 J Clin Aesthet Dermatol. 2018;11(8):21–28
J Clin Aesthet Dermatol. 2018;11(8):21–28
by Teo Soleymani, MD; Julien Lanoue, MD; and Zakia Rahman, MD
Drs. Soleymani, Lanoue, and Rahman are with the Department of Dermatology at Stanford University School of Medicine in Redwood City, California.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Background. Chemoexfoliation, also known as chemical peeling, is a method of targeted cutaneous ablation using specific caustic agents that allow for rapid, predictable, and uniform thickness of chemoablation to a desired cutaneous depth, ultimately resulting in an improved appearance of skin.
Objective. In this review, we provide an up-to-date analysis of all currently available chemical peels for dermatologic use, as well as a step-by-step instructional protocol for an algorithmic approach to treatment.
Methods. A comprehensive search of the Cochrane Library, MEDLINE, and PUBMED databases was performed to identify relevant literature investigating chemical peeling agents. In addition, a search of all commercially available, prescription-based peeling agents was performed to identify all products currently available in the United States market.
Results and Conclusion. Chemical peels are the third most commonly performed noninvasive cosmetic procedure in the United States, with over 1,300,000 procedures performed in 2016 alone. There has been a paradigm shift in recent years, with lasers largely supplanting deep peels. Despite this shift, superficial peels have proliferated in both popularity and product diversity. When used for the appropriate indication and with proper technique, nearly all peeling agents have demonstrated excellent clinical efficacy and remain an indispensable cost-effective tool in the dermatologist’s aesthetic toolbox.
Keywords: Chemical peel, Peel, Glycolic, Trichloroacetic acid, Phenol, Glogau, Aging, Photoaging, Wrinkles, Lentigo, Rhytides
Introduction
Chemoexfoliation, colloquially referred to as chemical peeling, is a method of targeted cutaneous ablation induced by specific caustic agents that allows for a rapid, predictable, and uniform thickness of chemoablation to a given desired cutaneous depth, ultimately resulting in improvement in the clinical appearance of skin. The goal of a chemical peel is to remove a predictable, uniform thickness of damaged skin, which subsequently allows for normal wound healing and skin rejuvenation to occur, while simultaneously minimizing complications, such as scarring and unwanted pigmentary change.
The caustic agents used for chemical peels cause controlled keratocoagulation and denaturation of the proteins within the epidermis and dermis, resulting in the release of proinflammatory cytokines and chemokines.1–7 Such targeted inflammation activates the normal healing signal cascade, including stimulation, development and deposition of new dermal collagen and elastin, reorganization of structural scaffold proteins and dermal connective tissue, and regeneration of new keratinocytes.1–10 This results in rejuvenation and thickening of the epidermis and an increase in dermal volume.1–10 Simultaneously, the keratocoagulation and subsequent exfoliation result in improvement in superficial and medium-depth dyspigmentation.1–10 While there might be subtle variability between the types of chemical agents used and their intended cosmetic outcome (i.e., reduction of redness vs. dyspigmentation vs. scarring), the general goal of a chemical peel is to improve the clinical appearance of skin by decreasing the quantity and quality of rhytides and/or acne scars, reducing inflammatory and noninflammatory acne lesions, improving dyspigmentation, and producing an overall more youthful appearance.3
In recent years, there has been a paradigm shift in the mechanism of action and technique by which exfoliation is performed. Lasers largely have supplanted deep chemical peels because of their improved control of ablative depth, their ease of use, and their relative lack of systemic toxicity and side effects.2–4,11–15 However, superficial peels have simultaneously increased in popularity, primarily due their relatively mild properties, minimal side effects, and relative cost efficiency compared to laser devices.2–4,11–15 According to recently published data from the American Society of Plastic Surgeons, chemical peels are the third most commonly performed noninvasive cosmetic procedure, after botulinum toxins and soft tissue fillers, with over 1,300,000 procedures performed in 2016 alone.16 The popularity of superficial peels has led to the inclusion of glycolic and/or lactic acids in many over-the-counter cosmetic products.1,2,17,18
Indications and Considerations
The indications for a chemical peel are primarily cosmetic (Table 1) and thus should be tailored to each patient’s specific concerns and wishes for aesthetic improvement of their skin, their ability to tolerate the post-procedural recovery period, and their Fitzpatrick skin type. As mentioned earlier, the type of chemical agent used varies according to condition severity and type and the wishes of the patient. Importantly, a patient’s desires should be ultimately tempered with a discussion of realistic expectations and judicious clinical judgment of appropriate treatment options. Indications for chemical peels can be broken down into four broad categories: 1) rejuvenation of chronic chrono- and photoaging; 2) acne and acneiform eruptions; 4) dyspigmentation; and 3) pre-malignant epidermal neoplasms (Table 1).1–3,5–7,11,13,17,19–23
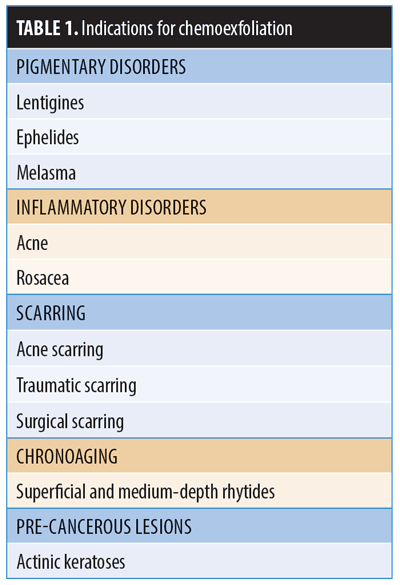
A step-by-step instructional outline for the algorithmic approach to treatment is detailed in Figure 1. Clinicians should take into consideration the condition and the depth of the involved tissue being treated. For example, superficial epidermal issues, such as solar lentigines, can be treated with superficial peels, while deeper defects, such as mild-to-moderate dermatoheliosis, require a medium or deep depth peel. In addition, it is important to determine whether the cosmetic concern is treatable by resurfacing. For example, deep rhytides, laxity of the jowls, and actual ptosis are unlikely to respond to chemical peeling, regardless of treatment depth, and will respond best to surgical intervention. The most common indications for chemical peeling are chronic photoaging and hyperpigmentation.1–3,5–7,11,13,17,19–23 For chronic photoaging and hyperpigmentation, the Glogau Scale of Photoaging5 can be useful in stratifying patients (Figure 1).
