to continually assess the tissue under the dressing in the earlier stages of healing without causing damage to the wound bed.30
WHS 2: proliferation. As the wound begins to heal, angiogenesis takes place and granulation tissue begins to form. The granulation stage typically lasts 2 to 4 weeks depending on the size and depth of the wound bed. For the wound to enter this stage, it must have been treated correctly during the inflammation stage with topical agents that promote a moist wound healing environment, as well as be free of devitalized, necrosed tissue, eschar, and infection.20 If eschar is present on the wound bed and autolytic debridement has failed or the wound is not responding to the topical use of dressings, it is important to refer the patient to a wound care specialist for input. Granulation tissue will always form from the base of the wound upwards, and healthy granulation tissue will always appear pink in color. If the wound is bleeding, dark red, or friable, it indicates an abnormality and can be an indication of infection or that the wound has been disturbed by overcleansing or dressing. When healthy granulation tissue has formed, new capillaries can start to grow. Capillaries are extremely fragile, and it is important to ensure dressing changes are as atraumatic to the tissue as possible to prevent further damage. The wound should only be redressed according to the exudate levels.24
To aid in granulation tissue formulation, a high protein diet and good oral hydration for the patient are needed. Poor water intake leads to less elasticity in the skin. The body continually deposits protein into new cells to enable them to repair and grow. During the proliferation stage, protein deficiency can delay the wound from progressing from the inflammatory phase to the remodeling stage, and fibroblast activity may be reduced.31 The patient should also be educated on the risks of smoking. Nicotine is a vasoconstrictor and reduces nutritional blood flow to the skin and tissues. It also increases platelet adhesiveness and the longevity of tissue ischemia.32
WHS 3: Epithelialization. Once healthy granulation tissue has reached the height of the wound, collagenesis will occur and elastin will form, which will enable the skin to start meeting at the edges. A moist environment is essential for this stage to occur quickly. If the wound is treated optimally during the inflammation and proliferation stages, epithelial cells will form more quickly, which will lead to a better long-term outcome for the patient regarding scar tissue formation.12,13,20,22 Winter, who was the first to understand the benefit of moist wound healing, published his initial findings in 1962. The study demonstrated that healing rates were slower if wounds were left uncovered compared to wounds that were occluded with a polymer dressing to promote a moist environment.14,15 These findings broadened our understanding of an optimal healing environment to increase the rate of reepithelialization.
WHS 4: Remodeling. This is the final stage of the wound healing process in which collagen fibers are synthesized. Elastin formation begins to take place, and the extracellular matrix is restored. The remodeling phase can begin as soon as 21 days after the initial injury or it can be delayed for years depending on the degree of trauma to the tissue and the level and duration of ischemia. There is no consensus as to the timeframe that defines when a wound becomes chronic; chronic wounds, however, fail to proceed through the normal phases of wound healing in a timely manner.20,22 Such wounds are challenging to heal and are at risk of re-occurring infections and biofilm formation. It is important that correct treatment is commenced as early as possible to ensure optimum healing. There is less vascularity during the maturation stage. To ensure collagen synthesis continues, epithelial cells need to be protected and the skin barrier needs to be strengthened by using a good-quality, medical-grade, humectant-based moisturizer and a broad-spectrum sunscreen.12,13,33,34,35
Unless there is considerable tissue loss at the point of presentation, aesthetic ischemic wounds should heal quickly. When presented with an actual or potential wound, clinicians should review the patient’s medical history and identify risk factors that may impair healing.
