 J Clin Aesthet Dermatol. 2023;16(3):21–26.
J Clin Aesthet Dermatol. 2023;16(3):21–26.
by Leon Kircik, MD; Emil A. Tanghetti, MD; Adam Friedman, MD, FAAD; Kristine Kucera, PA-C, MPAS, DHS; and Abby Jacobson, MS, PA-C
Dr. Kircik is with the Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Tanghetti is with the Center for Dermatology and Laser Surgery in Sacramento, California. Dr. Friedman is with the George Washington School of Medicine and Health Sciences in Washington, DC. Dr. Kucera is with US Dermatology Partners Richardson in Richardson, Texas. Ms. Jacobson is with Ortho Dermatologics (a division of Bausch Health US, LLC) in Bridgewater, New Jersey.
FUNDING: Writing and editorial support was funded by Ortho Dermatologics, a division of Bausch Health US, LLC.
DISCLOSURES: LK has served as an investigator, a speaker, an advisory board member, or a consultant for Abbott, Aclaris Therapeutics, Allergan, Amgen, Anacor Pharmaceuticals, Assos Pharmaceuticals, Astellas Pharma US, Asubio Pharma, Bayer Healthcare, Berlex Laboratories (Bayer Healthcare Pharmaceuticals), Biogen, BioLife, Biopelle, Blue Willow Biologics, Boehringer Ingelheim, Breckinridge Pharmaceutical, Celgene Corporation, Centocor, ColBar LifeScience, CollaGenex Pharmaceuticals, Combinatrix Molecular Diagnostics, Connetics Corporation, Coria Laboratories, Dermik Laboratories, Dermira, Dow Pharmaceutical Sciences, DUSA Pharmaceuticals, Eli Lilly, Embil Pharmaceutical, EOS Pharmaceutical, Ferndale Pharma Group, Galderma Laboratories, Genentech, GlaxoSmithKline, Healthpoint, Idera Pharmaceuticals, Innocutis Medical, Innovail, Johnson & Johnson, Laboratory Skin Care, LEO Pharma, L’Oréal, 3M, Maruho, Medical International Technologies, Medicis Pharmaceutical, Merck, Merz Pharma, Novartis AG, Noven Pharmaceuticals, Nucryst Pharmaceuticals, Obagi Medical Products, Ortho Neutrogena, Pediapharma, Pfizer, PharmaDerm, Promius Pharma, PuraCap Pharmaceutical, QLT, Quatrix, Quinnova Pharmaceuticals, Serono (Merck-Serono International), SkinMedica, Stiefel Laboratories, Sun Pharmaceutical Industries, Taro Pharmaceutical Industries, TolerRx, Triax Pharmaceuticals, UCB, Valeant Pharmaceuticals North America, Warner Chilcott, XenoPort, and ZAGE. ET is a consultant and speaker for Ortho Dermatologics (a division of Bausch Health US, LLC) and a speaker for AbbVie, Eli Lilly, Galderma, Novartis, and Pfizer. AF is a speaker for Abbvie, BMS, Incyte, Janssen, Lilly, Regeneron, Sanofi, and UCB and is a consultant for Abbvie, Aveeno, BMS, Galderma, Janssen, La Roche Posay, Mino Labs, Novartis, Novocure, Regeneron, Sanofi, and Zylo. KK has served as a speaker for AbbVie, Amgen, Beiersdorf, LEO Pharma, Ortho Dermatologics, Pfizer, and Sun Pharma and as an advisor for Jannsen, Novartis, and UCB. AJ is an employee of Ortho Dermatologics (a division of Bausch Health US, LLC).
ABSTRACT: Objective: The fixed-dose corticosteroid/retinoid combination halobetasol propionate (0.01%) and tazarotene (0.045%) lotion (HP/TAZ) is approved for topical treatment of plaque psoriasis in adults. In addition to its current indication for plaque psoriasis, a growing body of clinical data suggests that HP/TAZ may be a beneficial therapy for palmoplantar and scalp psoriasis. Here, we discuss the efficacy and safety of HP/TAZ in various psoriatic phenotypes and related conditions.
Methods: Three studies (one post-hoc analysis, two open-label reports) of HP/TAZ were identified for this discussion.
Results: A post-hoc analysis demonstrated that once-daily HP/TAZ was associated with sustained efficacy and clinically meaningful quality-of-life improvements in participants with psoriatic disease who had low quality of life and 3% to 5% affected body surface area at baseline. In open-label reports, HP/TAZ was associated with improvement in scalp psoriasis measures, as well as quality of life. Additionally, HP/TAZ was efficacious in patients with palmoplantar psoriasis and in one report of a patient with palmoplantar pustulosis.
