 by Kelly Segars, DO; Hassie Cooper, DO; Daniel J. Hogan, MD, FAAD; Richard Miller, DO, FAOCD; Michael Heaphy, Jr., MD, FAAD; and James Spencer, MD, FAAD
by Kelly Segars, DO; Hassie Cooper, DO; Daniel J. Hogan, MD, FAAD; Richard Miller, DO, FAOCD; Michael Heaphy, Jr., MD, FAAD; and James Spencer, MD, FAAD
Dr. Segars is with NSU-COM/Largo Medical Center Dermatology Residency in Largo, Florida. Dr. Cooper is with NSU-COM/Largo Medical Center Dermatology Residency in Largo, Florida. Dr. Hogan is with the C.W. Bill Young Bay Pines VA Medical Center in Bay Pines, Florida. Dr. Miller is with Largo Medical Center in Largo, Florida. Dr. Heaphy is with Gulf Coast Dermatopathology Laboratory, Inc. in Tampa, Florida, and is Clinical Assistant Professor of the Department of Dermatology at NSU-COM in Largo, Florida. Dr. Spencer is with Spencer Dermatology in St. Petersburg, Florida.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest to relevant to the content of this article. The views and opinions expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Abstract: Introduction: Basaloid follicular hamartoma (BFH) is a rare, benign neoplasm of the hair follicle, characterized by multiple brown papules involving the face, scalp, and trunk. It is described by multiple clinical forms, and can present as localized or generalized. Diagnosis is made histologically via biopsy, which is important in order to distinguish BFH from basal cell carcinoma (BCC) or other malignant epithelial neoplasms. Correct diagnosis allows for the avoidance of unnecessary surgeries to remove benign lesions. While benign, lesions can be cosmetically unacceptable. Case Report: A 68-year-old man with a two-year history of brown, homogenous papules on his face presented to discuss treatment options. A physical examination revealed hundreds of dark brown, 1- to 3mm verrucous papules distributed throughout the face. Two punch biopsies revealed histologic features consistent with BFH. Discussion: BFHs classically present with multiple 1- to 2mm tan-to-brown-colored papules distributed on the face, scalp, neck, axilla, trunk, and pubic area. Differential diagnoses can include nevus sebaceous, lichen striatus, linear epidermal nevus, and basal cell nevus. BFH arises from a mutation in the patch gene, the same gene thought to cause nevoid BCC syndrome. Histologic examination of BFH lesions is essential to diagnosis. No standard of care exists for BFH; treatment options remain limited. This patient was treated with three rounds of pulsed dye laser (PDL) therapy and showed marked improvement in the treated areas. The authors propose PDL to be a safe, effective, and novel cosmetic treatment for BFH and potentially other adnexal tumors.
Keywords: Basaloid follicular hamartoma, patch gene, pulsed dye laser, treatment of adnexal tumors
J Clin Aesthet Dermatol. 2018;11(3):39–41
Basaloid follicular hamartoma (BFH) is a benign neoplasm of the hair follicle.1 On histologic exam, BFH consists of epithelial proliferation of basaloid cells in anastomosing strands and cords.1,3
BFH currently has five described clinical forms: 1) individual or multiple papules; 2) localized plaques accompanied by alopecia; 3) localized linear papules or plaques; 4) generalized autosomal dominant type without accompanying disorders; and 5) generalized papules accompanied by alopecia and myasthenia gravis.3–7 It is characterized by multiple brown papules typically on the face, scalp, and trunk.1 It can present in a localized or generalized distribution and can occur as a familial syndrome, a congenital condition, or an acquired condition.1 Here we report a case of hundreds of BFHs on the face of a 68-year-old man, and we provide a review of the literature.
