 by Benji Dhillon, MBBS, MRCS, BSc, and Tapan Patel, MBBS, MRCP
by Benji Dhillon, MBBS, MRCS, BSc, and Tapan Patel, MBBS, MRCP
Drs. Dhillon and Patel are with the Phi Clinic in London, England, United Kingdom.
Funding: This article was supported by a contract sponsor grant from Lumenis Ltd.
Disclosures: Dr. Patel and Dr. Dhillon received payment for investigator services from Lumeinis Ltd.
Abstract: Objective. The goal of this study was to evaluate the safety and the efficacy of the SlimME™ (Lumenis AB, Lumenis, Israel) ultrasound device for noninvasive body contouring.
Design. This was an open-label, single-arm, exploratory study.
Participants. Twenty adult patients presenting with subcutaneous target region adipose fat thickness greater than 2.5cm were included in this study.
Measurements. Responses to a single treatment session with the ultrasound device were assessed. Change from baseline in abdominal circumference was evaluated at one, two, and three months posttreatment. Before and after treatment, the physician scored improvements, patients completed a self-improvement assessment questionnaire, and photographs were evaluated by three blinded reviewers. Immediate skin responses were recorded for up to 30 minutes posttreatment, and adverse events were recorded throughout the study.
Results. A statistically and clinically significant reduction in abdominal circumference was observed at three months posttreatment, with a mean of -2.19±1.95cm, -2.14±1.94cm, and -1.83±2.00cm reduction from baseline in the umbilicus, under the ribs, and anterior superior iliac spine (ASIS) circumference measurements, respectively. Physician-based assessments classified 89.5 percent of subjects as “improved” within three months of treatment, and 89.5 percent of patients indicated their conditions to be either improved or much improved within this same time period. Immediate skin reactions were all expected, short-term, and self-resolving.
Conclusions. A single treatment session using the ultrasound study device appears safe and effective in achieving noninvasive body contouring. Trial registry: NCT02849847
Keywords: SlimME™, body contouring, circumference reduction, ultrasound, noninvasive
J Clin Aesthet Dermatol. 2018;11(6):41–45
Introduction
The noninvasive body sculpting market continues to be one of the fastest growing segments of aesthetic medicine,1 largely as a result of the increased emphasis being placed on overall well-being and lifestyle. In the past few decades, multiple treatment modalities have been developed in an attempt to reduce the volume of stubborn pockets of fat.2 Current surgical options, such as liposuction, carry the drawbacks of hospitalization, anesthesia, pain, swelling, and relatively long recovery periods, as well as the risks inherent to any surgical procedure. Therefore, safer and less-invasive alternatives would be desirable.2 The majority of nonsurgical modalities allow for selective adipocytosis, while sparing the overlying skin.3 When compared to more invasive modalities, devices using high-frequency ultrasound, radiofrequency energy, laser light, and cryolipolysis have the potential to improve efficiency, minimize adverse consequences, and shorten posttreatment recovery time. This is achieved through thermal and cavitational destruction, or the creation of a temporary adipocyte cell membrane pore, leading to reduced adipocyte counts, which, when translated over millions of fat cells, results in a measurable reduction of fat.4
While noninvasive body contouring treatments are both safe and effective,5 they are still not risk-free, with reports of pain, bruising, tenderness, paresthesia, neuropraxia, burns, scarring, adipose tissue hyperplasia, and fat atrophy following such procedures.6–8 The recently marketed SlimME™ body contouring device (Lumenis AB, Hagersten, Sweden) is a noninvasive body-sculpting tool that incorporates uniform ultrasound for the thermal destruction of fat cells and multi-polar radiofrequency (RF), which elicits tissue heating and subsequent increased rates of fat metobolism, circulation, and lymphatic drainage of the fat cell debris.9 This feature ensures focused energy transmission into the subcutaneous fat only, and not into deeper structures. Furthermore, upon suction into the therapeutic head, the epidermis is cooled to a precise temperature of 18°C to 21°C to ensure skin protection. In addition, following the completion of energy transmission, the pulsatile vacuum feature performs a deep lymphatic massage, which temporarily improves local blood circulation, promoting clearance of the destructed fat cells.10,11
The study device’s ultrasound energy is transmitted via one of two therapeutic heads that differ in their technical parameters and sizes. The hexagonal handpiece is the smaller of the two and thus treats smaller body areas and requires longer procedure times compared to the larger rectangular handpiece. A single treatment session with the hexagonal head of the device has been shown to induce a statistically and clinically signficant decrease in abdomen circumference within three months, with the most prominent reduction recorded for the umbilicus height circumference.12 The current study sought to evaluate the safety and efficacy of a single combined treatment session with the bodysculpting device’s hexagonal and rectangular therapeutic heads.