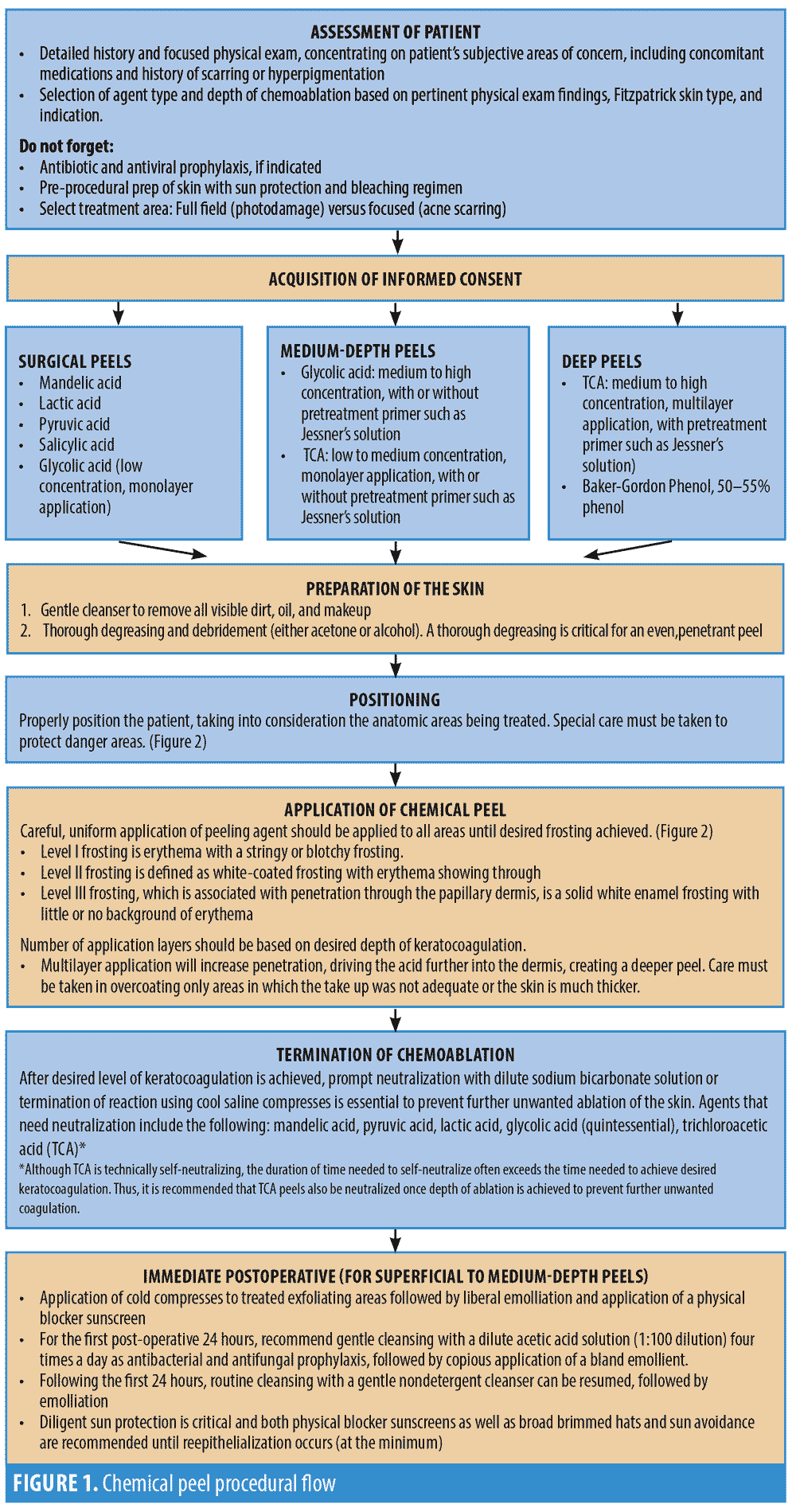
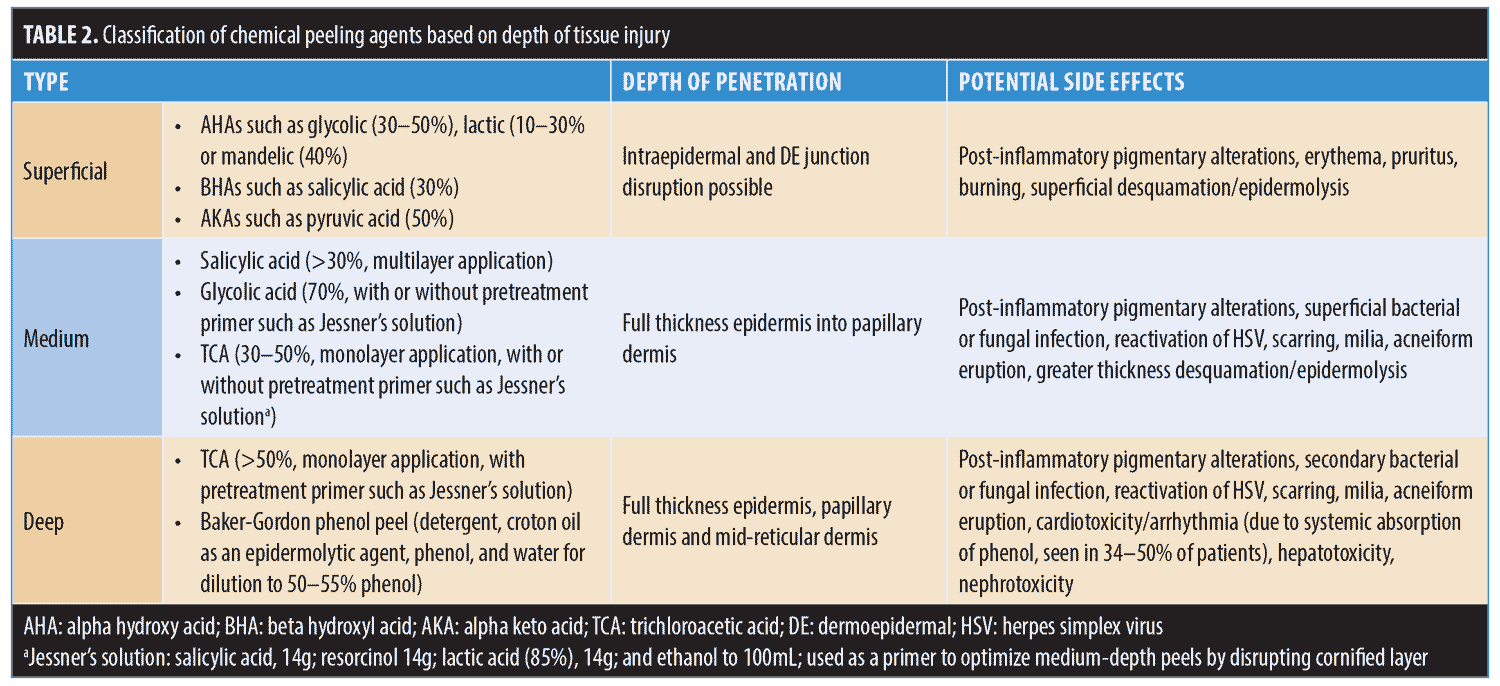
Chemical peels are divided into three categories, depending on the depth of the wound created by the peel (Table 2, Figure 2). Superficial peels penetrate the epidermis only, medium-depth peels affect the entire epidermis and papillary dermis, and deep peels allow for controlled tissue injury to the level of the midreticular dermis (and sometimes subcutis, if not used properly) (Figure 3). The depth of the peel is dictated by a number of factors, including type of caustic chemical, concentration, mode and number of applications, skin type, and the dermatologic condition being treated. Of particular note, depth of chemoexfoliation is cumulative dose-dependent; a monolayer of application allows for a more superficial level of anticipated exfoliation, with subsequent multiple layers or “passes” resulting in additive deeper peeling (Figure 3).5–7,19-21,24 However, multiple applications or layers of a superficial peel, for example, are not the same as a monolayer or single application of a medium-depth chemical peel. Furthermore, time of exposure is inversely proportional to concentration. For example, higher concentrations achieve targeted depth of keratocoagulation in shorter exposure times. In addition, the associated downtime, healing rate, and potential for side effects are directly proportional to the depth of the peel and inversely proportional to the cosmetic outcome; deeper peels will have longer recovery times and pose greater risks of scarring and dyspigmentation; however, they will also result in more dramatic improvements in skin tone and texture (Table 2).5–7,19–21,24
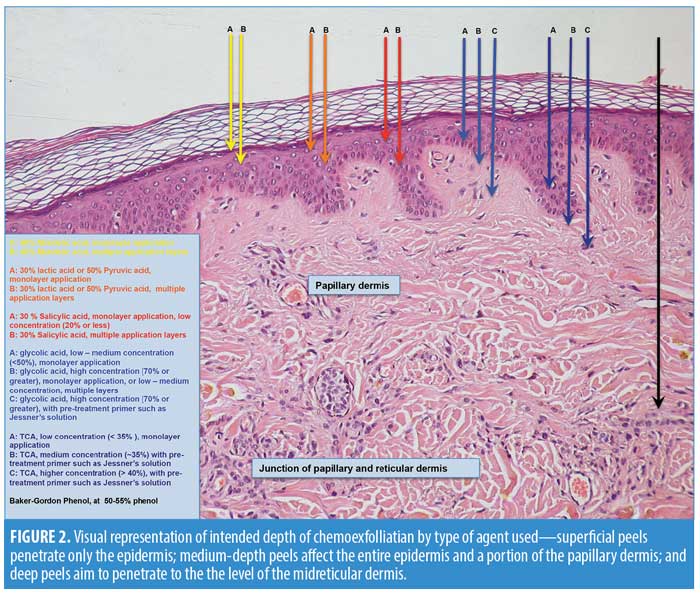
When used for the appropriate indication in the correct setting with the ideal technique, nearly all peels have demonstrated excellent clinical success in improving the tone and texture of facial skin and should remain an indispensable tool in the dermatologist’s aesthetic toolbox, particularly with the rising healthcare costs in the United
States.1–3,5– 7,11,13,17,19–23,25,26
Superficial Peels
Superficial peels result in controlled keratocoagulation and liquefaction of the cells confined to the epidermis, which range from very mild chemoexfoliation of the corneal cell layer down to the basal cell layer.1,2,3,7,17,24 The goal of superficial peeling is to treat conditions confined to the epidermis, while minimizing recovery downtime and risk of side effects. Currently, superficial peels largely comprise low-to-medium strength alpha-hydroxy acids (AHA) (e.g., 30–50% glycolic and 10–30% lactic acid) and, more recently, 40% mandelic acid.1,2,3,27–31 Additionally, low concentrations or monolayer applications of beta-hydroxy salicylic acid (30%) or alpha-keto pyruvic acid (50%) will provide excellent superficial chemoexfoliation as well (Table 2, Figure 1).1–3, 21,32–34