Factors that Affect Wound Healing
Nutrition. Wound healing requires vitamins, minerals, fatty acids, proteins, and carbohydrates to effectively regenerate. Malnutrition impairs the progress through the stages of wound healing. This may be an issue if the patient has an eating disorder or is otherwise malnourished.12,33
Infection. When the skin barrier is breached, there is the possibility of bacterial infection, which can delay wound healing. S. aureus, S. Spp. and P. aeruginosa are the primary bacteria responsible for wound infections.33 Though inflammation is part of the initial response to a wound, continued inflammation caused by infection can result in leukocyte migration and cytokine release. The leukocytes, being phagocytic, release endotoxins upon engulfing the bacteria, which can result in local necrosis and chronic inflammation. Chronic inflammation affects epithelialization, delays wound retraction, and affect the skin’s ability to mature and remodel.33,34
Age. Age is a major risk linked to impaired healing.34 As we age, the epidermal layer becomes thinner and the inflammatory response decreases. An inadequate inflammatory response results in delayed leukocyte activity, reduced phagocytosis, and decreased growth factor and cytokine release, resulting in delayed angiogenesis, epithelialization, and remodeling.34
Chronic disease. Many chronic diseases can impact wound healing, but diabetes mellitus (DM) is the most problematic given its multifactorial nature. DM impairs leukocyte migration and induces the release of proinflammatory cytokines, which results in chronic inflammation. DM also impacts the microenvironment of the wound, reducing oxygen tissue concentrations and decreasing angiogenesis. It impacts the development of keratinocytes and fibroblasts, which delays epithelialization and wound remodeling.33,34
Obesity is another chronic issue, though less problematic in a face wound. However, obesity leads to reduced microperfusion of the skin, increased release of pro-inflammatory cytokines, and decreased immune response to the area.34
Medication. Drugs that typically interfere with wound healing include those that affect coagulation, have an impact on inflammatory mechanisms, and those that impact on cell proliferation.32 Corticosteroids are of concern if there is a wound; not only do they modulate the immune response, but the anti-inflammatory effect of steroids also decrease in the release of growth factors and cytokines (which modulate other mechanisms of healing) and decrease fibroblast activity.11 Disease modifying antirheumatic drugs and biologic agents may also have a negative effect on wound healing. Chemotherapeutic drugs decrease cellular metabolism and proliferation in cells with a high turnover, specifically the skin. Clinicians should use significant caution when considering dermal filler treatment in patients undergoing chemotherapy.33
Smoking. It is well known that smoking impairs wound healing. Tissue hypoxia is a particular issue. It decreases the proliferation of erythrocytes, oxygenation, blood flow, and angiogenesis in the wound tissue.33,36 Smoking also increases platelet aggregation and adhesiveness, increasing blood viscosity, which results in higher risk of thrombosis. Further, cigarette compounds are involved in decreased fibroblast migration, proliferation, and collagen remodeling. Smoking also augments the immune system by decreasing neutrophils, macrophage, and lymphocyte activity, resulting in a higher risk of infection.32
Genetic predisposition. Cutaneous would healing can be impacted by genetic factors. Keloid scarring, for example, has a strong occurrence in patients with African, Asian, or Hispanic ancestry, with a minor occurrence in the Caucasian population. Ehlers-Danlos syndrome is a cluster of several disorders that affect connective tissue and collagen synthesis, characterized by skin fragility, hyperflexibility, joint hypermobility, and impaired wound healing. PIH is an issue that can manifest due to trauma. It results from the overproduction of melanin or an irregular placement of pigment after cutaneous inflammation. Aesthetic clinicians should take a careful history of all patients prior to initiating cosmetic procedures to determine if a patient is at high risk of complications should an ischemic event or other procedure-related complication occurs that results in a wound. Clinicians should also be equipped to address any PIH that may result.11
Key steps to wound assessment and management
Recognizing factors that might impact wound healing can allow the clinician to foresee possible complications in their patients, as well as explain why healing might be delayed, which may assist clinicians in augmenting the wound management plan accordingly. When ischemic injuries are situated on the face, quality wound care is vital to limit scarring and permanent damage to the tissues. Patients seeking aesthetic treatment are looking for an improvement compared to baseline and may be more sensitive to poor outcome that leave them looking “worse.” To safeguard against potential litigation, thorough contemporaneous notes and standardized photographic images should be taken during each assessment. There should be regular patient follow up with clear communication and instructions. Professionalism should always be maintained. Consider the following recommendations:
- Assess and document the wound bed using the wound assessment tool.
- All wounds should be cleansed using a topical antimicrobial cleansing agent to breakdown the bioburden.
- All wounds should be photographed at each dressing change. Any changes should be documented.
