 J Clin Aesthet Dermatol. 2024;17(5):15–22
J Clin Aesthet Dermatol. 2024;17(5):15–22
by Sara Ahmed Galal, MD; Sawsan Khalifa El-Sayed, MD; and Manar Mohamed Hasan Henidy, MBBCh
Drs. Galal and El-Sayed are with the Dermatology and Venereology Department and Faculty of Medicine at Al-Azhar University in Cairo, Egypt. Dr. Henidy is with the Faculty of Medicine at Al-Azhar University in Cairo, Egypt.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors have no conflicts of interest relevant to the contents of this article.
ABSTRACT: Objective. We sought to detect additional underlying hair loss disorders in patients with postpartum telogen effluvium.
Methods. We completed clinical and dermoscopic evaluations on 200 female participants experiencing postpartum hair loss.
Results. 9.5 percent of patients were diagnosed with telogen effluvium (TE), 56.0 percent patients were diagnosed with TE with androgenetic alopecia (AGA), 6.5 percent patients were diagnosed with TE and TA, and 28.0 percent patients were diagnosed with TE, AGA, and TA. In the central area, patients with TE displayed upright regrowing hair and single pilosebaceous unit in 100 percent and 94.7 percent of patients, respectively. While patients with TE and AGA, displayed upright regrowing hair, single pilosebaceous unit, and hair diameter diversity greater than 20 percent. In patients diagnosed with TE and TA, the trichoscopic findings were similar in the TE group to the patients diagnosed with TE, AGA, and TA were also similar to the patients with TE and AGA. Regarding the area of traction, there was no difference observed between the patients with TE and TA and patients with TE, AGA, and TA. The frequent findings were hair diameter diversity, empty follicles, and vellus hair.
Conclusion. Postpartum TE may be associated with other hair loss disorders. Awareness of this is critical to appropriate diagnosis and treatment.
Keywords: Telogen effluvium, postpartum, female androgenetic alopecia, traction alopecia
Introduction
Telogen effluvium (TE) is a type of diffuse hair shedding that is not associated with scarring or inflammation and is usually triggered by physiological or emotional stress.1 TE can be classified as acute (lasting less than six months) or chronic (lasting more than six months).2 The hallmark feature of TE is excessive shedding of telogen or resting hair, which typically occurs 2 to 3 months after exposure to a trigger. Hair regrowth is expected once the underlying stressor is removed.3 In normal individuals, the scalp contains approximately 85 percent anagen and 15 percent telogen hair, whereas in TE, up to 30 percent of hair follicles shift to telogen, leading to significant hair loss.4 The exact prevalence of TE is unknown, but it can occur in individuals of any age, gender, or race, with women being more commonly affected due to postpartum hormonal changes.5
Pregnancy and the postpartum period are associated with hormonal changes that can affect hair cycling. During pregnancy, a prolonged anagen phase is observed due to the effects of progesterone, which increases hair shaft diameter and inhibits androgen secretion leading to anagen maintenance. After delivery, progesterone levels decrease, while prolactin levels increase, leading to premature induction of catagen and simultaneous entry into the telogen phase, resulting in increased shedding of telogen hair.6
Regular clinical and trichoscopical examinations are very important for evaluating various types of hair loss. Trichoscopy is a non-invasive technique used to visualize hair shafts, hair follicle openings, and perifollicular epidermis.7
The postpartum period is defined as the 6 to 8 weeks following delivery.8 Excessive hair shedding during this period may reveal other underlying hair loss disorders.9 Therefore, the aim of our study was to detect how postpartum TE can unmask underlying hair loss disorders, such as female androgenetic alopecia and traction alopecia.
Methods
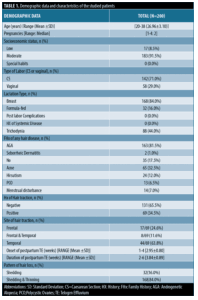
Patients. This observational, descriptive, cross-sectional, non-interventional study included two hundred female patients experiencing postpartum hair loss. They ranged in age from 20 to 38 years. All patients were enrolled from a dermatology outpatient clinic of our university hospital over a period from April 2022 to February 2023. The study was conducted following approval of the research ethical committee of the faculty of medicine. Consent was acquired from all patients.