With application of any caustic agent to the skin, subsequent keratocoagulation (i.e., protein denaturation of keratin and collagen) results in a “white frost” to appear on the skin where the chemical agent has been applied. This is an important clinical indicator of peel depth and a marker of duration of exposure. Level I frosting appears clinically as erythema with a stringy or patchy light frosting. Level II frosting appears as a uniform, white-coated frosting with underlying erythema showing through. Level III frosting, which is associated with penetration through the papillary dermis, appears as solid white enamel frosting with little to no background erythema.5,7,17–20 With superficial peels, the goal is to achieve little to no frosting (Level I at most), as clinically evident frosting often indicates exfoliation into the dermis (Figure 1).1,2 ,5 7,21
Commonly used superficial peels. Glycolic acid. The most popular and time-tested superficial peeling agent is glycolic acid, an AHA derived from sugar cane. It is the smallest and simplest AHA in terms of chemical structure, and is also a highly hydrophilic molecule with the greatest bioavailability of all the AHAs.35–37 When properly used, superficial exfoliation with glycolic acid at concentrations of 30 to 50% has demonstrated excellent clinical efficacy in the treatment of superficial hyperpigmentation, mild-to-moderate chrono- and photoaging, and fine rhytides.1–3,5,7,9,15,17,18,21 Glycolic acid is often considered the first-line choice of chemical peeling agents for treatment of melasma.1–3,5,7,9,15,17,18,21,38,39 For superficial chemoexfoliation, most concentrations range from 20 to 50%, with higher concentrations (70%) entering the medium-depth category. Glycolic acid is the prototypical non-self-neutralizing AHA (i.e., keratocoagulation will continue to occur as long as the caustic agent remains on the skin). Thus, careful application and diligent observation of time and clinical signs of reaction completion (e.g., erythema and frosting) are essential. Reaction completion of glycolic acid is achieved by introducing an alkaline neutralizing agent (e.g., sodium bicarbonate neutralization). Clinicians should note that neutralization of any acid with any base is an exothermic process; thus, patients should be warned that a transient increase in warmth, burning, or stinging will likely occur during neutralization.