Materials and Methods
The study was conducted according to the principles of the Declaration of Helsinki and was approved by The Cosmetic Research Ethics Committee Ltd. (East Sussex, United Kingdom). All subjects provided their written informed consent prior to participation.
This study was a three-month, open-label, single-arm, exploratory study wherein all participants were subjected to a single abdominal and/or flanks treatment session with the device. The study was conducted between July 2016 and December 2016. Assessments were performed at one, two, and three months following treatment.
 Participants. Male and female patients between the ages of 18 and 65 years (Table 1) were eligible for inclusion in the study if their body mass index (BMI) was 32 or less and their fat thickness in the treated area (abdomen or flanks) was greater than 2.5cm, as measured by caliper. Heavy smokers (more than one pack of cigarettes per day), patients with a history of surgery or planned surgery in the treated area, those who had undergone previous fat reduction/circumference reduction or skin rejuvenation treatments in the treated area within the last six months, those with any permanent or temporary (including injectable) implant in the treatment area, and/or those who had undergone weight change fluctuations exceeding 4.5kg (10 pounds) within the preceding six months were excluded from the present study. Similarly, pregnant or breastfeeding women were excluded, as were subjects who had fewer than eight suitable (fat thickness ?2.5cm) treatment zones. Subjects were instructed to maintain their diet, exercise, and lifestyle habits throughout the study.
Participants. Male and female patients between the ages of 18 and 65 years (Table 1) were eligible for inclusion in the study if their body mass index (BMI) was 32 or less and their fat thickness in the treated area (abdomen or flanks) was greater than 2.5cm, as measured by caliper. Heavy smokers (more than one pack of cigarettes per day), patients with a history of surgery or planned surgery in the treated area, those who had undergone previous fat reduction/circumference reduction or skin rejuvenation treatments in the treated area within the last six months, those with any permanent or temporary (including injectable) implant in the treatment area, and/or those who had undergone weight change fluctuations exceeding 4.5kg (10 pounds) within the preceding six months were excluded from the present study. Similarly, pregnant or breastfeeding women were excluded, as were subjects who had fewer than eight suitable (fat thickness ?2.5cm) treatment zones. Subjects were instructed to maintain their diet, exercise, and lifestyle habits throughout the study.
Treatment procedure. Subjects were asked to shave the treatment area 72 hours prior to the treatment, if necessary. Treatment began with a caliper evaluation of the fat thickness of the target body area (abdomen/flanks). Zones in which fat thickness was 3.5cm or greater were treated by the rectangular handpiece, while zones in which fat thickness was between 2.5 and 3.5cm were treated by the hexagonal handpiece. A thin layer of coupling lotion, designed to optimize ultrasound transmission, was applied to each zone immediately prior to treatment initiation. The theraputic head was placed on the skin surface to ensure acoustic contact, and treatment was initiated. A vacuum level of 18InHg was employed to lift the subcutanous fat tissue into the applicator and ultrasound energy was transmited. The technical parameters used in the treatments are listed in Table 2.
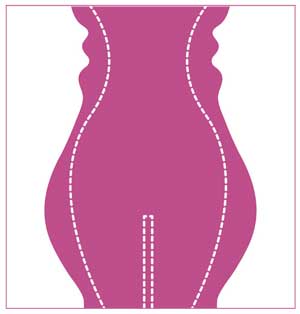
All subjects underwent a single treatment session, in which the entire treated region (abdomen/flanks) was tiled according to its anatomy (Figure 1).

Circumference measurement. Circumference was measured using a standardized technique by means of a validated apparatus (AccuFitness, Greenwood Village, Colorado, USA) that provides measurements at a constant height and under constant tension. The technique considers patient positioning, posture, and breathing6 at the anterior-superior iliac spine (ASIS), the umbilicus, and under the rib cage (maximal circumference). The height at which each measurement was taken was recorded with a measuring tape fixed to the wall. This allowed for standardization of each circumference measurement at each visit (i.e., at one, two, and three months). In order to assure that the measuring tape was placed horizontally in a straight line, a laser system projecting a perpendicular beam was used. The measuring tape was placed on top of the beam projection. Each circumference measurement was taken twice at each visit, and the average was used as the final measurement. The measurement at three months posttreatment was defined as the primary endpoint.