This study included postpartum female patients 6 to 8 weeks after delivery. We excluded patients with autoimmune and systemic diseases, patients on drugs that cause hair loss, such as anticonvulsants, antidepressants, and beta-blockers, for a duration beyond three months, patients with heat-treated hair, and patients with scalp or hair disorders, such as tinea capitis and alopecia areata. History was collected for all patients, which included the following information:
- Age, number of pregnancies, socioeconomic status, special habits, type of delivery (Caesarean section or vaginal), lactation type, and post-labor complications
- Present illness, including onset and duration of postpartum telogen effluvium, pattern of hair loss (shedding or thinning) and if there is hair traction, scalp burning or itching, or trichodynia
- History of acne, hirsutism, polycystic ovary syndrome, or menstrual irregularities
- History of systemic or autoimmune diseases
- History of drug intake as oral hormonal contraception or drugs for any medical problem
- Family history of any hair disease
General examinations were performed to exclude associated systemic diseases. A dermatological examination was performed to identify any skin disease and scalp or hair disorders. Hair pull test was performed by grasping about 60 hairs between the thumb and fingers then pulling gently but firmly from root to tip. Patients were asked to refrain from shampooing for 24 hours before examination.10
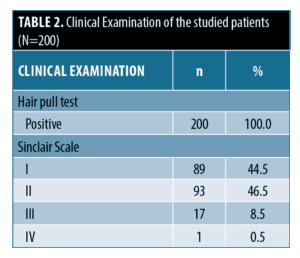
Clinical evaluation of all patients was done using Sinclair scale grading, as follows: Grade 1 is normal. Grade 2 shows a widening of the central part. Grade 3 shows a widening of the central part and thinning of the hair on either side of the central part. Grade 4 reveals the emergence of a diffuse hair loss over the top of the scalp. Grade 5 indicates advanced hair loss.11
Trichoscopic examination. A noncontact polarized dermoscopy (Dermlite HUD, the USA made, 2016 by 3GEN INC) connected to SAMSUNG Galaxy A52s 5G with 64 Megapixels camera and magnification power x10 was used to evaluate trichoscopic findings of telogen effluvium and other underlying hair loss disorders such as FAGA or TA.
Evaluation of the patients. Photographs of the hair were taken clinically from both central area and area of traction if present from a 20cm distance by SAMSUNG Galaxy A52s 5G with 64 Megapixels camera & magnification power (x10). The most common sites of traction are the frontal and temporoparietal areas, although any area of the scalp can be affected. Trichoscopic evaluation of both central area and area of traction if present to detect underlying hair disorders.
Statistical analysis. Data were collected, revised, coded, and entered into the Statistical Package for Social Science using SPSS Inc.’s, version 20.0 (Chicago, Illinois). Mean, standard deviation (SD) and ranges were used to express quantitative data, while numbers and percentages were used to express qualitative data. The confidence interval was set to 95 percent, and the margin of error accepted was set to 5 percent. So, the P-value was considered significant as the following: P-value >0.05: nonsignificant (NS); P-value <0.05: significant (S); and P-value <0.001: highly significant (HS).
Results
This observational, descriptive, cross-sectional study was conducted on 200 female patients experiencing postpartum telogen effluvium. The age of the studied patients ranged from 20 to 38 years with a mean SD 26.96±3.10 years. The number of pregnancies ranged from 1 to 4 (median=2). Demographic data and characteristics of the study participants are listed in Table 1.
Clinical examination of the studied patients. The clinical examination of the studied patients showed that all patients (N=200, 100%) had a positive hair pull test. As for the Sinclair Scale, 89 patients (44.5%) were Class I, 93 patients (46.5%) were Class II, 17 patients (8.5%) were Class III and one patient (0.5%) was Class IV (Table 2).
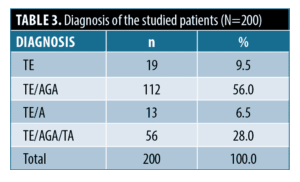
Diagnosis of the studied patients. The diagnosis of the studied patients was as follows: 19 patients (9.5%) were diagnosed as TE, 112 patients (56.0%) had TE with AGA (TE/AGA), 13 patients (6.5%) had TE with TA (TE/TA) and 56 patients (28.0%) had TE with AGA and TA (TE/AGA/TA) (Table 3).
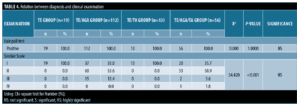
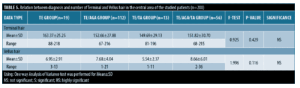
Relation between diagnosis and clinical examination. Regarding the relation between Sinclair scale grades and diagnosis; there was a high statistically significant presentation of Grade I in the TE group and the TE/TA group, while Grade II and Grade III were presented in the TE/AGA/TA group and TE/AGA group with p-value <0.001. There was no statistically significant relation between hair pull test and diagnosis with p-value=1.0000 (Table 4).
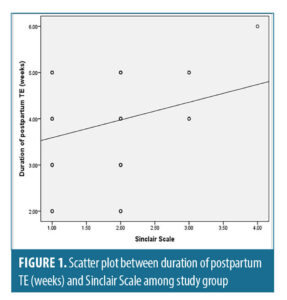
Correlation between Sinclair Scale and different parameters. There was a highly statistically significant positive correlation between duration of postpartum TE (weeks) and Sinclair scale, with (r-value 0.283 and p-value<0.001) (Figure 1).