After neutralization, the skin may be rinsed or cleansed gently. Following application, an initial erythema might become red and is often accompanied by edema. Stringy or patchy light frosting (Level I) might subsequently develop, indicating epidermolysis with separation of the epidermis from the underlying dermis. Development of frank or uniform frosting (?Level II) indicates deeper destruction into the dermis and is not desirable, as this is meant to be a relatively superficial peel. Exfoliation typically occurs over several days, and reepithelialization is complete within 7 to 10 days.1,2,7,8,9,21
Lactic and mandelic acids. More recently, lactic and mandelic acids have emerged as popular single agents for superficial peeling, largely due to their equivalent efficacy compared to gold-standard glycolic acid, which has a relatively mild discomfort profile with minimal associated downtime and
risk.1–3,17,27, 28,30,39
Lactic acid, which is structurally identical to glycolic acid with the exception of an additional methyl group at the ?-carbon end, has a lower pKa and thus a lower pH than glycolic acid at equivalent concentrations, allowing for efficient chemoexfolation at lower concentrations.35–37,40 Clinically, lactic acid has demonstrated comparable efficacy in the treatment of photodamage, superficial hyperpigmentation, and fine rhytides compared to standard glycolic acid peels.Because lactic acid has a lower pH than glycolic acid, a lower concentration is often used to achieve an equivalent depth of keratocoagulation compared to glycolic acid, which allows a favorable side effect profile and recovery time. As with glycolic acid, neutralization is necessary, and exfoliation after treatment typically occurs over several days with complete reepithelialization in 7 to 10 days.1,2,17,27,28,39
Mandelic acid, a simple phenolic alpha-hydroxy acid, is essentially an aromatic glycolic acid with a benzene ring attached to the alpha-carbon where the hydroxyl group is attached.29 Given this unique structure, mandelic acid is soluble in both water and polar organic solutions, which results in a more uniform penetration through lipid-rich areas of skin.29,35–37 Clinically, mandelic acid has demonstrated efficacy in the treatment of superficial erythema and dyspigmentation, as well as efficacy in the reduction of cutaneous sebum production.1,3,30,39 Comparatively, the results of mandelic acid peels are more subtle than that of superficial glycolic acid peels; however, the side effects and subsequent downtime of mandelic acid are comparatively less, which allows more frequent “touch ups” and shortened intervals between treatment sessions.1,3,30,39 Patients often experience minimal desquamation, and reepithelialization is often complete within 3 to 5 days.