Limitations: Limitations include the small sample sizes of open-label reports of HP/TAZ; larger studies are needed to confirm findings.
Conclusion: Taken together, this evidence suggests that HP/TAZ may address unmet needs in psoriatic disease as a therapy for patients with all levels of psoriasis severity who experience daily challenges associated with their disease and that it may be a candidate for treating a variety of forms of psoriasis.
Keywords: Corticosteroid, plaque psoriasis, retinoid, palmoplantar psoriasis, scalp psoriasis, quality of life
Plaque psoriasis is a chronic inflammatory disease that affects about two percent of the population and manifests with numerous phenotypes.1,2 Although most patients with plaque psoriasis have limited disease involvement (e.g., <5% body surface area [BSA]),1 their quality of life may still be substantially reduced. Further, disease severity can be underestimated with objective measurements alone (e.g., BSA or psoriasis area and severity index).3 Patients with “objectively” milder disease (e.g., <10% BSA involvement) but substantially reduced quality of life may also encounter problems with insurance coverage or provider perceptions of severity. Unfortunately, little consensus has been reached on how to address the needs of patients with lower BSA involvement and disease characteristics that negatively influence quality of life.3
Another challenge that influences treatment of psoriasis and the patient experience is the location of lesions, including special areas.3 Certain disease locations may be difficult to treat or treatment resistant, despite a large selection of currently available therapies. For instance, scalp psoriasis is a common disease phenotype that negatively affects quality of life, is difficult to manage, and often resists treatment.1,4 Frequent scratching can lead to Koebnerization of the scalp and lesions that are difficult to control.1 Additionally, unpleasant or messy formulations on a highly visible body area such as the scalp may limit patient adherence. Another phenotype is palmoplantar psoriasis, which presents as well-defined erythematous plaques on the palms and soles, either in isolation or in combination with generalized plaque psoriasis.5 A disease with similar pathologic features is palmoplantar pustulosis (PPP), an inflammatory disorder that affects the palms and soles of the feet. Sterile pustulation is present in both pustular psoriasis and PPP, which complicates diagnosis and classification of these conditions. Both conditions are considered challenging to treat, as the thicker skin on palmoplantar surfaces may not allow for adequate absorption of topical therapies, thereby reducing efficacy.5
Patients with chronic, inflammatory conditions such as psoriasis often require long-term treatment; however, high-potency topical corticosteroids, which are mainstays of psoriasis treatment, are only recommended for short-term use. Long-term use of these therapies may contribute to an increased risk of adverse events, including skin atrophy and, as suggested more recently, osteoporosis and bone fracture.6,7 Additionally, use of multiple topicals may increase the risk of contact hypersensitivity in patients with palmoplantar involvement.8 To mitigate side effects from corticosteroids, current treatment guidelines recommend concomitant nonsteroidal topicals.6 However, the need remains for an efficacious and well-tolerated topical treatment for difficult-to-treat disease.
Fixed-combination halobetasol propionate 0.01% and tazarotene 0.045% (HP/TAZ) is approved for the treatment of plaque psoriasis in adults.9 The efficacy and safety of HP/TAZ in plaque psoriasis has been demonstrated in two Phase III trials and in a 52-week open-label study.10,11 The purpose of this review is to highlight additional psoriatic diseases and conditions for which HP/TAZ may provide symptomatic relief. Here, we review the efficacy and safety of HP/TAZ in a subgroup of clinical trial patients with BSA involvement of 3% to 5% and poor quality of life, as well as open-label reports of HP/TAZ in scalp and palmoplantar disease.
PATIENTS WITH POOR QUALITY OF LIFE DESPITE LOW BSA INVOLVEMENT
Patients with low BSA involvement can still experience substantial negative effects of psoriasis, including reduced quality of life.3,12 It is difficult for providers to adequately capture the needs of these patients, as severity scoring tools cannot reliably measure patient-specific or subjective responses. The classification of disease severity is also informed by response to topical therapy. For instance, international guidelines define severity based in part on lack of symptom control with topical therapy.13,14 Therefore, response to topical therapy in patients who have objectively lower BSA involvement yet reduced quality of life may represent a key benchmark of treatment success.