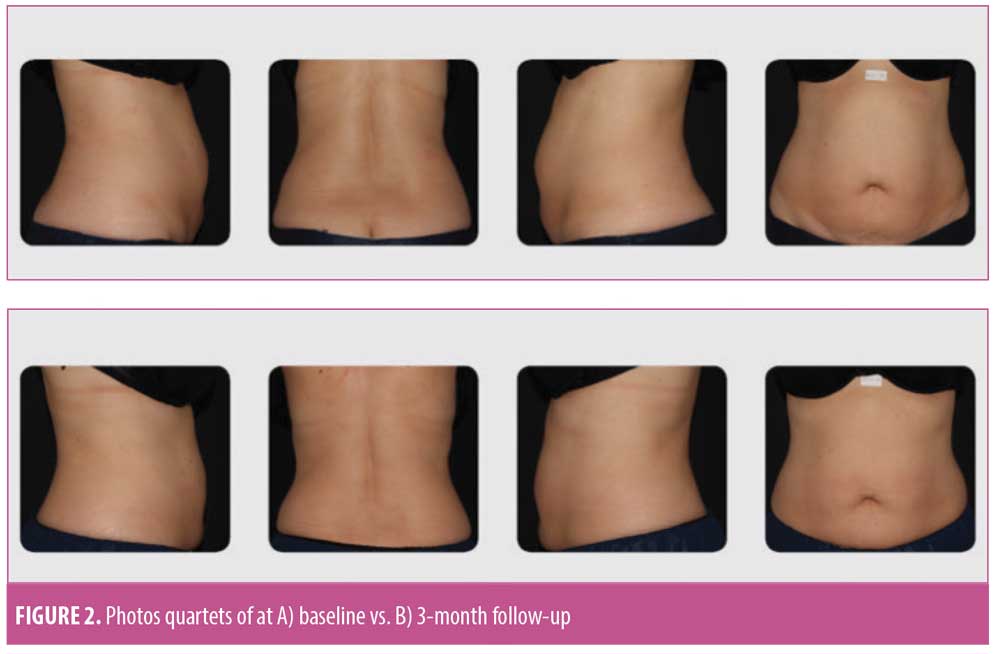
Clinical photographs. Subjects were photographed with their feet separated at a fixed distance, using a foot positioning guide fixture. At baseline and all follow-up visits, photographs were acquired using a standardized photography set-up (FotoFinder Systems, Columbia, Maryland, USA) to ensure consistency. At the completion of the study, clinical photographs were reviewed by three blinded, independent physicians. The blinded assessors were presented with randomized quartets of anterior, posterior, left, and right photographs of each subject and were asked to determine which quartets of photographs were taken pretreatment and which were taken posttreatment.
Subject and physician assessments of improvement. Subjects assessed the improvement in their abdominal circumference using the Subject Assessment of Improvement Questionnaire. The assesment is based on the Global Aesthetic Improvement (GAI) scale, where “0” indicates worsened and “4” indicates very much improved. The physician completed the Physician Assessment of Improvement Evaluation, which also uses the GAI scale. Both questionnaires were filled out at the one, two, and three-month follow-up visits.
Subject satisfaction. Subject satisfaction was evaluated at the one, two, and three-month follow-up visits using the five-point Likert scale, in which 0 represents very dissatisfied and 4 represents very satisfied.
