 J Clin Aesthet Dermatol. 2020;13(8):17–22
J Clin Aesthet Dermatol. 2020;13(8):17–22
by Dirk Freiherr von Dalwig-Nolda, MD and Glynis Ablon MD, FAAD
Dr. Freiherr von Dalwig-Nolda is with the DermaVen Center in Bad Salzuflen, Germany. Dr. Ablon is with the University of California, Los Angeles in Los Angeles, California and the Ablon Skin Institute & Research Center in Manhattan Beach, California.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective: To assess the effectiveness of the amiea med (amiea med, MT.DERM GmbH, Berlin, Germany) automated microneedling device in reducing facial atrophic acne scars.
Study design: Open label, single center.
Participants: Healthy males and females, aged 18 to 65 years, with signs of facial atrophic acne scarring were selected. After consenting and satisfying inclusion criteria, each subject underwent four microneedling sessions 30 days apart. Subjects were assessed at baseline and three months after the last treatment.
Measurements: Acne scars were classified according to Jacob classification. Physician assessment of acne scarring severity was carried out using the Goodman and Baron grading scale. Subjects graded their redness, pain and discomfort on the evening of the treatment and up to seven days posttreatment using a subject diary.
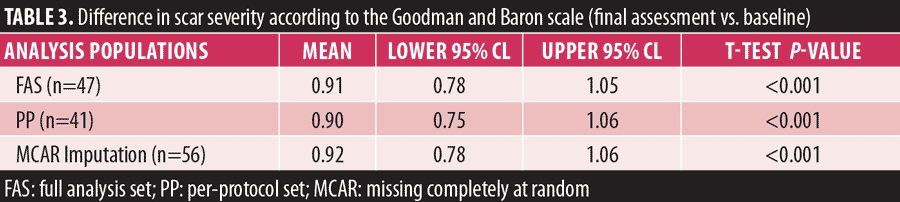
Results: Three months after the last treatment, facial acne scars had improved by 0.91 of a grade [CI. 0.78-1.05] according to Goodman and Baron Grading Scale (p<0.001). Improvement in acne scarring was not restricted to severity of grade,with no difference seen in Fitzpatrick skin types. According to Jacob classification, rolling scars showed the greatest improvement, with a mean improvement of 1.06 of a grade at the final assessment (p<0.001).
Conclusion: This study demonstrates that four microneedling treatments of facial skin, spaced four weeks apart, significantly improves the appearance of facial acne scarring. The treatment is well tolerated with minimal pain, discomfort, and downtime. Side effects appear minor and easily managed compared to other more aggressive technologies.
Study Registration: German Register for Clinical studies (DRKS) No [DRKS00013187]
Keywords: Microneedling, acne scars, safety, effectiveness, single-center, open-label, minimally invasive, percutaneous collagen induction, tissue remodeling
Acne vulgaris is one of the most common skin diseases and a dermatological disorder of adolescents, affecting 80 percent of individuals 11 to 30 years of age.1–3 Scarring due to facial acne is a result of excessive inflammation, acne severity, physical manipulation of the skin, and a delay in seeking adequate treatment. While epidemiologic data on acne scarring vary, many patients with acne experience some degree of scarring.3,4 Due to the physical disfigurement and associated psychological incumbrance of atrophic acne scarring, many patients with scarring seek treatment to improve the condition.5
Management of acne scarring remains a therapeutic challenge. Aggressive treatments, such as carbon dioxide laser resurfacing and deep chemical peels, yield significant improvement, but are associated with considerable downtime, permanent hypopigmentation, and the risk of pigmentation, especially in patients with higher Fitzpatrick skin types. Nonablative lasers and microdermabrasion are associated with much less downtime but do not achieve the same levels of treatment efficacy.6 In recent years, fractionated microneedling and laser technologies—which remove or perforate the skin in a pinpoint pattern, inducing neocollagenesis—have shown encouraging results.7
Microneedling, also known as percutaneous collagen induction (PCI), is a minimally invasive technique first described as a principle by Orentreich and Orentreich,8 who described “subscision” as a way of building up collagen beneath retracted scars and wrinkles. Later, Camirand and Doucet introduced PCI as a treatment for acne scars performed using a tattoo gun.9,10 Independently, Fernandes used a similar technique of inserting a 15-gauge needle into the skin, inducing PCI.11,12 This was followed by the development of the dermal roller. Generically, a dermal roller is a sterile plastic cylinder with stainless steel needles protruding between 1mm and 3mm from the surface of the cylinder. The dermal roller is rolled vigorously over the skin to create numerous needle pricks, which leads to thousands of microscopic wounds in the dermis, initiating the natural posttraumatic inflammatory response i.e., the release of growth factors and the formation of collagen and elastin.11,12
Modern automated microneedling devices have increasingly replaced the dermal roller. Typically, the needle cylinder is replaced by single-use, sterile needle cartridges with a range of different needle configurations. Rather than relying on the operator to physically roll the device over the skin, these automated devices allow the operator to define the penetration depth and frequency of the needle penetration and control the treatment area and coverage. Current automated microneedling devices contain multiple fine sterile needles, typically 0.5 to 3mm in length.