Case report
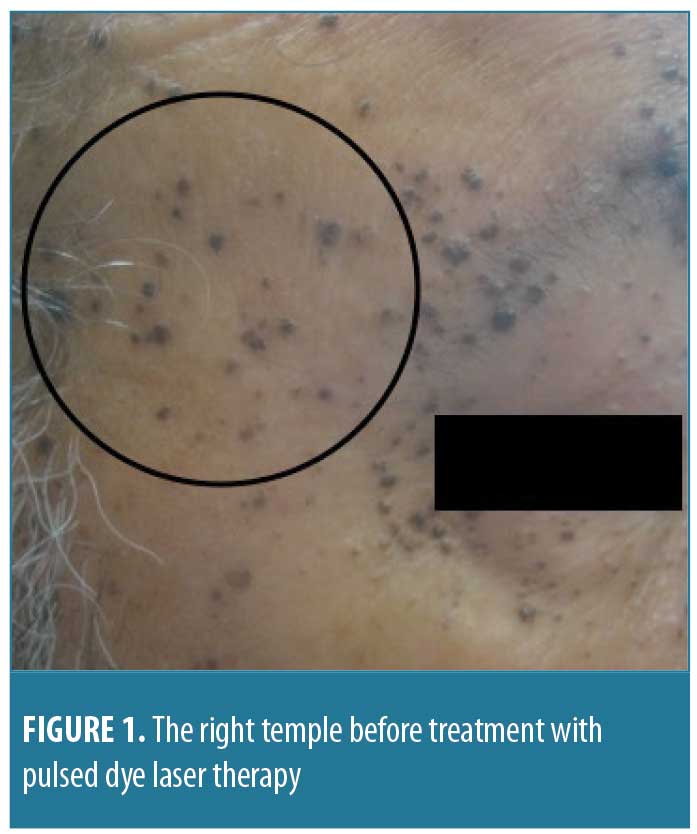
A 68-year-old man presented with a two-year history of brown, homogenous papules on his face. He presented to discuss available treatment options for the removal of the papules, as they were cosmetically bothersome. His past medical history included atrial fibrillation, chronic kidney disease, dilated cardiomyopathy with defibrillator, obstructive sleep apnea, congestive heart failure, and hyperlipidemia. On physical examination, he had hundreds of dark brown, 1- to 3mm warty papules distributed on his face, with higher concentrations of papules in the nasolabial folds, forehead, and periorbital regions (Figure 1). The patient’s physical examination was otherwise unremarkable, and no palmar pits were noted. Two punch biopsies were taken: one in the left preauricular area and one in the left nasolabial fold. The nasolabial fold biopsy revealed histologic features consistent with the suspected diagnosis of BFH (Figure 2).
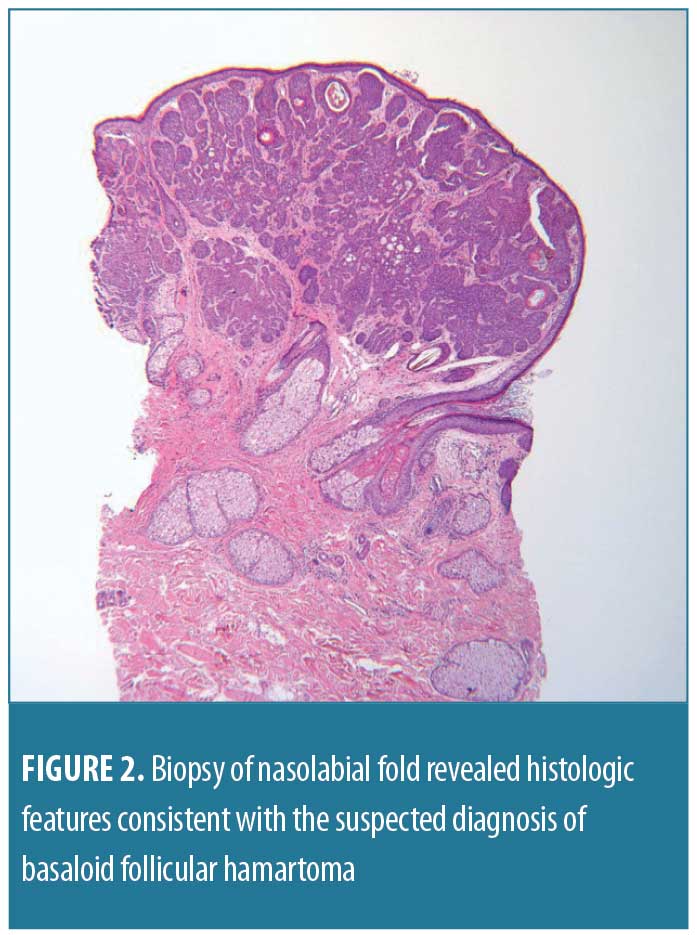
Small collections of basaloid cells, growing as anastomosing strands and forming cystic structures, were seen. There was no stroma-epithelium retraction and no cellular positivity with androgen receptor. However, there was positivity with B-cell lymphoma 2 (Bcl-2), the stroma was CD34-positive, and CK20 revealed scattered positive cells within the growth.