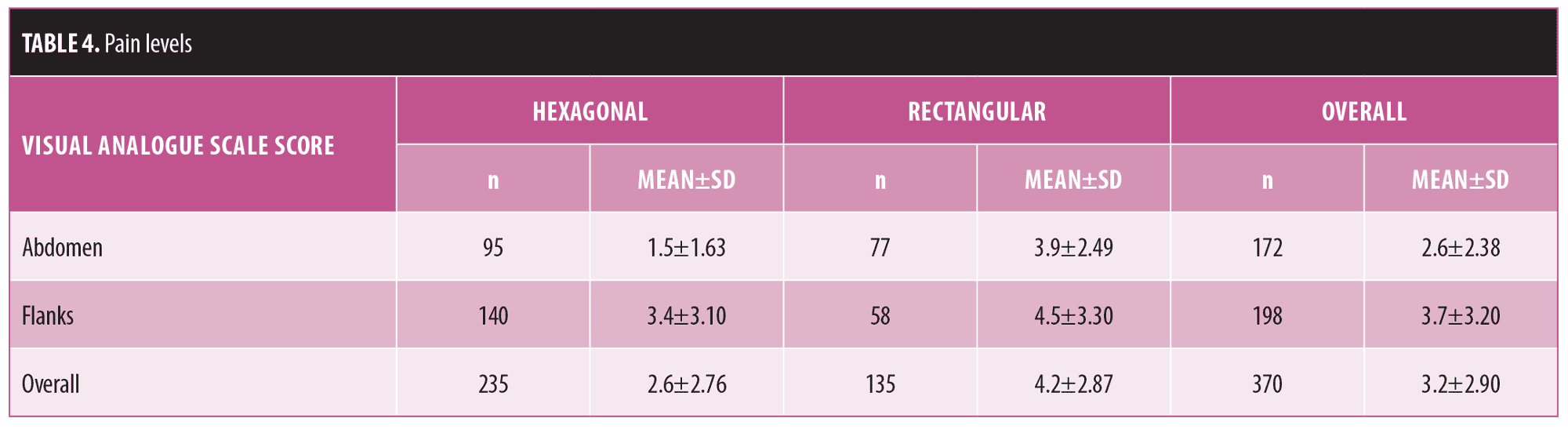
Pain and discomfort assessment. Procedure-related pain and discomfort were evaluated immediately and at 30 minutes following treatment using a Visual Analogue Scale (VAS). Subjects were presented with a 10cm horizontal line, where “0” indicated “no pain” and 10 indicated “intolerable pain.” Subjects were asked to make a mark along the scale to rate their pain level. A number was derived by the research staff by measuring up to the point of the subject marking versus the length of the entire line.
Immediate skin response. Skin responses occurring up to 30 minutes following treatment were evaluated and documented by the study physician. Expected responses included erythema, edema, purpura, and hematoma. Response severity was rated by the physician, wherein “0” indicated none and “4” indicated severe.
Adverse events monitoring. The incidence of adverse events (AEs) and serious AEs occurring at any time during the trial or follow-up period was recorded and monitored.
Statistical analysis. All statistical analyses and data presentations were performed using the SAS version 9.4 software (SAS Institute, Cary, North Carolina, USA). The level of significance indicates whether the adjusted means difference was significantly different from baseline (zero). All statistical tests were two-sided and tested at a five-percent level of significance. The statistical analyses were performed by BioStats Statistical Consulting Ltd. (Modi’in-Maccabim-Re’ut, Israel).
A mixed model repeated measurements analysis was conducted to characterize the change in circumference over time with the following covariates: baseline circumference, sex, baseline weight, and change in weight. The analysis used the data collected up to the point of three months after treatment and calculated the circumference reductions at the ASIS, umbilicus, and under the ribs separately.
Results
Subject baseline demographics and characteristics. Twenty (17 women and 3 men) with a mean age of 37.6±7.1 years and BMI of 25.1±3.8kg/m2 met the entry criteria and were enrolled in the study. Both abdomen and flank regions were found to be eligible for treatment in all 20 subjects. All 20 subjects were included in the safety evaluation.
Statistically and clinically significant reductions from baseline in circumferences at the umbilicus (-2.14±1.94 cm), ASIS (-1.83±2.00cm), and under the ribs (-2.19±1.95cm) were observed at three months following treatment (Table 3). These improvements were reflected in the physician’s assessments, who classified 89.5 percent of cases as showing improved contour within three months of treatment. In addition, 75 percent of “baseline” versus “post-treatment” photo quartets were correctly identified by blinded assessors (Figure 2). In parallel, at the three-month follow-up visit, 89.5 percent of the subjects classified their appearance as improved from baseline. Overall, subjects were satisfied with the treatment results; 47.37 percent of subjects rated their satisfaction level as “satisfied” or “very satisfied” at one month post-treatment; the percentage of satisfied patients rose to 89.5 percent by the three-month follow-up visit.