In addition to acne scarring, there is a growing list of indications that respond to PCI, including wrinkles and laxity, dyspigmentation, alopecia, and hyperhidrosis. The primary advantage of PCI is that the technique preserves the epidermis while stimulating collagen deposition, thereby reducing the risk of posttreatment complications and decreasing downtime. The second author has previously reported on the safety and effectiveness of this type of device for the treatment of age-related skin conditions.13
The purpose of the present study was to evaluate the efficacy and safety of an automated microneedling device when used for acne scar treatment.
Methods
The study protocol was approved by the ethics committee of Westfallen-Lippe and Westfallen Wilhelms-University Münster, Germany (2017-366-f) and registered in the German Register for Clinical Studies (no. DRKS00013187).
This was a single-center, open-label study involving 56 healthy subjects. Screening data were reviewed to determine eligibility. After informed consent, confidentiality, and photographic release forms were completed, volunteers who met all inclusion criteria and none of the exclusion criteria entered the study. Each subject underwent four microneedling sessions, four weeks apart.
Subjects were assessed at baseline and three months after the last treatment. The number, type, and severity of adverse events were recorded during the duration of the study period.
Subject selection. Healthy males and females, aged 18 to 65 years, with signs of facial atrophic acne scarring, including icepick, rolling, and boxcar, were selected. Volunteers were excluded if their facial scars were not older than six months, they had active acne vulgaris, or were pregnant or lactating. Volunteers were also excluded if they suffered from hemophilia or other bleeding disorders or were taking an anticoagulant or using aspirin. Volunteers with systemic infections, acute skin infections of the face, inflammatory skin conditions, uncontrolled diabetes mellitus, or dermatosis (e.g., keloid scars), birth marks, or eczema in the treatment area were also excluded. Further exclusion criteria were known malignancy or volunteers undergoing chemotherapy, radiotherapy, or high-dose corticosteroid therapy. In addition, volunteers who had undergone plastic surgery within the previous 12 months, had a known allergy to the topical anesthetic, or who had undergone filler injections within the previous six months were also excluded.
Microneedling device. The chosen microneedling device (amiea med, MT.DERM GmbH, Berlin, Germany) consists of a handpiece, a safety needle cartridge, and a control unit. The handpiece contains the motor that moves the needles of the safety needle cartridge and a needle depth gauge that allows the user to control the depth of needle stitches from 0 to 1.5mm. The control unit allows the user to adjust the frequency of the needle stroke from 50 to 150Hz. The safety needle cartridge contains six stainless steel microneedles (maximum of 1.5mm in length, 0.35-mm gauge), is supplied sterile, and is single-use (Figure 1).

Treatment protocol. Each subject underwent four microneedling sessions, four weeks apart. After cleansing the face with Sterillium® (BODE Chemie GmbH, Hamburg, Germany), a topical anesthetic containing lidocaine and prilocaine (EMLA; AstraZeneca, Cambridge, United Kingdom) was applied to the entire face. After 30 minutes, excess anesthetic was removed by wiping the skin with a cotton pad soaked in 70% isopropyl alcohol and the skin was allowed to dry. The skin was then wiped again with Sterillium® and allowed to dry. A liberal quantity of the sterile ultrasound transmission gel Aquasonic® 100 (Parker Laboratories, Fairfield, New Jersey) was applied to the subject’s face to assist with the movement of the needle cartridge over the skin.