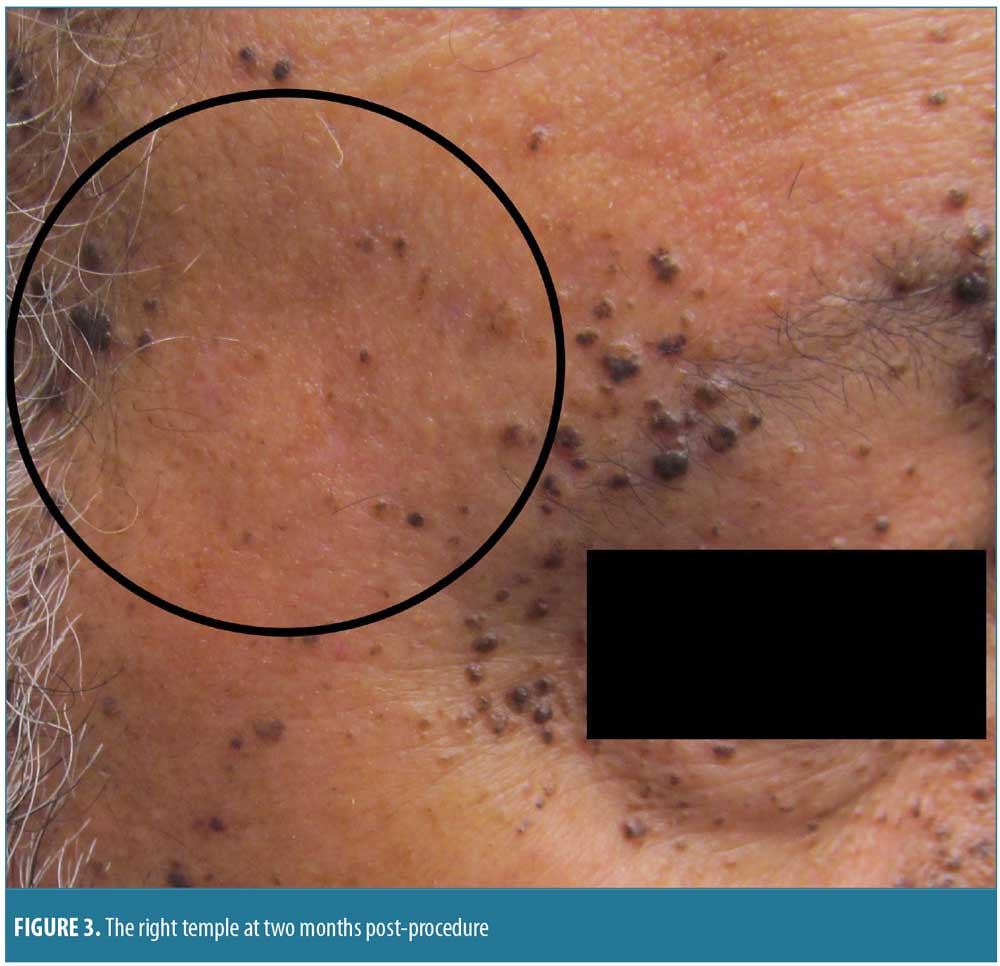
The patient was treated with pulsed dye laser (PDL) therapy in three areas of the face: the right temple, the right cheek, and the entire forehead. The right temple was treated for 1.5ms with 10J of cryogen and was found to be clear at two months post-procedure (Figure 3).
Discussion
A 1969 paper by Brown et al7 first characterized and described BFH, but called it generalized follicle hamartoma. The researchers presented a case report of a patient with multiple papules in the nasolabial folds and around the eyes. This patient also presented with diffuse alopecia and myasthenia gravis. The term basaloid follicular hamartoma was first used by Mehregan et al in a 1985 paper6 when describing a new adnexal tumor distribution that may be associated with an underlying disease or syndrome.
Clinically, most cases of BFH present with multiple 1- to 2mm tan-to-brown-colored papules on the face, scalp, neck, axilla, trunk, and pubic area.1,4 There might be other associated conditions in patients with BFH, including alopecia, palmar pitting, hypohidrosis, hypotrichosis, myasthenia gravis, systemic lupus erythematosus, and cystic fibrosis.1,4,7,9,14–16 Five types of BFH have been described in the literature: 1) solitary or multiple papules; 2) localized linear papules/plaques; 3) localized papules with associated alopecia; 4) generalized papules associated with myasthenia gravis and alopecia; and 5) a generalized autosomal dominant familial type without associated disorders.3–7 The patient in our case was deemed to have the multiple papules type, as he had no other relevant signs or symptoms and no family history that might place him in another subtype.