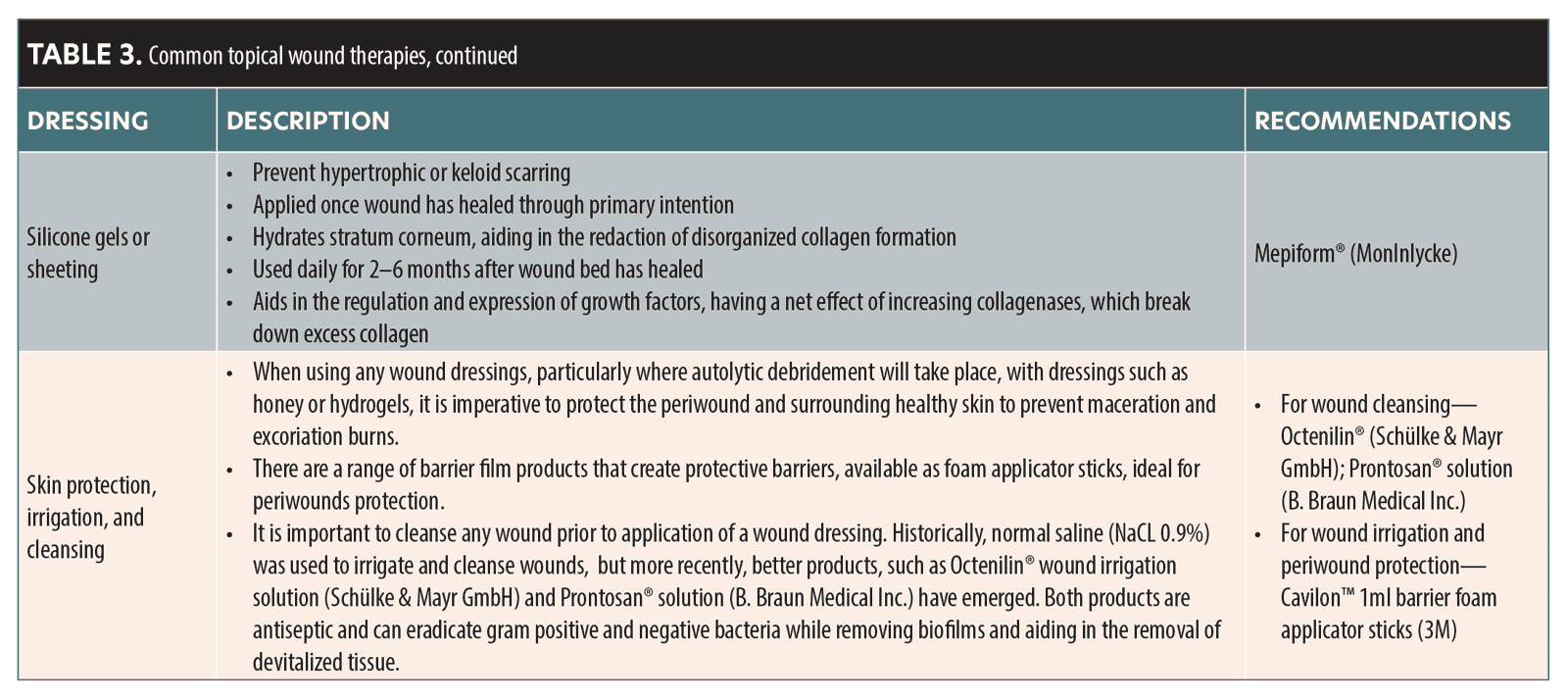
- Consult Tables 2 and 3 to aid in selection of appropriate dressings.
Typical Wounds Seen Post-Dermal Filler Ischemic Event and Recommended Management
The authors have put together a list of typical wounds that have been caused by both reversible and nonreversible dermal fillers. Table 2 gives suggested wound management following the principle of moist wound healing. The choice of dressings and topical agents are available in the UK market, with Table 3 detailing generic information about the dressings and topicals that are available. This should help guide choice in other respective countries. CMAC can guide on appropriateness of dressings and agents.