In a post-hoc analysis of two pooled Phase III trials of HP/TAZ,11 researchers assessed outcomes in adults with psoriasis, low BSA involvement (3%–5%), and poor quality of life (Dermatology Life Quality Index [DLQI] ≥11).15 Thirty-seven participants received HP/TAZ and 28 received vehicle. Treatment success (defined as ≥2-grade reduction from baseline in Investigator’s Global Assessment [IGA] score and a score of clear or almost clear [0 or 1]), change from baseline in BSA involvement, and clinically meaningful improvement in DLQI (≥4-point reduction in DLQI score) were assessed through eight weeks and a 4-week posttreatment visit. Outcomes were shown for the subgroup of participants with BSA 3%–5% + DLQI ≥11 in comparison with the overall study population.
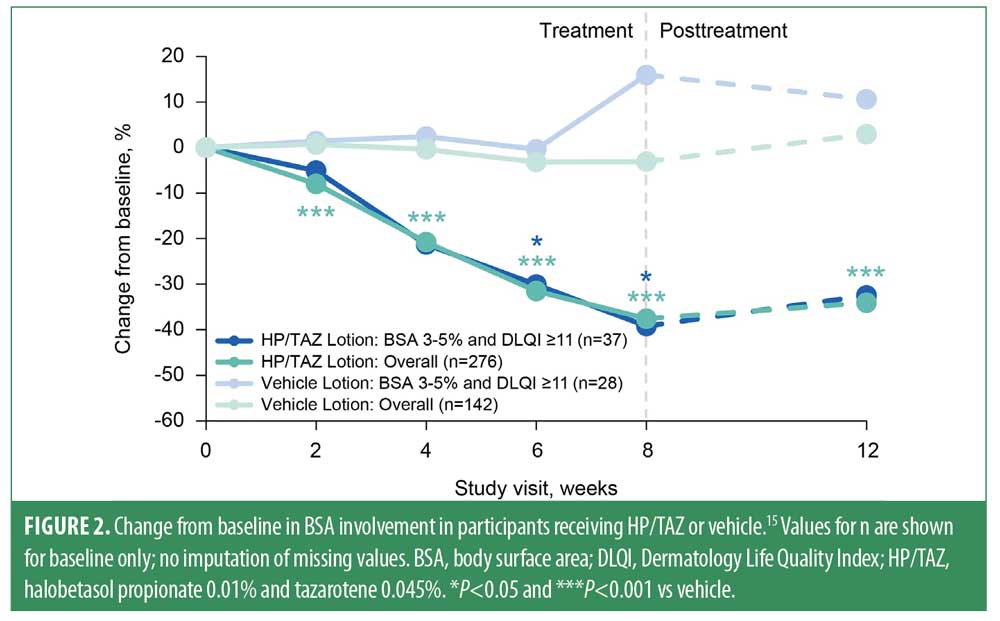
At Week 8, significantly more participants in the BSA 3%–5% + DLQI ≥11 subgroup who received HP/TAZ achieved treatment success versus those who received placebo (50.3% vs. 14.6%; P<0.05; Figure 1). Similarly, participants in the BSA 3%–5% + DLQI ≥11 subgroup who were treated with HP/TAZ experienced significantly greater reductions from baseline in BSA involvement than did those treated with placebo (-39.2% vs. +15.9%; P<0.05; Figure 2). Measures of treatment success and BSA involvement remained significantly better than baseline at four weeks’ posttreatment and were generally similar to those in the overall study population.11