Salicylic and pyruvic acids. In addition to the aforementioned AHAs, salicylic acid, a beta-hydroxy acid, and pyruvic acid, an alpha-keto acid, have also demonstrated clinical efficacy as single agents for superficial peeling when used in lower concentrations or with monolayer application technique.1–3,21,32–34
Salicylic acid has a chemical structure similar to that of mandelic acid, with the key difference being that the carboxyl group is directly attached to the benzene ring and the hydroxyl group is attached to the ?-carbon of the benzene ring, ortho to the carboxyl group.35–37 This makes salicylic acid poorly soluble in water, but highly lipophilic. In addition, given its low pKa and small molecular size, salicylic acid demonstrates easy, rapid, and deep penetration through the lipid barriers of the epidermis. Clinically, this translates into excellent efficacy in the treatment of cutaneous disorders involving excess sebum production, namely acne vulgaris. In fact, 30% salicylic acid is often considered the “gold standard” superficial peel for the treatment of acne and has demonstrated excellent clinical efficacy for the treatment of mild-to-moderate inflammatory papulopustular acne vulgaris and comedonal acne.1–3,7,18,21,33,34,41–43 It is less commonly used as an agent for photorejuvenation and hyperpigmentation, compared to the other aforementioned peels; however, clinical efficacy for those indications has been demonstrated.1–3,7,18,21,33,34,41–43 Given its high lipophilicity, salicylic acid exhibits potent cumulative dose effect; in other words, multiple layers or excessive application of the acid can cause rapid keratocoagulation beyond the epidermis into the papillary dermis. Salicylic acid, unlike glycolic acid and the other AHAs, is self-neutralized by the skin’s own endogenous lipoproteins.7,21,33,34, 41–43 However, cumulative dose exposure remains critical, and careful attention to length of application time and signs of reaction completion are necessary to prevent unwanted, excessive keratocoagulation. Exfoliation after treatment typically occurs over several days, and reepithelialization is complete within 7 to 10 days.7,21,33,34,41–43
Pyruvic acid, the smallest alpha-keto acid, is structurally a carboxylic acid with a functional ketone moiety. It has similar lipophilic and keratolytic properties as salicylic acid but has less lipophilicity.35–37 It is also partially hydrophilic, giving it properties of both salicylic acid and glycolic acid. Clinically, pyruvic acid peels have demonstrated efficacy in the treatment of acne vulgaris and associated disorders of excess sebum production, as well as mild photoaging and superficial hyperpigmentation.32 It is commonly used as a superficial peeling agent for inflammatory and comedonal acne. Comparatively, however, pyruvic acid is not as efficacious as that of salicylic acid in the treatment of acne vulgaris and associated disorders involving excess sebum production.32,41,42 This is likely due to salicylic acid’s greater lipophilic properties and easier penetration through the lipid barriers of the epidermis. Unlike salicylic acid but much like other AHAs, pyruvic acid is not self-neutralizing and will continue to cause keratocoagulation for the duration of exposure to the skin until it is neutralized with an alkaline solution. Exfoliation after treatment typically occurs over several days, and reepithelialization is complete within 5 to 10 days.32
Over-the-counter superficial peels. In addition to the aforementioned physician-grade chemical peeling agents, there are many over-the-counter, low concentration, superficial chemical peeling agents (e.g., 3–10% glycolic acid and mild fruit-derived acids [citric, tartaric, or malic]). These formulations cause mild, gradual exfoliation over several weeks and can be used as pre-peel primers to potentiate the effects of a higher concentration peel. Discussion of these peels is beyond the scope of this review.
Medium-depth Peels
Medium-depth peels allow for controlled keratocoagulation through the dermis and into the papillary dermis. This results in deeper regenerative changes that can target pathology both within the epidermis and the superficial dermis and can often be performed in a single setting. When used appropriately and with proper technique, medium-depth peels have demonstrated excellent clinical efficacy in the treatment of fine rhytides, chronic actinic photodamage, superficial hyperpigmentary disorders (e.g., melasma), superficial acne scars, and even actinic premalignant changes as field therapy. 2,3,5, 7–9,15,19–23
Commonly used medium-depth solutions. The original benchmark for medium-depth chemoexfoliation was a 50% TCA solution.2, 3,5, 7–9,15,19–23 It was, and to a certain extent still is, a popular and frequently used peel to treat fine rhytides, actinic photodamage, hyperpigmentation, and even actinic-related premalignant changes, such as actinic keratoses.2,3, 5,7–9, 15,19–23 However, TCA used at higher concentrations has a relatively high risk of complications, including dyschromia, scarring, and occasionally bacterial superinfection and cutaneous herpes simplex virus (HSV) reactivation.2,3, 5,7–9, 15,19–23,44 Thus, use of high-concentration TCA (>50%) has fallen out of favor as a single-agent chemical peel.
The most common chemical agents currently used for medium-depth peeling are 70% glycolic acid and 35 to 50% TCA, with or without adjuvant combination products (e.g., Jessner’s solution [comprising 14g resorcinol, 14g salicylic acid, 14mL lactic acid in ethanol constituted to 100mL] or solid carbon dioxide [CO2]).2,3, 5,7–9, 15,19–23 In addition, multiple layered applications of 20 to 40% salicylic acid and pyruvic acid are also used for medium-depth peeling, though not traditionally considered medium-depth peels.
Pre-, peri-, and post-treatment guidelines using medium-depth peels. As mentioned earlier, depth of peeling is directly correlated with cumulative dose exposure of the skin to a given acid. In other words, for any given length of exposure, higher concentrations of a given acid will cause deeper chemoexfolation, as will multiple layers of application or multiple “passes” over the same area. With medium-depth peels, this can be of critical concern, as exfoliation down into the papillary dermis is often achieved with a single first-pass application. Thus, combination treatments with a pre-treatment agent (e.g., Jessner’s solution or solid CO2), followed by a lower concentration of a medium-depth acid(e.g., 50–70% glycolic acid or 35% TCA) have become increasingly popular due to their demonstrated equal clinical efficacy to the original 50% TCA peels of the past. These combination treatments provide a more uniform and controlled depth peeling with a greater safety margin and a reduced incidence of dyschromias and scarring.2,3, 5,7–9,15,19–23
Application of Jessner’s solution or solid CO2 with acetone, an older technique, prior to the application of glycolic acid or TCA results in a more homogenous disruption of the epidermal barrier and a more thorough removal of natural oils from the skin, providing greater penetration of the acid and a more uniform frosting.2,3,5,7–9,15,19–23 Furthermore, the resultant frosting is often better controlled, with less risk of “hot spots” that can occur with higher concentrations of TCA or glycolic
acid.2,3,5,7–9,15,19–23
Medium-depth peels are often performed with mild pre-operative sedation and nonsteroidal anti-inflammatory agents (NSAIDs), as this depth of peeling does have considerably higher levels of associated pain. NSAIDs are particularly helpful in reducing swelling and pain and are often given pre-operatively to help mitigate post-operative inflammatory sequelae.6,7,15,19–21, 24,22–23 With medium-depth peels, proper application technique is critical, as is avoiding unnecessary, inadvertent reapplication with excess peel solution. It is recommended that the face be peeled sequentially: forehead to temples first, followed by cheeks and chin, and finally the delicate cutaneous lips and eyelids. Careful feathering of the solution into the hairline and around the rim of the jaw and brow conceals the demarcation line between peeled and nonpeeled areas. Clinicians should be particularly careful when applying peeling solution to the eyelid, and it is recommended to leave 2 to 3mm of lid margin as a “safety zone” to prevent solution from entering into the eyes. With medium-depth peeling, achievement of Level II to III frosting is the goal.6,7,15,19–21,24,22–23 Often, Level II frosting is sufficient for adequate depth of reaction. Occasionally, however, deeper Level III frosting is necessary in areas of thicker skin or heavier actinic damage. Proper technique will allow for uniform and even application, eliminating the need for unnecessary reapplication and the risk of excessive keratocoagulation. If frosting is incomplete or uneven, the peeling solution should be carefully reapplied to areas of need only. Most medium-depth chemical peels use a Level II frosting, and this is especially true over eyelids and areas of sensitive skin. Those areas with a greater tendency for scar formation, such as the zygomatic arch, the bony prominences of the jawline and chin, the eyelids, and the vermillion border, should only achieve a maximum of Level II frosting.