The operator was instructed to start with a minimum needle protrusion of 0.5mm and increase in increments until pinpoint bleeding was achieved. During the treatment, the operator was directed to stretch the skin to ensure even piercing of the needles. The handpiece was guided perpendicular to the skin in a rapid circular motion. Blood was wiped away with a sterile saline solution to prevent encrustation. At the end of the treatment, the face was cleansed again with sterile saline solution.
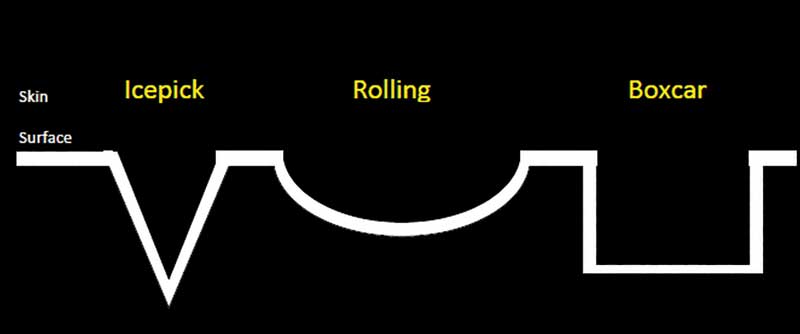
Recorded data. Acne scars were classified according to Jacob.2 The Jacob classification is a descriptive, simple, universally applicable acne scar classification system that includes three scar types: icepick, rolling, and boxcar.
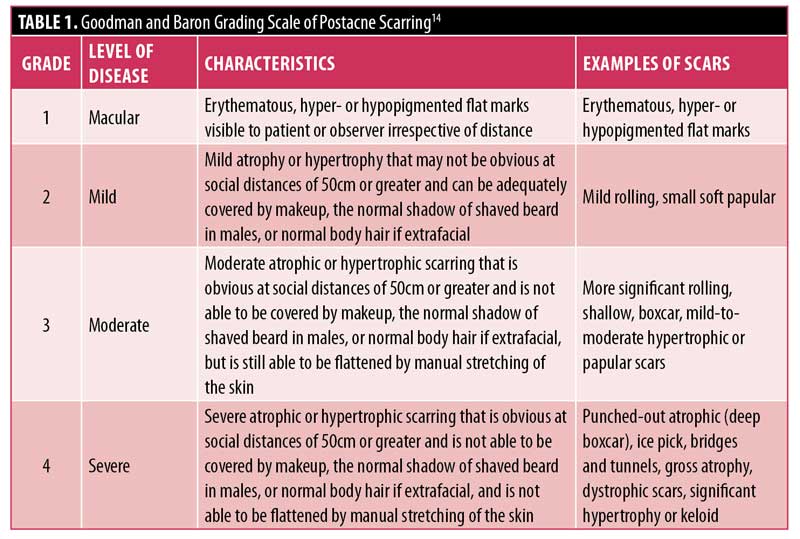
Physician assessment of acne scarring severity was carried out using the Goodman and Baron grading scale.14 The Goodman and Baron scale is a qualitative global acne scar grading system whose aim is to establish an index of severity of an individual’s condition that can be readily acknowledged, recorded, and compared over time or at a point in time in a clinic or between different clinics (Table 1).14 VISIA photography (Canfield Scientific, Parsippany, New Jersey) was performed at baseline and at the final assessment.
Subjects graded their redness, pain, and discomfort on the evening of the treatment and up to seven days posttreatment using a subject diary. A descriptive photonumeric grading scale was used to evaluate redness. Pain and discomfort were recorded using an 11-point visual analog scale (0–10 points), where zero points was equivalent to no pain/discomfort and 10 points represented the most intense pain/discomfort ever.
The number, type, and severity of adverse events were also recorded during the duration of the study period.
Objective and endpoints. The objective of the study was to assess the effectiveness of the amiea med automated microneedling device in reducing facial atrophic acne scars.