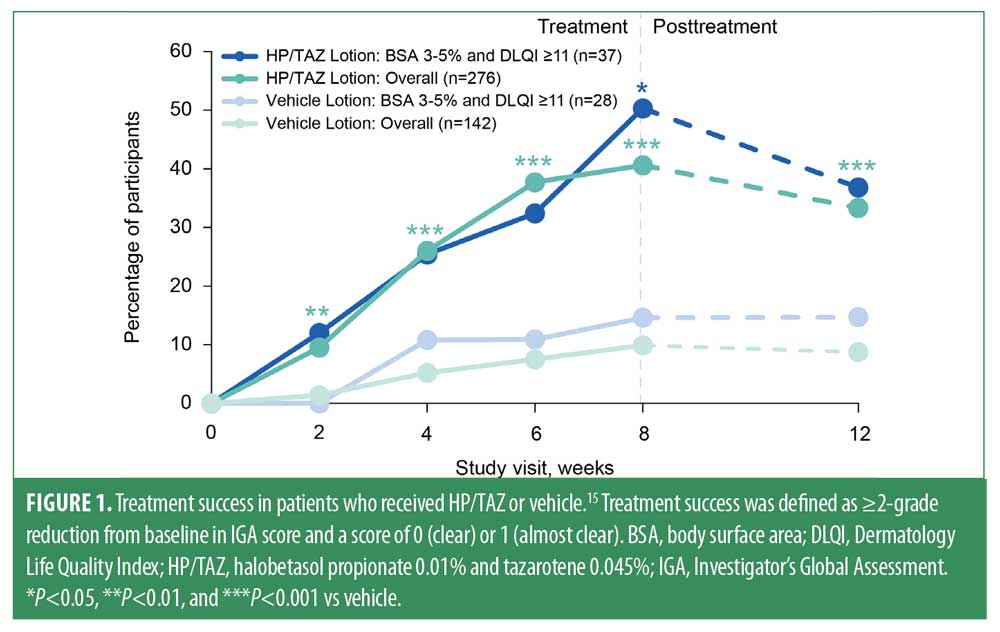
Baseline DLQI scores in this post-hoc analysis of participants with BSA 3%–5% and DLQI ≥11 were 15.9 (HP/TAZ, n=37) and 15.5 (vehicle, n=28). Baseline DLQI scores in the overall population were 8.2 (HP/TAZ, n=276) and 8.8 (vehicle, n=141). At Week 8, the proportion of participants in the HP/TAZ–treated BSA 3%–5% + DLQI ≥11 subgroup who experienced clinically meaningful improvement in DLQI score was greater than that in the vehicle-treated group (85.3% vs. 55.6%; Figure 3) and in the overall study population (63.4%). These improvements were maintained at four weeks’ posttreatment.
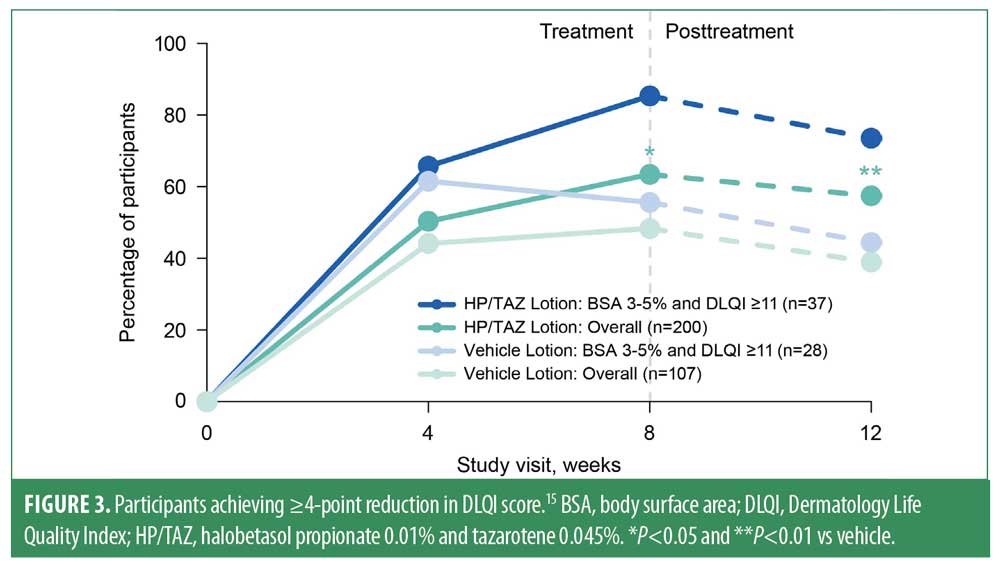
Although analyses were limited because of the small sample size, participants with low BSA involvement and poor quality of life who received HP/TAZ experienced clinically meaningful improvements in DLQI and greater efficacy versus those who received vehicle. Findings from this subgroup were similar to those of the overall study population, suggesting that HP/TAZ is a viable therapy for patients with relatively limited affected BSA and poor quality of life.