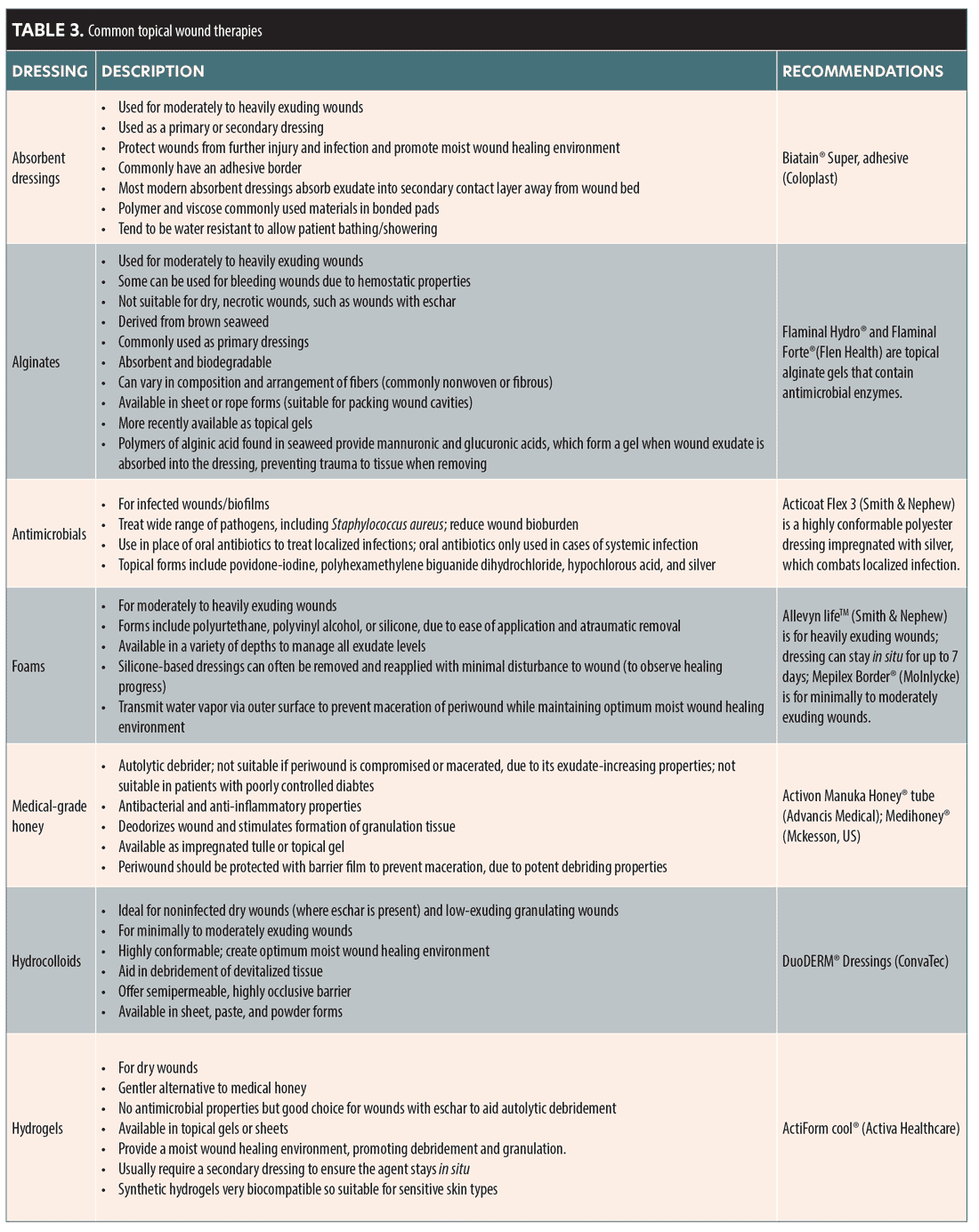
Common topical and wound dressings
Table 3 outlines common topicals and dressings that may be helpful in treating an ischemic wound. The table details the rationale behind the use and how they may be beneficial.