The differential diagnosis of BFH is partially dependent on whether it presents as individual or multiple papules or plaques; however, the differential diagnosis for both forms include: basal cell carcinoma, sebaceous hyperplasia, seborrheic keratosis, trichilemmoma, intradermal melanocytic nevus, syringoma, angiofibroma, steatocystoma nevus sebaceous, lichen striatus, linear epidermal nevus, and basal cell nevus.1,17
BFH arises due to a mutation in the patch (PTCH) gene located on chromosome 9q23, the same gene thought to cause nevoid basal cell carcinoma syndrome.4 Expression of the mutation is thought to milder in BFH, as these are benign tumors.4,8 PTCH codes for a receptor that binds the product of the sonic hedgehog gene (SHH).1 SHH encodes signaling proteins linked with patterning during embryonic development. 1 The PTCH receptor usually binds with the G-protein smoothened (SMO).4 When SHH is absent, the PTCH receptor inactivates SMO, thereby inhibiting any downstream signal.4 When SHH is present and binds to the PTCH receptor, SMO is no longer inhibited and hedgehog target genes downstream are upregulated through a cascade using Gli family transcription factors.1,9,10 This constant signaling results in unchecked and abnormal cell division and growth.4 It is important to distinguish BFH from other epithelial neoplasms with malignant potential such as infundibulocystic basal cell carcinoma (BCC). Immunohistochemistry is a helpful tool to demonstrate this distinction. The stromal cells are CD34+, while the BCC cells are CD34-; BFH has a low proliferative index (low Ki-67), whereas BCC expresses strong Ki-67 positivity.11,12,13 Bcl-2 expression is typically negative in BFH and positive in BCC, but negativity in BFH is inconsistent. The immunohistochemical staining of our patient’s biopsy was consistent with BFH: CD34+ stroma, with anastomosing strands of basaloid cells without retraction between the stroma and epithelium.
A histologic examination of BFH lesions is key in making the diagnosis. Brown et al originally described the lesion as basaloid cell proliferations of the entire hair follicle; however, this is questionable.7 The basaloid cells contained hyperchromatic nuclei with rare mitotic figures and sparse cytoplasm.7 More recent studies have characterized the histology of BFH as anastomosing strands and cords of basaloid cells that make up the affected hair follicles.1,3 Again, there are rare mitotic figures and there is minimal stroma.1 Since BFH is only found within hair follicles, the tissue is histologically normal in the dermis between follicles as well as in the reticular dermis.1,18
Treatment options for BFH are limited and experimental; no standard of care has been determined. There are reports of the use of 5-aminolevulinic acid (5-ALA) plus photodynamic therapy as a safe treatment for BFH. This is the treatment of choice for children with multiple BFH.1
PDL has traditionally been used to treat vascular lesions such as port-wine stains and has also been used for acne, scars, and photorejuvenation. PDL is a 595 nm laser that penetrates 1 mm to 2 mm into the epidermis, though larger spot sizes can achieve a greater depth of penetration.20
While PDL is traditionally utilized to treat vascular lesions, there have been reports discussing its effectiveness in treating nonvascular lesions, including pigmented lesions. There is minimal literature on the effects of PDL on BFH, but there are some reports of PDL used as an effective treatment for BCC and for other nonvascular lesions. PDL has been shown in multiple studies to be an effective treatment for pigmented lesions including lentigines,21 nevus spilus,21 and melanocytic nevi by selectively targeting and destroying the chromophore of melanin or hemoglobin. One review discussed the efficacy of PDL as a treatment for nonvascular lesions, including verrucae vulgaris, molluscum contagiosum, and several other nonvascular lesions.22 They hypothesized that PDL was effective in these cases because of its destructive effect on the surrounding vasculature supplying the lesions, thereby potentially reducing the nutrients available to the affected cells.22 A similar hypothesis exists regarding using PDL as a treatment for BCC. The mechanism of destruction of BFH in our case is proposed to be secondary to selective photothermolysis of the vasculature as well as targeting of melanin pigment in the tumors themselves.
In theory, other traditionally vascular lasers could also be used to treat BFH, including potassium-titanyl-phosphate laser and intense pulsed light (IPL) therapy. Furthermore, lasers that target deeper dermal pigmentation may also be effective, including ruby, alexandrite, and neodymium-doped yttrium aluminium garnet lasers. More studies are needed to evaluate the efficacy and side effects of these treatments.23
In summary, BFH is a rare benign adnexal tumor that has varying clinical presentations and can be an inherited or acquired condition. It is diagnosed histologically via biopsy, which is important to keep in mind when attempting to distinguish BFH from BCC. Correctly diagnosing BFH will help patients to avoid unnecessary surgeries to remove benign lesions.11,19 More research is needed to find effective treatments and medications for BFH, as these lesions can be cosmetically bothersome to affected individuals. PDL may be a safe and effective treatment for BFH, though more studies are necessary.