SCALP PSORIASIS
Poor quality of life, including embarrassment and emotional distress, is also a hallmark of scalp psoriasis.16,17 The scalp, a body area commonly affected by psoriasis, is difficult to treat and associated with itch, scarring, and alopecia.16 Scalp psoriasis presents as plaques on the hairline, which can extend to the forehead, ears, and neck; plaques may occur with other forms of psoriasis, or in isolation. Repetitive scratching may lead to Koebnerization of the scalp, which may result in difficult-to-control plaques and further resistance to therapy.1 Diagnosis may be delayed, as scalp psoriasis can share clinical features with seborrheic dermatitis and other papulosquamous conditions.17 Additionally, scalp psoriasis may serve as a predictor for the development of other disorders, such as psoriatic arthritis; in a cohort study of 1633 patients across 30 years, the risk of developing psoriatic arthritis was nearly quadrupled in patients with scalp psoriasis when compared with those without scalp lesions.18
Topical therapies are currently recommended as first-line treatment for scalp psoriasis, but the presence of hair can impair correct treatment application or penetration.6,16 Adherence to topical therapy is challenging because formulations may be greasy, difficult to apply, and difficult to remove from hair. Current formulations include shampoos, gels, oils, and foams1,16; however, proper selection of vehicle depends on hair type and patient preferences.6
Although HP/TAZ is currently approved for the treatment of plaque psoriasis,9 scalp psoriasis was not included in its pivotal trials.10,11 However, the lotion base of HP/TAZ may be suitable for application to hair-bearing areas such as the scalp.19 To assess the efficacy and safety of HP/TAZ in such patients, Ozyurekoglu and Kircik conducted a single-center, open-label pilot study of 21 adults who had moderate-to-severe psoriasis with scalp involvement through eight weeks of treatment and a follow-up visit at Week 12 (N=20).19 The primary endpoint was proportion of participants with an IGA score of 0 or 1 (clear or almost clear) at Week 8. Other outcomes through 12 weeks included change from baseline in IGA, 75%/90%/100% improvement in Psoriasis Scalp Severity Index (PSSI 75/90/100), decrease in BSA involvement, scalp IGA (sIGA) improvement, visual analog score (VAS) of pruritus improvement, and safety.
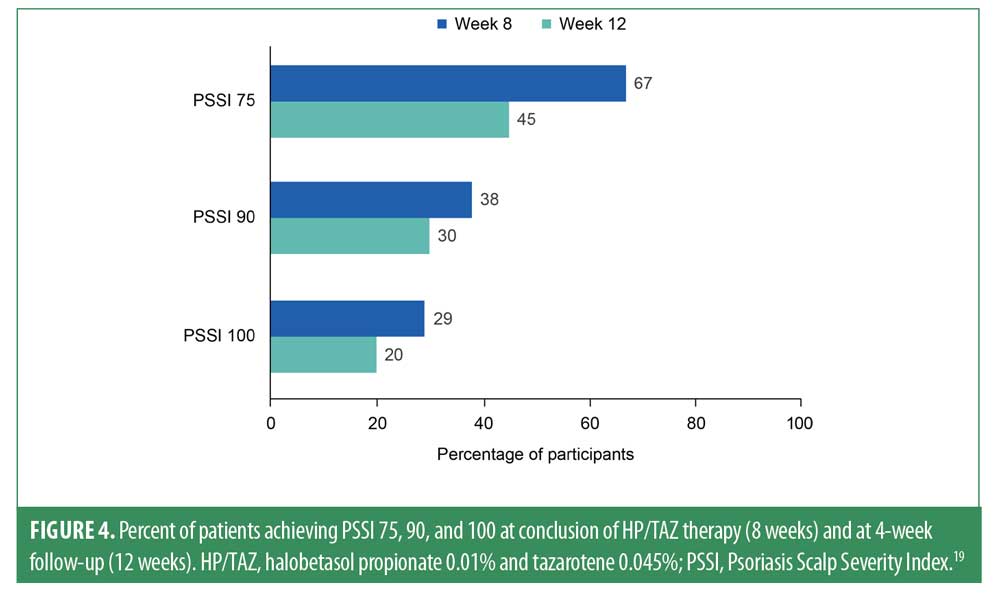
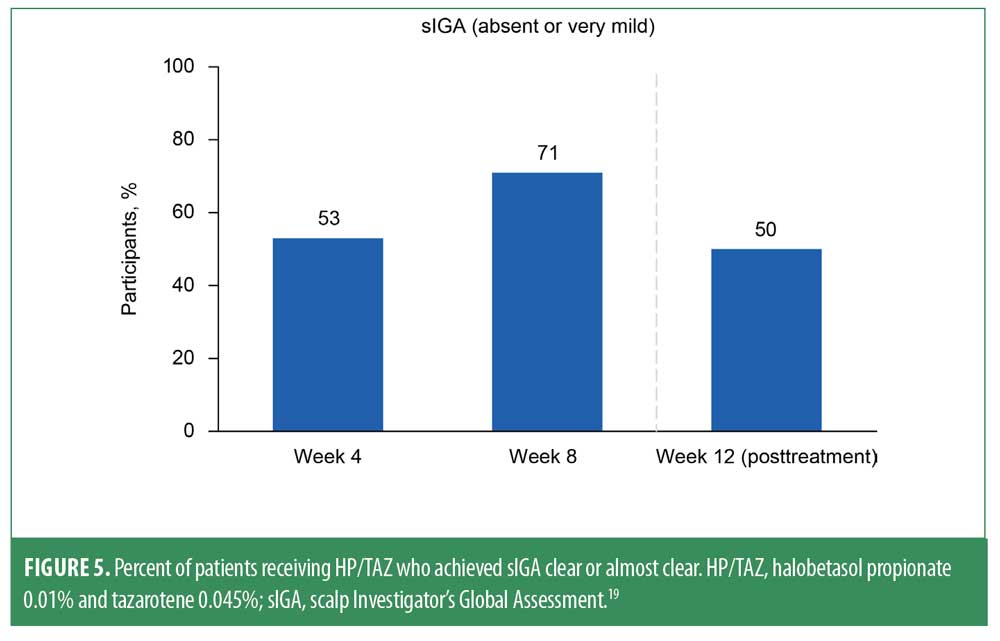
Measurements of PSSI 75 revealed significant improvements from baseline at Weeks 4, 8, and 12 (P≤0.0005 for all).19,20 Two-thirds of patients (67%) achieved PSSI 75 at Week 8, and 45% maintained PSSI 75 at Week 12 (Figure 4). Moreover, at the 8-week time point, 38% of participants achieved PSSI 90 (with 30% maintaining at Week 12), and 29% achieved PSSI 100 (with 20% maintaining at Week 12). Improvements in scalp psoriasis were also captured by sIGA; at the 8-week visit, 15/21 patients (>70%) achieved an sIGA score of 0 or 1 (Figure 5). Nearly one-third of patients (29%) had sIGA 0 at Week 8, and 20% maintained sIGA 0 at Week 12.