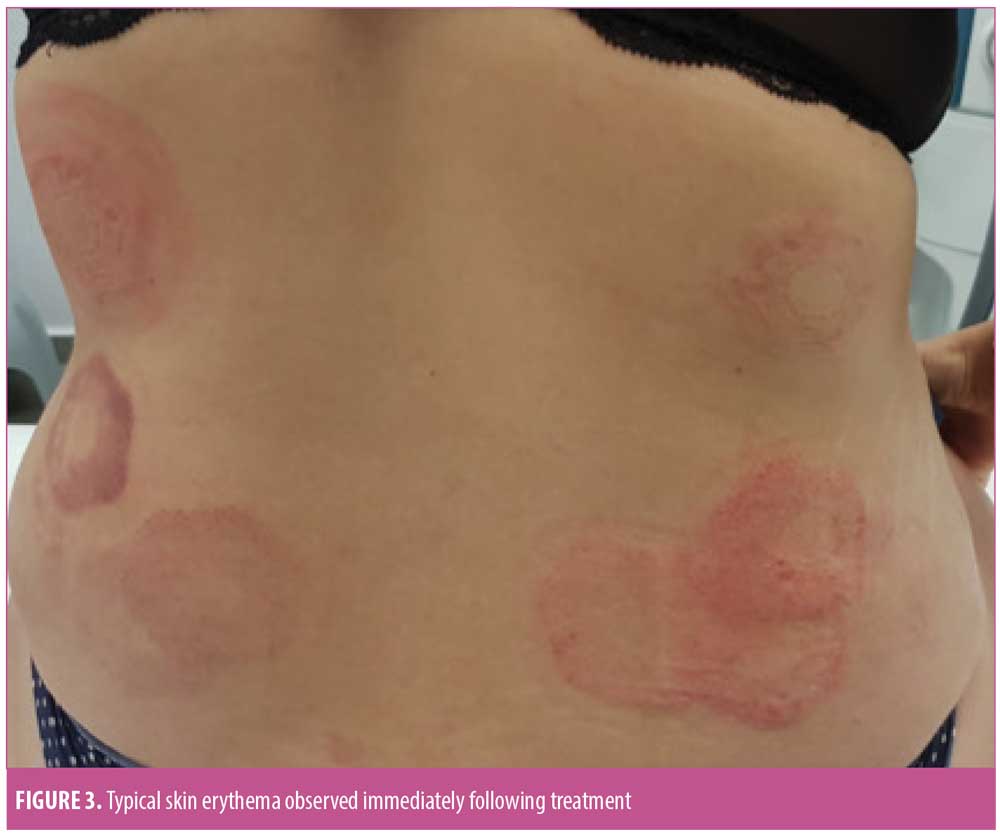
Safety outcomes. Mild erythema was the most common immediate skin response to treatment (Figure 3), with 70.35 percent of the treated zones demonstrating an erythemous response immediately after treatment. Other responses included edema, purpura, and hematoma. Erythema (11.63%) was the only skin reaction persisting for up to 30 minutes following abdominal treatment. Skin reactions persisting for up to 30 minutes following flank treatment included erythema (53.03%) and purpura (9.60%). Only one subject presented with a trace of erythema that persisted for one month post-treatment. No signs of skin responses were noted at the three-month follow-up visit.
Overall, treatment-related pain was mild and tolerable, with mean VAS scores of 2.6±2.8 and 4.2±2.9 for the hexagonal and rectangular handpieces, respectively (Table 4), and generally higher pain scores recorded for the flanks versus the abdomen.
No patients reported pain 30 minutes or one month post-treatment. Furthermore, no serious AEs were noted, and all subjects maintained good health throughout the study. Two unanticipated AEs were noted during the study; both were unrelated to the treatment procedure.
Discussion
The rising demand for body sculpting platforms has spurred the design of various noninvasive treatment devices, which are still limited by a gamut of associated AEs. The study device uses ultrasound energy, RF, and vacuum to induce hyperthermic temperatures, resulting in fat cell destruction and subsequent circumference reduction and improved aesthetic appearance. A single device treatment session induced a statistically and clinically signficant decrease in abdomen circumference within three months, with the most prominent reduction noted at the under-the-ribs height circumference. Both physician and patient assessments supported the clinical findings, with improvements in appearance recorded for 89.48 percent of subjects within three months of treatment. By and large, blindly assessed photographs were correctly categorized as pre- versus post-treatment, while some were mislabeled, which might be because photographs were taken with the treatment area concealed by disposable underwear and/or incorrect usage of the superimposing feature of the camera. Since this study aimed to evaluate the treatment-related change in body contouring, subjects were instructed to maintain their regular diet, exercise, and lifestyle habits; mean weight change over the study period was 0.1±0.02 percent. Nevertheless, the statistical model used for efficacy analysis was controlled for subject weight and weight change over time. In addition, the analysis enabled modeling and estimation of the within-subject dependence on treatment site, thereby providing a more accurate estimation of the treatment effect.