References
- Mills O, Thomas B. Basaloid follicular hamartoma. Arch Pathol Lab Med. 2010;134(8):1215–1219.
- Huang S, Hsiao T, Lee C. Basaloid follicular hamartoma: a case report and review of the literature. Kaohsiung J Med Sci. 2012;28(1):57–60.
- Wheeler C, Carroll M, Groben P, et al. Autosomal dominantly inherited generalized basaloid follicular hamartoma syndrome: report of a new disease in a North Carolina family. J Am Acad Dermatol. 2000; 43(2 Pt 1):189–206.
- Gumaste P, Ortiz A, Patel A, et al. Generalized basaloid follicular hamartoma syndrome: a case report and review of the literature. Am J Dermatopathol. 2015;37(3):e37–e40.
- Brownstein M. Basaloid follicular hamartoma: solitary and multiple types. J Am Acad Dermatol. 1992;27(2 Pt 1):237–240.
- Mehregan A, Baker S. Basaloid follicular hamartoma: three cases with localized and systematized unilateral lesions. J Cutan Pathol. 1985;12(1):55–65.
- Brown A, Crounse R, Winkelmann R. Generalized hair-follicle hamartoma, associated with alopecia, aminoacidura, and myasthenia gravis. Arch Dermatol. 1969;99(4):478–493.
- Grachtchouk V, Grachtchouk M, Lowe L, et al. The magnitude of hedgehog signaling activity defines skin tumor phenotype. EMBO J. 2003;22(11):2741–2751.
- Smith K, Skelton H. Basaloid follicular hamartomas associated with autoimmune disease: a possible role for retinoids in therapy. J Am Acad Dermatol. 2003;49(6):1067–1070.
- Villavicencio E, Walterhouse D, Lannaccone P. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67(5):1047–1054.
- Lee P, Lourduraj L, Palko M, et al. Hereditary basaloid follicular hamartoma syndrome. Cutis. 2006;78(1):42–46.
- Jih D, Shapiro M, James W, et al. Familial basaloid follicular hamartoma: lesional characterization and review of the literature. Am J Dermatopathol. 2003;25(2):130–137.
- Naeyaert J, Pauwels C, Geerts M, et al. CD-34 and Ki-67 staining patterns of basaloid follicular hamartoma are different from those in fibroepithelioma of Pinkus and other variants of basal cell carcinoma. J Cutan Pathol. 2001;28(10):538–541.
- Ramos-Ceballos F, Pahaei S, Kincannon J, et al. Bcl-2, CD34 and CD10 expression in basaloid follicular hamartoma, vellus hair hamartoma and neurofollicular hamartoma demonstrate full follicular differentiation. J Cutan Pathol. 2008;35(5):477–483.
- Mascaro J, Ferrando J, Bombi J, et al. Congenital generalized follicular hamartoma associated with alopecia and cystic fibrosis of three siblings. Arch Dermatol. 1995;131(4):454–458.
- Starink T, Lane E, Meijer C. Generalized trichoepitheliomas with alopecia and myasthenia gravis: clinicopathologic and immunohistochemical study and comparison with classic and desmoplastic trichoepithelioma. J Am Acad Dermatol. 1986;15(5 Pt 2):1104–1112.
- Patel A, Harting M, Smith-Zagone M, Hsu S. Familial basaloid follicular hamartoma: a report of one family. Dermatol Online J. 2008;14(4):14.
- Requena L, del Carmen Farina M, Robledo M, et al. Multiple hereditary infundibulocystic basal cell carcinomas. Arch Dermatol. 1999;135(10):1227–1235.
- Nelson B, Johnson T, Waldinger T, et al. Basaloid follicular hamartoma: a histologic diagnosis with diverse clinical presentations. Arch Dermatol. 1993;129:915–917.
- Hruza GJ. Lasers and Lights: Procedures in Cosmetic Dermatology. 3rd ed. Edinburg, TX: Elsevier Inc.; 2013: 10–11.
- Abrusci V, Benzecry V. Pulsed dye laser treatment of multiple common acquired melanocytic nevi: a novel approach. Dermatol Surg. 2016;42(7):914–917.
- Karsai S, Roos S, Hammes S, Raulin C. Pulsed dye laser: what’s new in non-vascular lesions?. J Eur Acad Dermatol Venereol. 2007;21(7):877–890.
- Hamilton M. Laser treatment of pigmented and vascular lesions in the office. Facial Plast Surg. 2004;20(1):63–69.