The primary endpoint of this study was to show a significant reduction, defined as a single-grade reduction in acne scarring according to the Goodman and Baron grading scale, three months after a course of four microneedling sessions relative to at baseline.
Secondary endpoints included a significant reduction of scarring at three months after the last treatment versus at baseline according to baseline severity using the Goodman and Baron grading scale and the Jacob classification.
Down time was defined as the time in which redness, pain, and discomfort after treatment decreased. The safety endpoints were incidence and the degree of any adverse events.
Data analyzed. All eligible subjects who were enrolled into the study and received at least one microneedling treatment were included in the safety analysis (safety analysis set; SAF). Also, all eligible subjects who were enrolled into the study and received at least one microneedling treatment were included in the analysis of efficacy (full analysis set; FAS).
The per-protocol set (PP) and missing completely at random (MCAR) imputation were used for sensitivity analysis.
Analysis of efficacy. For changes in acne scarring, 95% confidence intervals (CIs) were computed for the mean changes from baseline in the Goodman and Baron score. A one-sample, two-sided t-test was applied to test the null hypothesis (reduction=0). If the null hypothesis was rejected at the 0.05 level and a reduction was observed, the study was regarded as a success.
RESULTS
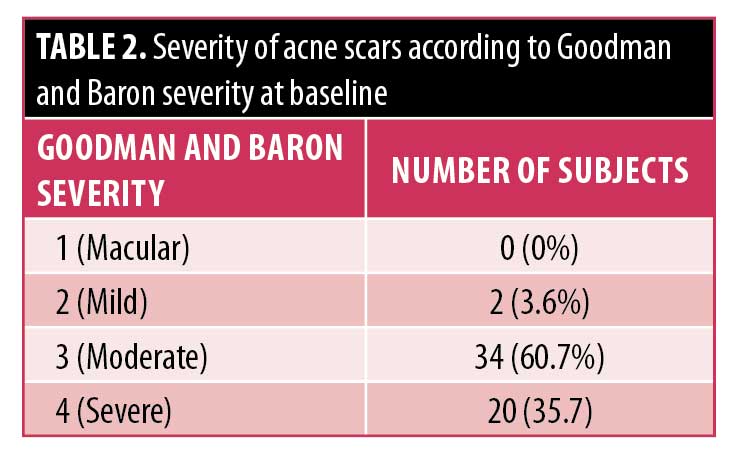
Study population. Fifty-six subjects were enrolled into this study and 47 subjects completed all treatment and assessment visits. Six subjects failed to attend all the treatment visits and were withdrawn and three subjects were lost to final follow-up. No subjects were withdrawn due to issues related to study protocol or to reported adverse events. The mean age of the subjects was 35.8 years with a range of 18 to 62 years and a male-to-female ratio of 18:38. Fitzpatrick Skin Types ranged from I to IV. According to the Jacob classification, at baseline, 42 subjects had ice-pick scars, all subjects presented with rolling scars, and 55 showed boxcar scars. The severity according to Goodman and Baron ranged from mild (two points) to severe (four points) (Table 2).2,14
Treatment. The average microneedling time over the four treatments was 15.7 minutes.Needle protrusion (i.e., the length of needle that protrudes out of the needle cartridge into the skin) was, on average, 1.39mm. The average frequency used in all four treatments was 147.2Hz.