At the Week 8 visit, 10/21 patients (48%) achieved a score of IGA 0 or 1. IGA decreased significantly from baseline to each of Weeks 4, 8, and 12 (P≤0.004 for all).19,20 BSA involvement was decreased significantly from baseline at Weeks 4, 8, and 12 by 31% (P=0.0002), 47% (P=0.0003), and 34% (P=0.007), respectively.
HP/TAZ was associated with a nominal decrease in pruritus score, as measured by VAS for pruritus. However, in local tolerability assessments, participants reported significantly less itching at Weeks 4 and 8 and less burning at Week 12. HP/TAZ was associated with significant absolute and relative improvement in quality of life at Week 8, as measured by DLQI (change from baseline, 44%; P=0.04).19,20
No patients experienced skin atrophy, striae, telangiectasia, or folliculitis. However, seven nonserious adverse events were reported in five patients. Six were of moderate severity, and one was severe.20 None appeared to be related to the study medication or any HP/TAZ–associated action. Of the seven events, two resolved by end of study and five were ongoing with no follow-up deemed necessary.20
These preliminary findings indicate that HP/TAZ may represent a novel therapy for scalp psoriasis, a challenging phenotype to treat. In this off-label study of HP/TAZ in 21 adults with scalp psoriasis, HP/TAZ produced significant and substantial improvements in measures of scalp psoriasis and itch and had a well-tolerated safety profile. Additionally, the lotion vehicle of HP/TAZ may be advantageous for application to the scalp and represent a favorable option for patient preference. Though these initial results are promising, additional studies are warranted to further investigate the efficacy, safety, and tolerability of HP/TAZ in scalp psoriasis.
PALMOPLANTAR PSORIASIS AND PALMOPLANTAR PUSTULOSIS
Palmoplantar psoriasis is a form of psoriasis characterized by symmetrical erythematous, scaly plaques, which may occur alone or with other forms of psoriasis.21 In contrast, patients with PPP do not develop psoriasis at other body sites and exhibit histologic involvement of the acrosyringium.22 PPP is characterized by localized pustules, scale, and patchy erythema on the palms and soles. It has a higher frequency in women and tobacco users and follows a chronic relapse-recurrence cycle with periods of flares and remission. For both palmoplantar psoriasis and PPP, itching and painful fissures contribute to poor quality of life and negative self-image.21 Discomfort experienced by those with palmoplantar involvement most likely stems from psoriatic lesions in locations used heavily for activities of daily living (e.g., hands and feet).23
Topical treatment of palmoplantar psoriasis and PPP usually involves steroid creams or ointments, tar ointments, topical and oral retinoids, and moisturizing creams; however both conditions are highly treatment-resistant, despite numerous available therapies.21,22 Thicker skin on the palms and soles may prevent adequate absorption of topical therapies and may explain the limited efficacy of certain treatments. Further, use of multiple topicals for resistant PPP lesions may lead to contact hypersensitivity.8 Evidence-based treatment guidelines and clinical data are currently lacking for palmoplantar psoriasis and PPP,21 highlighting the need for effective therapies.