Postoperatively, there is an immediate burning sensation as the peel solution is applied, but this subsides as frosting is completed. With glycolic and TCA peels, keratocoagulation will continue as long as the caustic agent remains on the
skin.6,7,15,19–21,24,22–23 Thus, careful technique and diligent observation of procedure time and clinical signs of reaction completion (e.g., erythema and frosting) are essential. Reaction completion is achieved by neutralizing with an alkaline agent such as sodium bicarbonate. It is important to note that neutralization of any acid with any base is exothermic; thus, during neutralization, patients might experience a transient increase in feeling sof warmth, burning, or stinging of the treatment area.35–37 Subsequent edema, erythema, and desquamation are expected. With peels that target the periorbital and forehead areas, significant eyelid edema can occur and might even result in temporary closure. For the first 24 hours, NSAIDs, soaks with dilute bleach or vinegar, and cool compresses with ice packs are useful in ameliorating some of the immediate postoperative swelling and pain. A bland emollient or mupirocin ointment should be applied to all treated areas for the first 24 hours post-treatment, and daily after that. Following treatment with medium-depth peels, the erythema initially intensifies, peaking 4 to 5 days post-treatment. Exfoliation is complete within 10 to 14 days.6,7,15,19–21, 24,22–23
With medium-depth peels, the concentration and amount of each agent that is applied modulates the intensity of keratocoagulation and thus the effectiveness of the peel. The nuances in concentration, variation of combination types, and treatment method can be adjusted according to the patient’s Fitzpatrick skin type and specific skin condition being treated. For a patient with advanced photoaging, such as crow’s feet or rhytides in the periorbital and/or perioral area, with medium-depth changes on the remaining face, a medium-depth peel can be used to as an adjuvant or neoadjuvant to laser resurfacing or deep chemical peeling.3,13,15
Deep Peels
With the advent and rapid improvements in lasers, deep peeling has fallen out of favor in recent years, as lasers allow for precise and predictable ablation that result in consistently reproducible and uniform thickness tissue vaporization with lower incidences of scarring and postoperative complications. Furthermore, the systemic toxicities associated with deep peeling are virtually nonexistent with lasers.4,11,12,13,15,24 However, deep peels have been used successfully for nearly half a century, and when used with appropriate technique in the proper setting, they have produced reliable and durable high-quality
results.2,5–7,10, 21,25,26,45 The ideal candidate for deep peeling procedures is a patient with moderate-to-severe chrono- and photoaging (e.g., chronic actinic damage with deep furrowed rhytides and/or significant hyperpigmentation, such as those in Glogau Group III or IV). 2,5,6,7,10,17,21,25,26
Commonly used deep-depth solutions. The two most commonly used deep peels are high concentration TCA (?50%), and the phenol peel. As previously discussed, high concentration TCA peels have fallen out of favor due to frequent complications, a high incidence of scarring, and significant unpredictability in ablative depth.2,4–7,10,21,24–26 When using pure phenol peels, the undiluted, high concentration of phenol causes rapid keratocoagulation, producing a liquefactive “plug” of denatured protein that inhibits further chemical peeling. As a result, pure phenol provides only medium-depth ablation and is rarely used in chemical peeling.5–7,10,21,25,26,45
The Baker-Gordon phenol peel, developed nearly 50 years ago, uses a phenol formulation consisting of a detergent, croton oil as an epidermolytic agent, phenol, and water for 50 to 55% dilution percent. This dilution allows for deeper and more uniform keratocoagulation than full strength phenol.7,10,21,25,26 The Baker-Gordon phenol peel, considered to be the original deep chemical peel, provides reliable and consistent deep chemoexfoliation into the level of the mid-reticular dermis and is the preferred agent of choice for deep peeling.5–7,10,21,25,26,45
The Baker-Gordon phenol solution has two methods of application: occluded and unoccluded. The occluded method is accomplished by applying an occlusive (waterproof) dressing (petroleum jelly dressing may also be used) over the treatment area immediately following application of the solution, thus achieving maximum penetration of the phenol acid. This penetration is particularly useful for treatment of deep, furrowed rhytides and severe Glogau Group IV photodamaged skin. It results in keratocoagulation that extends into the midreticular dermis and, if used incorrectly the subcutis and fascia, .7,10, 21,25–26 The unoccluded method involves more skin cleansing, lipid removal, as well as application of more peel solution, than the occluded method, but does not provide as deep of chemoexfoliation as the occluded method.7,10,20,21,25,26,42,43
Pre, peri-, and post-treatment guidelines using deep-depth peels. It is important to stress that any patient undergoing deep chemical peeling should be fully educated on and indicate an understanding of the risk factors, increased morbidity, and potential complications involved with the procedure, so that the benefits versus risks can be carefully weighed. With an experienced surgeon, deep peeling is a reliable and safe method of treating severely photoaged and actinic-damaged skin, including deep perioral and periorbital rhytides and furrowed rhytides along the forehead and cheek, and ameliorating pre-malignant changes that commonly occur in severe photoaged skin.5–7,10,21,25,26,45 Deep peeling should be performed in an ambulatory surgical setting or office surgical suite, as systemic toxicity resulting from cutaneous absorption of the chemical agent is a concern. Practitioners should provide adequate preoperative sedation and intravenous hydration, particularly when using phenol peels, to minimize the phenol concentration in the patient’s serum that results from inevitable cutaneous absorption. Phenol is highly arrhythmogenic, metabolized by the liver and excreted by the kidney, and any patient with a history of cardiac arrhythmia and/or hepatic or renal dysfunction should not undergo phenol peeling.4,6,7,10,21,24–26 Cardiac monitoring is recommended for potential periprocedural toxicity assessment.4,6,7,10,21,24–26