Experience with the study device in patients who had a preabdominoplasty demonstrated that the generated ultrasound energy was converted to heat, with subcutaneous adipose tissue temperatures reaching up to 48°C, leading to adipocystosis without harm to the upper skin layers, owing to the device’s cooling feature.13 In the study, the hexagonal therapeutic head was shown to reduce circumference in subjects with unwanted/excess abdominal fat. Similarly, the present study demonstrated that circumference reduction of both the abdominal and flank regions was achieved by the use of both the hexagonal and rectangular therapeutic heads.
The observed decrease in circumference stands in line with a porcine model treated with the study device, wherein a gradual increase in inflammation in the treated adipose tissue peaked at one month post-treatment and subsided within three months after treatment.13 The inflammation was characterized by adipocyte apoptosis, granulomatous panniculitis, and macrophage accumulation. At the onset of inflammation, the fat layer underwent massive fat cell destruction concomitant to increased recruitment of immune cells. This process reached its peak within one month of injury, after which point, a wound healing process was initiated and lasted for the next two months. During this period, the destroyed fat cells were cleared from the tissue and the overall thickness of the layer was reduced. These findings might explain the moderate circumference reduction observed here at one month following treatment and the clinically significant mean reduction registered following three months.
Safety evaluations demonstrated that the treatment was well-tolerated, elicited short-lived and minimal post-treatment skin reactions, and induced no serious AEs. Treatment by either hexagonal or rectangular therapeutic heads was associated with a mean VAS score of approximately 3 (mild pain). We assume that the pain reported during flank treatment was slightly higher than that during abdominal treatment due to the proximity of the flanks to bony landmarks of the pelvis.
Taken together, the primary efficacy endpoint, which was defined as a circumference reduction at three months following treatment, was met. This was supported by high investigator ratings of aesthetic improvement, subject satisfaction, and the percentage of photographs correctly categorized as before and after treatment. The treatment was well-tolerated and triggered no serious AEs. Thus, the study device appears to be an effective and safe means of reducing fat with minimal discomfort.
References
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14(4):e3727.
- Mulholland RS, Paul MD, Chalfoun C. Noninvasive body contouring with radiofrequency, ultrasound, cryolipolysis, and low-level laser therapy. Clin Plast Surg. 2011;38(3):503–520.
- Franco W, Kothare A, Ronan SJ, et al. Hyperthermic injury to adipocyte cells by selective heating of subcutaneous fat with a novel radiofrequency device: feasibility studies. Lasers Surg Med. 2010;42(5): 361–370.
- Ingargiola MJ, Motakef S, Chung MT, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg. 2015;135(6):1581–1590.
- Kennedy J, Verne S, Griffith R, et al. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29(9):1679–1688.
- Coleman KM, Coleman WP 3rd, Benchetrit A. Non-invasive, external ultrasonic lipolysis. Semin Cutan Med Surg. 2009;28(4):263–267.
- Krueger N, Mai SV, Luebberding S, Sadick NS. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;26;7:201–205.
- Coleman SR, Sachdeva K, Egbert BM, et al. Clinical efficacy of noninvasive cryolipolysis and its effects on peripheral nerves. Aesthetic Plast Surg. 2009;33(4):482–488.
- Manuskiatti W, Wachirakaphan C, Lektrakul N, Varothai S. Circumference reduction and cellulite treatment with a TriPollar radiofrequency device: a pilot study. J Eur Acad Dermatol Venereol. 2009;23(7): 820–827.
- Bayrakci Tunay V, Akbayrak T, et al. Effects of mechanical massage, manual lymphatic drainage and connective tissue manipulation techniques on fat mass in women with cellulite. J Eur Acad Dermatol Venereol. 2010;24(2):138–142.
- Lach E. Reduction of subcutaneous fat and improvement in cellulite appearance by dual-wavelength, low-level laser energy combined with vacuum and massage. J Cosmet Laser Ther. 2008;10(4):202–209.
- Otto MJ. The safety and efficacy of thermal lipolysis of adipose tissue via ultrasound for circumference reduction: an open label, single-arm exploratory study. Lasers Surg Med. 2016;48(8):734–741.
- Ferrando G. Safety, tolerability, and efficacy evaluation of the SlimME device for circumference reduction. Lasers Surg Med. 2018; published online DOI 10.1002/lsm.22796.