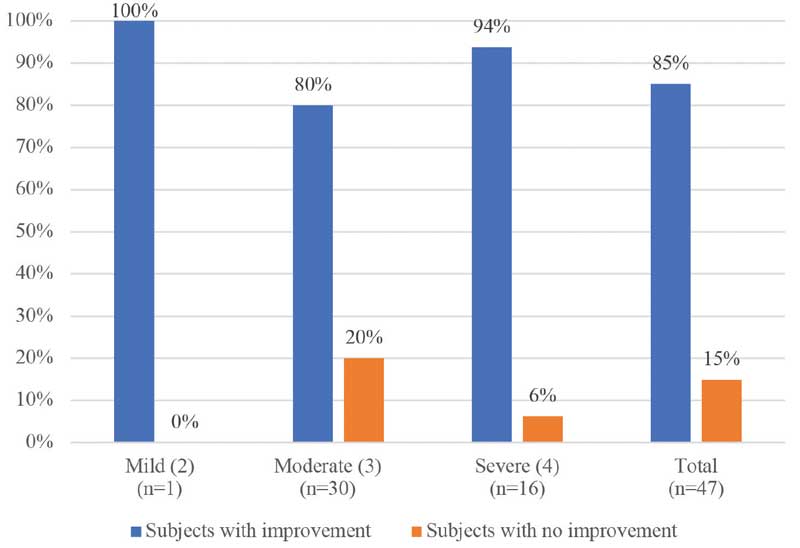
Efficacy. At final assessment, three months after the four microneedling sessions, facial acne scars had improved by 0.91 of a grade (95% CI: 0.78–1.05) according to the Goodman and Baron grading scale (p<0.001).14 Forty subjects (85.1%) saw some reduction in facial acne scarring relative to baseline (Figure 3). Seven subjects (14.9%) saw no improvement in their acne scarring. Worsening of scarring was not reported in any of the subects over the course of the treatment. These results were confirmed by sensitivity analyses.
PP analysis showed a significant reduction in scarring (p<0.001) with a mean of 0.90 (95% CI: 0.75–1.06). MCAR imputation showed a significant reduction in scarring (p<0.001) with a mean of 0.92 (95% CI: 0.78–1.06) (Table 3)
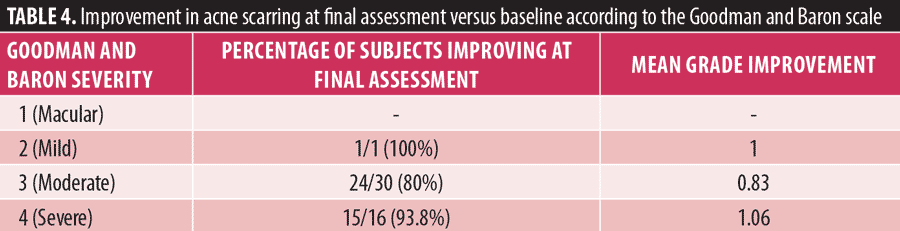
Improvement according to acne scar severity at baseline. In relation to the secondary endpoint, Table 4 and Figure 3 illustrate the number of subjects who saw improvements in acne scarring after the last treatment compared to at baseline according to the severity based on the Goodman and Baron grading scale.
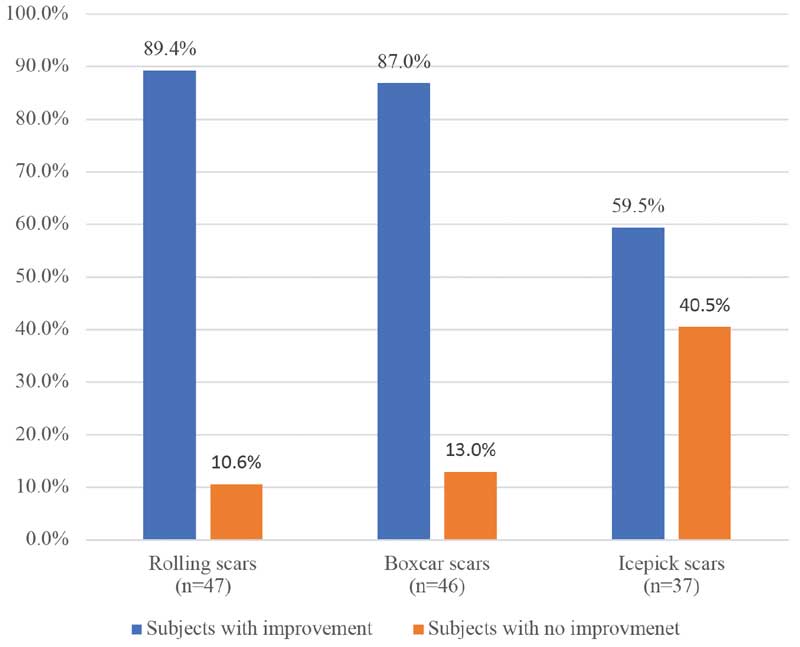
Improvement according to acne scar type. Within acne scarring types according to the Jacob classification, the greatest improvement was seen in the rolling scars subgroup, with 42 of 47 (89.4%) of subjects improving at final assessment. Meanwhile, 30 subjects (30/46; 65.2%) within the boxcar subgroup saw an improvement and 22 of 37 (59.5%) of subjects within the icepick subgroup saw an improvement (Figure 4).