Following a deep-peel procedure, the postoperative inflammatory phase of wound healing commences immediately, with deep, dark, dusky edematous erythema that will evolve into full-thickness epidermal necrosis with serosanginous exudate, crusting, and sterile pyoderma within 24 to 48 hours.4,6,7,10,21,24–26 Inflammation might be severe, and the eyes might swell shut. During this initial phase, it is important for the patient to use cool compresses, ice packs, and NSAIDs to control inflammation, and gentle debridant soaks with dilute vinegar solution to remove necrotic epidermal debris and prevent thick crust formation from the serosanguinous
exudate.4–7, 21,24–26 Because the skin has lost its entire epidermal barrier, transmembrane water loss is significant. Routine, repetitive application of a bland emollient and oral hydration are essential to avoiding potential complications.4–7,21,24–26,44 With deep chemical peels, reepithelialization does not commence until Day 3 or 4, post-procedure, after the inflammatory response has subsided, and continues for 14 days or longer.4–7,10,21,24–26,44,45 Maintaining a moist occlusive barrier with bland emollients allows for rapid reepithelialization and reduces the likelihood of delayed wound healing or scar contracture.4–7,10,21,24–26,44,45 Because deep peeling causes keratocoagulation and exfoliation down to the mid-reticular dermis, both bacterial and candidal superinfections of the wound site and potential HSV reactivation are serious potential complications. Patients with a history of orolabial HSV should begin prophylactic antiretroviral therapy 1 to 2 days prior to peeling and should continue it until full reepithelialization has occurred, generally 10 to 14 days post-treatment.4–7,10,21,24–26,44,45 For prevention of bacterial superinfection, mupirocin ointment can be used as the bland emollient for the first few days after treatment, and debridant soaks with dilute vinegar might be of added antimicrobial effect. Candidal superinfection is uncommon but should be astutely monitored during follow-up. If there is any suspicion of candidal superinfection, prompt oral antifungals, such as fluconazole, should be initiated. Fibroplasia, neoangiogenesis, and neocollagen formation will continue well beyond the initial treatment period, upwards of six months post-treatment.10,25,26, 44,45
Summary
Analysis of the available literature reveals the following: chemical peels are the third most commonly performed noninvasive cosmetic procedure in the United States, with over 1,300,000 procedures performed in 2016 alone. Indications for treatment can be classified into four categories: chronic chrono- and photoaging, acne and acneiform eruptions, dyspigmentation, and pre-malignant epidermal neoplasms. Selection of agent type is determined by a number of factors, including treatment indication, desired depth of ablation, pertinent exam findings, Fitzpatrick skin type, and relevant dermatologic history of the patient. When used for the appropriate indication with the proper technique, nearly all peel solutions and depths have demonstrated excellent clinical success in improving skin tone and texture, and are cost-effective compared to invasive procedures. Chemical peels should remain indispensable tools in the dermatologist’s aesthetic toolbox, particularly in light of the current rising healthcare costs in the United States.
References
- Berson DS, Cohen JL, Rendon MI, et al. Clinical role and application of superficial chemical peels in today’s practice. J Drugs Dermatol. 2009;(9): 803–11.
- Fischer TC, Perosino E, Poli F, et al. Cosmetic Dermatology European Expert Group. Chemical peels in aesthetic dermatology: an update 2009. J Eur Acad Dermatol Venereol. 2010; 24(3): 281–292.
- Hassan KM, Benedetto AV. Facial skin rejuvenation: ablative laser resurfacing, chemical peels, or photodynamic therapy? facts and controversies. Clin Dermatol. 2013;31(6):737–740.
- Nikalji N, Godse K, Sakhiya J, et al. Complications of medium-depth and deep chemical peels. J Cutan Aesthet Surg. 2012;(4):254–260.
- Glogau RG. Chemical peeling and aging skin. J Geratr Dermatol. 1994;2(1):30–35.
- Glogau RG, Matarasso SL. Chemical peels. trichloroacetic acid and phenol. Dermatol Clin. 1995;13(2):263–276.
- Baker TJ, Gordon HL. Chemical face peeling. In: Baker TJ, Gordon HL (eds). Surgical Rejuvenation of the Face. Maryland Heights, MO: C.V. Mosby;1986;230–232.
- Nelson BR, Fader DJ, Gillard M, et al. Pilot histologic and ultrastructural study of the effects of medium-depth chemical facial peels on dermal collagen in patients with actinically damaged skin. J Am Acad Dermatol. 1995;32(3):472–478.
- Tse Y, Ostad A, Lee HS, et al. A clinical and histologic evaluation of two medium-depth peels. glycolic acid versus Jessner’s trichloroacetic acid.Dermatol Surg.1996;22(9):781–786.
- Kligman AM, Baker TJ, Gordon HL. Long-term histologic follow-up of phenol face peels. Plast Reconstr Surg. 1985;75(5):652–659.
- Puri N. A study on fractional erbium glass laser therapy versus chemical peeling for the treatment of melasma in female patients. J Cutan Aesthet Surg. 2013;(3):148–151.
- Alexiades-Armenakas M. Fractional laser resurfacing. J Drugs Dermatol. 2007;(7):750–751.