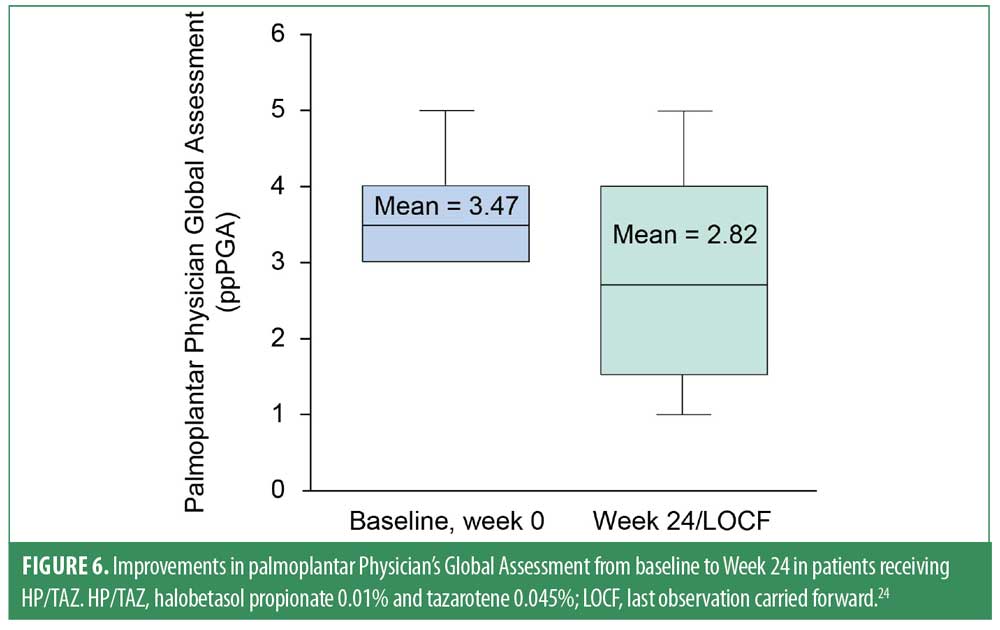
Topical corticosteroids and tazarotene are each used individually for the treatment of palmoplantar psoriasis and PPP.21 To expand on this treatment selection, Campbell et al examined the efficacy and safety of HP/TAZ in an open-label study of 17 patients with moderate-to-severe palmoplantar psoriasis, as assessed by the Palmoplantar Physician Global Assessment (ppPGA). Patients received HP/TAZ daily for 24 weeks.24 Mean ppPGA score at baseline was 3.47 and was significantly reduced (improved) to 2.82 at Week 24 (P=0.0165; Figure 6). Treatment-emergent adverse events were mild and limited to application site dermatitis (n=3), which did not lead to discontinuation.
Additionally, a recent case report documented successful use of HP/TAZ to treat PPP in a 54-year-old Caucasian woman.25 The patient presented with a pustular lesion on her right palm near the base of her thumb that had continued to relapse and substantially affect her work and quality of life. Once a diagnosis of PPP was established, a variety of treatments were discussed with the patient; while awaiting approval for systemic biologic therapy, she started treatment with once-daily HP/TAZ. Because symptoms improved after one week, the patient elected to forego systemic therapy at that time and continue HP/TAZ. At a 6-week follow-up visit, the patient’s palm was generally clear (some erythema remained). No side effects were reported and HP/TAZ was tapered and discontinued.
Improvement was maintained for five weeks after discontinuation of HP/TAZ, until the lesion recurred; HP/TAZ was then resumed twice daily. Her palm was clear at a one-month follow-up visit, but she elected to start systemic biologic therapy, owing to concerns about long-term control. After receiving systemic biologic therapy for four weeks, the patient was restarted on HP/TAZ because erythema had re-emerged with a single pustule. These symptoms resolved within two weeks of HP/TAZ treatment. During the COVID-19 pandemic, the patient discontinued her biologic after six months of use and, as of last correspondence, is using HP/TAZ two to three times a week at night and sleeping with cotton gloves (unpublished follow-up).