With respect to absolute improvement on the Goodman and Baron grading scale, rolling scars showed the greatest improvement according to the Goodman and Baron scale with a mean improvement of 1.06 of a grade at the final assessment. Improvements in ice pick and boxcar scars were not as significant (0.68 and 0.72 of a grade, respectively).
According to Fitzpatrick Skin Type, there was no significant difference in treatment outcome among Fitzpatrick Skin Types I to IV at three months after last treatment relative to at baseline.
The time in which redness, pain, and discomfort after treatment decreased was recorded by the subject from the evening of the treatment until Day 7. Subject-reported evaluation of pain on the evening of the treatment received a mean pain score of 2.8. Mean pain scores receded to less than one by Day 3 and remained less than one until Day 7. Subject-reported discomfort was highest on Day 1 with a mean score of 3.2. Discomfort had receded to less than one by Day 4 and remained there until Day 7. Finally, 49 percent of subjects reported their skin to be moderately red on the evening of the treatment; the remainder reported their skin redness to be mild or less than mild. By Day 4, no subjects reported their skin redness as moderate. One subject reported their redness to be mild, with the remainder reporting minor, minimal, or absent redness. By Day 5, 94 percent of subjects reported their skin redness as either minimal or absent.
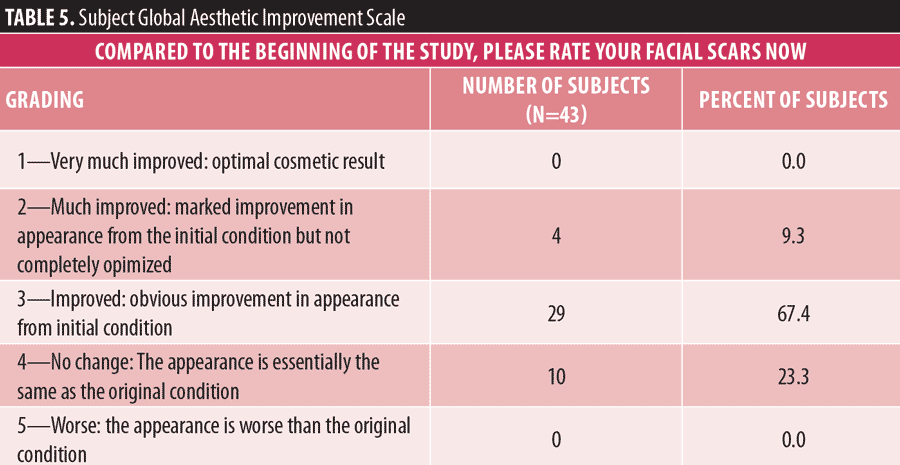
Subject assessment of treatment outcome. At final follow-up, subjects assessed their facial acne scarring relative to at baseline using the Subject Global Aesthetic Improvement Scale (SGAIS).15 Of those subjects responding, 33 of 43 (77%) reported visible improvement in their acne scarring, while 10 subjects (23%) reported no change. No subjects reported that the appearance of their acne scars was worse than the original condition (Table 5). Thirty-eight subjects (88%) said that they would recommend the treatment to family and friends.