- Hassan KM, Benedetto AV. Facial skin rejuvenation: ablative laser resurfacing, chemical peels, or photodynamic therapy? facts and controversies. Clin Dermatol. 2013;(6):737–740.
- Brauer JA, Patel U, Hale EK. Laser skin resurfacing, chemical peels, and other cutaneous treatments of the brow and upper lid. Clin Plast Surg. 2013;40(1):91–99.
- Monheit GD. Zeitouni NC. Skin resurfacing for photoaging: laser resurfacing versus chemical peeling. Cosmetic Dermatol. 1997;(4):11–22.
- The American Society of Plastic Surgeons. 2016 National Plastic Surgery Statistics: Cosmetic and Reconstructive Procedure Trends. 2017. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/2016-plastic-surgery-statistics-report.pdf. Accessed 1 Nov 2017.
- Briden ME. Alpha-hydroxy acid chemical peeling agents: case studies and rationale for safe and effective use. Cutis. 2004;(2 Suppl):18–24.
- Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;(7):32–43.
- Brody HJ. Trichloracetic acid application in chemical peeling, operative techniques. Plast Reconstr Surg. 1995;2(2):127–128.
- Brody HJ. Variations and comparisons in medium-depth chemical peeling. J Dermatol Surg Oncol. 1989;(9):953–963.
- Rubin M. Manual of chemical peels. Philadelphia: Lippincott. 1995.
- Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15(9):945–950.
- Coleman WP 3rd, Futrell JM. The glycolic acid trichloroacetic acid peel. J Dermatol Surg Oncol. 1994;20(1):76–80.
- Anitha B. Prevention of complications in chemical peeling. J Cutan Aesthet Surg. 2010 Sep;(3): 186–188.
- Asken S.Unoccluded Baker-Gordon phenol peels—review and update. J Dermatol Surg Oncol. 1989;15(9):998–1008.
- Alt TH. Occluded Baker-Gordon chemical peel: review and update. J Dermatol Surg Oncol. 1989;15(9):980–993.
- Prestes PS, Oliveira MM, Leonardi GR. Randomized clinical efficacy of superficial peeling with 85% lactic acid versus 70% glycolic acid. An Bras Dermatol. 2013;88(6):900–905.
- Sharquie KE, Al-Tikreety MM, Al-Mashhadani SA. Lactic acid as a new therapeutic peeling agent in melasma. Dermatol Surg. 2005;31(2):149–154.
- Aston JG, Newkirk JD, Jenkins DM, Dorsky J. Mandelic acid. Org Synth. 1952;3:538
- Wójcik A, Kubiak M, Rotsztejn H. Influence of azelaic and mandelic acid peels on sebum secretion in ageing women. Postepy Dermatol Alergol. 2013;30(3):140–145.
- Vavouli C, Katsambas A, Gregoriou S, et al. Chemical peeling with trichloroacetic acid and lactic acid for infraorbital dark circles. J Cosmet Dermatol. 2013;12(3):204–209.
- Berardesca E, Cameli N, Primavera G, Carrera M. Clinical and instrumental evaluation of skin improvement after treatment with a new 50% pyruvic acid peel. Dermatol Surg. 2006;32(4): 526–31.
- Kligman DE, Draelos ZD. Combination superficial peels With salicylic acid and post-peel retinoids. J Drugs Dermatol. 2016;15(4):442–450.
- Dayal S, Amrani A, Sahu P, Jain VK. Jessner’s solution versus 30% salicylic acid peels: a comparative study of the efficacy and safety in mild-to-moderate acne vulgaris. J Cosmet Dermatol. 2017;16(1):43–51.
- Gerhartz, W. (exec ed.), Ullmann’s Encyclopedia of Industrial Chemistry. 5th ed.Vol A1: Deerfield Beach, FL: VCH Publishers, 1985 to Present., p. VA13 509.
- Haynes, WM. CRC Handbook of Chemistry and Physics. 92nd ed. 2011. Boca Raton, Fl: CRC press. ISBN 1439855110.
- Lehninger AL, Nelson DL, Cox MM. Principles of Biochemistry (5th ed.). New York: W. H. Freeman and Company; 2008:528. ISBN A978-0-7167-7108-1.
- Kumari R, Thappa DM. Comparative study of trichloroacetic acid versus glycolic acid chemical peels in the treatment of melasma. Indian J Dermatol Venereol Leprol. 2010;76(4):447.
- Sarkar R, Garg V, Bansal S, et al. Comparative evaluation of efficacy and tolerability of glycolic acid, salicylic , mandelic acid, and phytic acid combination peels in melasma. Dermatol Surg. 2016;42(3):384–91.
- Silva, AM, Kong X, Hider RC. Determination of the pKa value of the hydroxyl group in the alpha-hydroxycarboxylates citrate, malate and lactate by 13C NMR: implications for metal coordination in biological systems. Biometals. 2009;22(5):771–778.
- Kessler E, Flanagan K, Chia C, et al. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008 Jan;34(1):45–50.
- Dréno B, Fischer TC, Perosino E, et al. Expert opinion: efficacy of superficial chemical peels in active acne management—what can we learn from the literature today? evidence-based recommendations. J Eur Acad Dermatol Venereol. 2011 Jun;25(6):695–704.
- Levesque A, Hamzavi I, Seite S, et al. Randomized trial comparing a chemical peel containing a lipophilic hydroxy acid derivative of salicylic acid with a salicylic acid peel in subjects with comedonal acne. J Cosmet Dermatol. 2011;10(3):174–178.
- Monheit GD. Facial resurfacing may trigger the herpes simplex virus. Cosmetic Dermatol. 1995;8(7):9–16.
- Fitzpatrick RE, Tope WD, Goldman MP, Satur NM. Pulsed carbon dioxide laser, trichloroacetic acid, Baker-Gordon phenol, and dermabrasion: a comparative clinical and histologic study of cutaneous resurfacing in a porcine model. Arch Dermatol. 1996;132(4):469–471.