Oxygen therapy
Oxygen is required by all cells. A microenvironment of hypoxia is important for angiogenesis, epithelialization, and synthesis of growth factors. However, it leads to the synthesis of reactive oxygen species and pro-inflammatory cytokines that can impair healing. The correct oxygen concentration is needed to create balance, prevent wound infection, improve angiogenic response, increase fibroblast and keratinocyte production, and ensure proliferation and migration of the skin cells.33
The evidence base for use of hyperbaric oxygen therapy (HBOT) in acute filler complications is weak. It has been effective in treating idiopathic cilioretinal artery (CLRA) and central retinal vein occlusion,36 and provided some benefit in treating visual impairment linked to extraocular muscle ischemia following calcium hydroxyapatite filler injection.37 HBOT appeared particularly efficacious in treating visual impairment due to anterior segment ischemia following HA injections, and, when included in therapy, two cases of skin necrosis.38,39 Zhang40 further reported inclusion of HBOT in treating three patients with visual loss and skin necrosis; all showed improvements in skin healing but not in terms of sight recovery. HBOT is often included as part of a treatment strategy, but there is a lack of head-to-head research focused on this intervention. As a result, it is still unknown whether the use of additional HBOT is beneficial or at which stage its use would be optimal. The authors have seen patients with wounds after a vascular occlusion where HBOT has been prioritized, and the wound has been left uncovered to dry out. This is not optimal management.
If concentrated oxygen therapy is deployed, clinicians may have the option to use a continuous oxygen therapy (COT) device that is provided with a dressing. Water-based topicals can be applied underneath to stop the wound from drying. Devices are available in the US and UK and may be a practical treatment option if the clinician deems oxygen therapy appropriate. In the US, the company, EO2 Concepts® (San Antonio, Texas), allows the clinician to prescribe an inexpensive COT device that can be shipped to the patient and worn continually until the wound has healed.
Conclusion
Wounds that occur due to a dermal filler ischemic injury are not addressed in the literature or managed optimally in practice. This guideline serves to provide a basic guide to wound healing, create awareness of basic wound management principles, and recommend management approaches to typical wound presentations following a vascular ischemic event. For further support please contact CMAC for specialist advice.
References
- Belezney K, Carruthers J, Humphrey S, et al. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019; 39 (6): 662-674.
- Alam M, Kakar R, Dover JS et al. Rates of vascular occlusion associated with using needles vs cannula for filler injection. JAMA Dermatol. 2021;157(2):174-180.
- Murray G, Convery C, Davies E, Walker L. Guideline for the management of hyaluronic acid filler- induced vascular occlusion. J Clin Aesthet Dermatol. 2021;14(5):E61-69.
- Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498–510.
- Pierrefeu A, Brosset S, Lahon M, et al. Transverse facial artery perforators: anatomical, two- and three-simensional radiographic study. Plast Reconstr Surg. 2019;143(4):820e-828e.
- Kalogeris T, Baines G, Krenz M, Korthuis R. Cell biology of ischaemia/reperusion injury. Int Rev Mol Biol. 2012;298:229-317.
- Hong JY, Seok J, Ahn GR, et al. Impending skin necrosis after dermal filler injection: A ‘golden time’ for first-aid intervention’. Dermatol Ther. 2017;30:e12440.
- Khalil H, Cullen M, Chamnbers H, et al. Elements affecting wound healing time: an evidence-based analysis. Wound Rep and Reg. 2015;23:550-556.
- McGarr G, Hodges G, Mallette M, Cheung S. Ischaemia-reperfusion injury alters skin microvascular responses to local healing of index finger. Microvascular research. 2018;(118):12-19.
- Yousef H, Alhajj M, Sharma S. Anatomy, Skin (Integument), Epidermis.[updated 2021 Jul26]. In:StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3(7):20-31.
- Benbow M. Exploring the concept of moist wound healing and its application in practice. Br J Nurs. 2008;17(15).
- Flanagan M. Wound Healing and Skin integrity. Philadelphia, PA: John Wiley and Sons;2013.
- Winter G. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200:378-379.
- Winter G. Formation of the scab and rate of epithelialisation of superficial wounds in the skin of the young domestic pig. Nature;193(4812): 293-294.