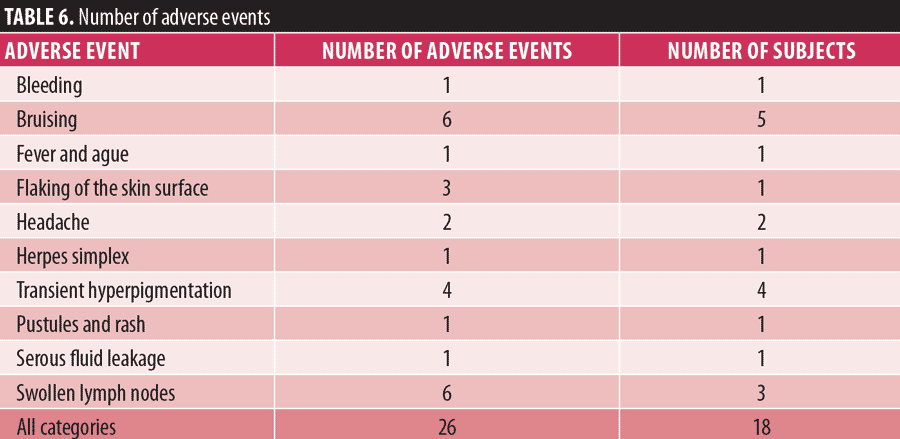
Safety. No serious adverse events were reported during the study period. However, there was a total of 26 adverse events reported by 18 subjects (Table 6). Twenty-two of these were defined as expected and four as unanticipated or unexpected. Three of the unexpected adverse events were attributable to the device, including headache (n=2) and excessive bleeding of the treatment area two days postintervention (n=1). One event, fever and rigors, was not attributable to the use of the device. Twenty-three (88.5%) adverse events were recorded as mild and three (11.5%) were recorded as moderate. No adverse event resulted in study discontinuation. No events were unresolved at the end of the study period. Bruising (six events in five subjects) and swollen lymph nodes (six events in three subjects) were the most common adverse events. Transient hyperpigmentation was recorded in four subjects. There were no atypical skin observations made immediately after the treatment session or reported by subjects during follow-up.
Discussion
Facial acne scars are composed of the same protein collagen as the normal tissue it replaces; however, the structure of fibers differs from the normal random lattice weave. Instead, the collagen crosslinks and forms a pronounced configuration in a single direction. This unidirectional arrangement is functionally inferior.
Previous efficacious treatments of facial acne scarring, such as ablative laser resurfacing, induces collagen synthesis, but at the price of an overbearing inflammatory reaction and a higher risk of adverse events. In microneedling, however, the epidermis remains relatively intact; this is believed to induce transforming growth factor (TGF) ?3, which is associated with scarless healing and normal lattice-weave formation, rather than TGF ?1 and ?2, which are associated with collagen scar deposition.16 Synthesized collagen deposition in acne-scarred skin has also been visually demonstrated by El-Dawela et al16 using picrosirius red stain under polarized light to confirm the abundance of newly synthesized lattice-weave collagen replacing preoperative matured unidirectional collagen as a result of PCI.
Microneedling has been extensively studied for acne scar treatment. A recent review concluded that, although the studies analyzed were varied in structure, there was acceptable evidence available that supported the use of microneedling and PCI for acne scarring, while criticizing study design.17,18
Here, we present a suitably sized study reporting on the use of an automated microneedling system for the successful treatment of facial acne scarring in Fitzpatrick Skin Types I to IV. These data were used for successful clearance of a microneedling device for acne scar treatment in the United States (K182407) and Europe.
Microneedling is a comparatively simple and relatively quick office procedure that is effective in the treatment of facial acne scars. In this study, subjects demonstrated a significant improvement in facial acne scarring three months after a course of four treatments, with 85 percent of subjects seeing improvements in their acne scars. Results show that improvements in acne scarring were not restricted to any severity grade according to the Goodman and Baron grading scale; indeed, 94 percent of subjects with severe acne experienced improvement within the study, with their facial acne scars improving by 0.91 of a grade (95% CI: 0.78–1.05) according to the Goodman and Baron grading scale (p<0.001). Of scar types classified by the Jacob classification scheme, rolling and boxcar scars appeared to show greater improvements relative to icepick scars when using this type of cartridge. This pattern is similar to the results seen by Majid,6 who reported excellent improvements in rolling and boxcar scars with moderate improvements in icepick after a course of microneedling. Icepick scars are typically harder to treat and resolve because of the depth and anatomical nature of the scar shape. However, most subjects suffer from mixed types of acne scars and the improvement of the overall appearance of acne scars was observed in 85 percent of the subjects. Improvements in acne scars were observed equally by physicians and patients.