These results demonstrate that HP/TAZ is associated with improvement in patients with palmoplantar psoriasis through 24 weeks of treatment and rapid resolution of symptoms and quality of life improvement in a patient with PPP. Despite this patient experiencing a flare, which is typical of PPP, restarting treatment with HP/TAZ was successful, suggesting that it may be an effective option for PPP. Additional studies are warranted to further examine the efficacy and safety of HP/TAZ in patients with palmoplantar involvement.
Conclusion
Despite the wealth of topical therapies available for the treatment of psoriasis, numerous challenges still exist. Patients with low BSA involvement still report substantial reductions in quality of life, and other conditions such as scalp psoriasis and palmoplantar disease are difficult to treat owing to the specialized location of lesions. Fixed-combination HP/TAZ represents a viable treatment option that has been shown to improve quality of life in patients with low BSA involvement. Additionally, HP/TAZ has shown efficacy in treating scalp psoriasis and palmoplantar disease in open-label reports. Taken together, these findings provide support for continued investigation of HP/TAZ as a viable therapy for other forms of psoriatic disease.
Acknowledgments
Writing and editorial support was funded by Ortho Dermatologics and provided under the direction of the authors by Deirdre Rodeberg, PhD, CMPP™, and Claire F. Levine, MS, ELS, of MedThink SciCom. Ortho Dermatologics is a division of Bausch Health US, LLC.
References
- American Academy of Dermatology Work Group, Menter A, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137–174.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850.
- Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1):117–122.
- Egeberg A, See K, Garrelts A, et al. Epidemiology of psoriasis in hard-to-treat body locations: data from the Danish skin cohort. BMC Dermatol. 2020;20(1):3.
- Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349–358.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470.
- Egeberg A, Schwarz P, Harslof T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157(3):275–282.
- Al-Mutairi N, Abdalla TO, Nour TM. Resistant palmoplantar lesions in patients of psoriasis: evaluation of the causes and comparison of the frequency of delayed-type hypersensitivity in patients without palm and sole lesions. Med Princ Pract. 2014;23(6):561–567.
- Duobrii [package insert]. Bridgewater, NJ: Bausch Health US, LLC; 2020.
- Lebwohl MG, Stein Gold L, Papp K, et al. Long-term safety and efficacy of a fixed-combination halobetasol propionate 0.01%/tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis: phase 3 open-label study. J Eur Acad Dermatol Venereol. 2021;35(5):1152–1160.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17(8):855–861.
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–139.
- Papp K, Gulliver W, Lynde C, et al. Canadian guidelines for the management of plaque psoriasis: overview. J Cutan Med Surg. 2011;15(4):210–219.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10.
- Stein Gold L, Lain E, Bagel J, et al. Fixed-combination halobetasol propionate 0.01%/tazarotene 0.045% lotion for the treatment of plaque psoriasis in patients with 3-5% body surface area (BSA) and poor quality of life (QoL). Presented at: American Academy of Dermatology Virtual Meeting Experience 2021; April 23-25, 2021; Virtual.
- Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33–40.
- Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61(2):233–239.
- Ozyurekoglu E, Kircik L. An open-label pilot study to investigate safety and efficacy of fixed combination tazarotene 0.045% and halobetasol propionate 0.01% lotion for the treatment of scalp psoriasis. J Drugs Dermatol. 2021;20(11):1191–1194.
- Data on file, Ortho Dermatologics.
- Nair LV. Management of recalcitrant palmoplantar psoriasis. Journal of Skin and Sexually Transmitted Diseases. 2019;1(1):8–12.
- Freitas E, Rodrigues MA, Torres T. Diagnosis, screening and treatment of patients with palmoplantar pustulosis (PPP): a review of current practices and recommendations. Clin Cosmet Investig Dermatol. 2020;13:561–578.
- Chung J, Callis Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623–632.
- Campbell et al. A polymeric emulsion of halobetasol propionate and tazarotene in the treatment of palmoplantar psoriasis. Poster presented at: American Academy of Dermatology Annual Meeting; March 25-29, 2022; Boston, MA.
- Kucera KJ, Miller JE. Treatment of palmoplantar pustulosis using fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion: a case report. J Psoriasis Psoriatic Arthritis. 2020;5(4):164–167.