- Dyson M, Young S, Pendle CL, et al. Comparison of the effects of moist and dry conditions on dermal repair. J Invest Dermatol. 1988;91(5):434-439.
- Chen WY, Rogers AA, Lydon MJ. Characterization of biologic properties of wound fluid collected during early stages of wound healing. J Invest Dermatol. 1992;99(5):559-564.
- Kunugiza Y, Tomita T, Moritomo H, Yoshikawa H. A hydrocellular foam dressing versus gauze: effects on the healing of rat excisional wounds. J Wound Care. 2010;19(1)10-14.
- Garvier MH, Bass LM, Fitzgerald R, et al. Differentiating non permanent injectable fillers: prevention and treatment of filler complications. Aesth Surg J. 2018;38(1):S29-40.
- Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflam. 2019;2019:3706315.
- Dealey C. The Care of Wounds: A Guide for Nurses, 4th ed. Hoboken, NJ: John Wiley and Sons Ltd.;2012.
- Lawrence JC. Moist wound healing: critique I. J Wound Care. 1995;4(8):368-370.
- Farber JL, Chien KR, Mittnacht Jr. Myocardial ischaemia:the pathogenesis of irreversible cell injury in ischaemia. Am J Pathol. 1981;102(2):271.
- Kamolz LP, Wild T. Wound bed preparation: the impact of debridement and wound cleansing. Wound Med. 2013;1:44-50.
- Patel GK. How to diagnose and treat a hemorrhagic skin necrosis. Wounds UK. 2007;34.
- Makrantonaki E, Ganceviviene R, Zouboulis C. An update on the role of the sebaceous gland in pathogenesis of acne. Dermato-Endocrinology. 2011;3(1):41-49.
- Cutting KF. Wound exudate: composition and functions. Br J Community Nurs. 2003;8(9 Suppl):4-9.
- Percival SL, Suleman L. Slough and biofilm: removal of barriers to wound healing by desloughing. J Wound Care. 2015;24(11):498-510.
- Cummings P, Del Beccaro MA. Antibiotics to prevent infection of simple wounds: a meta-analysis of randomized studies. Am J Emerg Med. 1995;13(4):396-400.
- Meuleneire, Rüknagel . Soft silicone dressings made easy . Wounds International. 2013.
- Barchitatta M, Maugeri A, Favara G, et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin.Int J Mol Sci. 2019;20: 1119
- Ellis P. The impact of smoking on wound healing: the role of the nurse. Br J Nurs. 2018;27(6):S10-S14.
- Gushiken LFS, Beserra FP, Bastos JK, Pellizson CH. Cutaneous wound healing: an update from physiopathology to current therapies. Life. 2021;11;665-684.
- Khalil H, Cullen M, Chambers H, et al. Elements affecting wound healing time: an evidence-base analysis. Wound Rep and Reg. 2015;23:550-556.
- Bryan J. Moist wound healing: a concept that changed our practice. J Wound Care. 2004;13(6):227-228.
- Celebi AR, Kilavuzoglu AE, Altiparmak UE, et al. Hyperbaric oxygen for the treatment of the rare combination of central retinal vein occlusion and cilioretinal artery occlusion. Diving Hyperb Med. 2016;46(1):50-53.36)
- Sung WI, Tsai S, Chen LJ. Ocular complications following cosmetic filler injection. JAMA Ophthalmol. 2018;136(5):e180716.
- Worley N, Lupo M, Holcomb K et al. Hyperbaric oxygen treatment of keratitis following Facial hyaluronic acid injection. Ochsner J. 2020;20(2):193–196.
- Darling MD, Peterson JD, Fabi SG. Impending necrosis after injection of hyaluronic acid and calcium hydroxylapatite fillers: report of 2 cases treated with hyperbaric oxygen therapy. Dermatol Surg. 2014;40:1049–1052.
- Zhang L, Pan L, Xu H et al. Clinical observations and the anatomical basis of blindness after facial hyaluronic acid injection. Aesthetic Plast Surg. 2019;43(4):1054-1060.