Since the epidermis remains structurally intact during treatment, with no damage to the basal membrane, side effects associated with the technique appear manageable and somewhat predictable. The treatment appeared to be well-tolerated by patients, with adverse events predominantly limited to transient bruising and swollen lymph nodes of the neck. Hyperpigmentation was reported in four individuals with Fitzpatrick Skin Types II, III, and IV, but this occurrence was transient and resolved prior to the next treatment and did not reoccur. This finding is supported by Bonati et al.19 whose review identified only a handful of cases of postinflammatory hyperpigmentation after microneedling. Conversely, Aust et al.20 reported that the number of melanocyctes remained stable in subjects undergoing microneedling, resulting in the absence of post inflammatory hyperpigmentation.
No serious adverse events were recorded. There appeared to be no adverse events related to the topical anesthetic or to the sterile gel used as a lubricant. There were no atypical skin observations made during or immediately after the treatment by the physician.
Future clinical evaluation of the technique would benefit from a more ethnically diverse study group, particularly those subjects with phototypes V and VI who might benefit from the lower risk of hyperpigmentation offered by the treatment. Studies using three-dimensional profilometry and different cartridge types and longer follow-up would be beneficial, particularly to identify the efficacy of this technique in the treatment of icepick scars.
Conclusion
Microneedling is a quick, relatively simple, and effective in-office procedure. This technology offers a number of benefits to the provider and patient. The device is cost-effective, with a relatively small recurring cost to the provider and the treatment is well-tolerated with minimal pain, discomfort, and minimal patient downtime. Side effects appear minor and easily manageable as compared to those of other, more aggressive treatments, such as laser ablation and strong chemical peels.
In this study, we have demonstrated that four microneedling treatments of facial acne scarring with a microneedling device, spaced four weeks apart, produce noticeable improvements in facial acne scars (p<0.001), irrespective of severity.
References
- Uslu G, ?endur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22(4):462–469.
- Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45(1):109–117.
- Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19(4):303–308.
- Connolly D, Vu HL, Mariwalla K, Saedi N. Acne scarring—pathogenesis, evaluation, and treatment options. J Clin Aesthet Dermatol. 2017;10(9):12–23.
- Newman MD, Bowe WP, Heughebaert C, Shalita AR. Therapeutic considerations for severe nodular acne. Am J Clin Dermatol. 2011;12(1):7–14.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2(1):26–30.
- Alam M, Dover JS, Arndt KA. To ablate or not: a proposal regarding nomenclature. J Am Acad Dermatol. 2011;64: 1170–1174.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21(6):543–549.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21(1):48–51.
- Dover JS, Hruza GJ. Laser skin resurfacing. Semin Cutan Med Surg. 1996;15(3):177–188.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26(2):192–199.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17(1):51–63, vi.
- Ablon G. Safety and effectiveness of an automated microneedling device in improving the signs of aging skin. J Clin Aesthet Dermatol. 2018 Aug;11(8):29–34.
- Goodman GJ, Baron JA. Post acne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32(12):1458–1466.
- Fabi SG, Massaki A, Eimpunth S, Pogoda J, Goldman MP. Evaluation of microfocused ultrasound with visualization for lifting, tightening, and wrinkle reduction of the décolletage. J Am Acad Dermatol. 2013;69(6):965–971.
- El-Dawela RE, Kerim MA. Formation of new collagen after dermal roller in atrophic acne scars. MJMR. 2013;24(1):15–28.
- Harris AG, Naidoo C, Murrell DF. Skin needling as a treatment for acne scarring: an up-to-date review of the literature. Int J Womens Dermatol. 2015;1(2):77–81.
- Hou A, Cohen B, Haimovic A, Elbuluk N. Microneedling: a comprehensive review. Dermatol Surg. 2017;43(3):321–339.
- Bonati LM, Epstein GK, Strugar TL. Microneedling in all akin rypes: a review. J Drugs Dermatol. 2017;16(4):308–313
- Aust MC, Reimers K, Repenning C, et al. Percutaneous collagen induction: minimally invasive skin rejuvenation without risk of hyperpigmentation—fact or fiction? Plast Reconstr Surg. 2008;122(5):1553–1563.