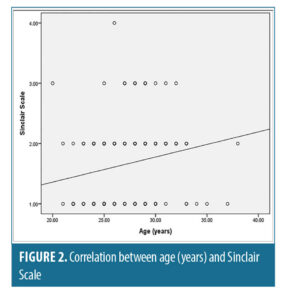
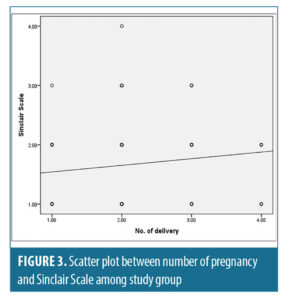
There was a statistically significant positive correlation between age “years” and number of pregnancies with Sinclair scale with (r-value=0.197, r-value=0.283 and p-value=0.005, p-value<0.05) respectively (Figures 2 and 3).
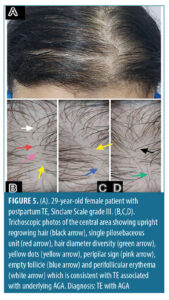
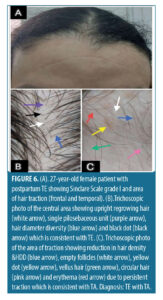
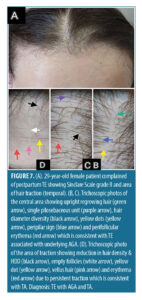
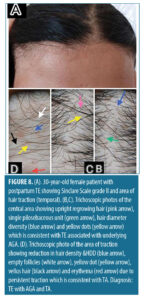
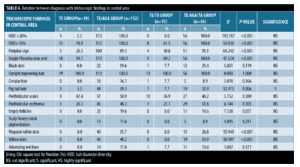
Trichoscopic findings in the central area. There was no statistically significant difference between the diagnostic groups with regard to number of terminal and vellus hair (p-value>0.05) (Table 5).
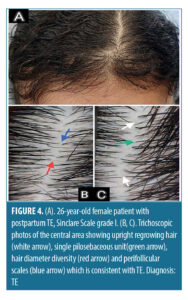
The most frequent trichoscopic findings in the central area in patients diagnosed with TE (n=19, 9.5%) were upright regrowing hair and single pilosebaceous unit (100%, 94.7%), followed by hair diameter diversity greater than 10 percent and perifollicular scales (78.9%, 47.4%).
In the TE /TA group (n=13, 6.5%), the most frequent trichoscopic findings in the central area were upright regrowing hair and perifollicular scales (100.0%, 76.9%), followed by single pilosebaceous unit and hair diameter diversity greater than 10 percent (69.2%, 61.5%).
In patients diagnosed with TE/AGA (n=112, 56.0%), upright regrowing hair, single pilosebaceous unit and hair diameter diversity greater than 20 percent were detected in all patients in this group, followed by peripilar sign (89.3%), perifollicular scales (50.9%), perifollicular erythema (40.2%), yellow dots (40.2%), pig tail hair (39.3%), pinpoint white dots (35.7%), black dots (17.0%), and circular hair (16.1%).
The trichoscopic findings of TE/AGA/TA (n=56, 28.0%) in the central area were almost similar to that of TE/AGA (Table 6).
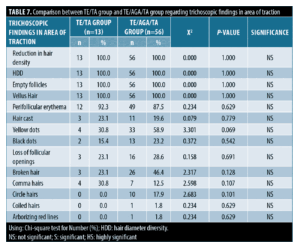
Trichoscopic findings in area of traction. There was no statistically significant difference between the TE/TA group and the TE/AGA/TA group, with p-value >0.05.
The most frequent trichoscopic findings in 100 percent of patients were reduction in hair density, hair diameter diversity, empty follicles, vellus hair, and perifollicular erythema. The less frequent trichoscopic findings were yellow dots, comma hairs, hair cast, broken hair, loss of follicular openings, and black dots (Table 7).
Discussion
Postpartum telogen effluvium (PPTE) is a commonly described entity, but few studies deal with the real incidence and pathogenesis of this claimed common disease.12
Postpartum TE is characterized by an increase in the proportion of hair follicles that remain in the prolonged anagen phase, rather than cycling into telogen phase. When finally released from anagen, the clinical sign of increased shedding of telogen hair will be found. Telogen hair is also known as “club hair” due to the shape of the root. Anagen hair are actively growing hair, while telogen hair, in contrast, are resting hair.4 In post-partum telogen effluvium (PPTE) the excessive hair loss may “unmask” the underlying hair loss disorders, such as female androgenetic alopecia (FAGA) and traction alopecia (TA). Realization of this phenomenon is critical for proper treatment of patients.8 The diagnosis of hair loss disorders depends on correlations of different diagnostic modalities such as clinical examination, hair pull test, trichoscopy, trichogram, and histopathology.
To the best of our knowledge, there were no previous reports in the literature concerning PPTE and associated hair loss disorders on Egyptian patients. Our study was inspired a report by Samrao and Mirmirani9 on three patients with postpartum telogen effluvium who were found to have an underlying condition of TA.We conducted this study to detect how postpartum telogen effluvium can unmask other underlying hair loss disorders such as FAGA and TA.
In our study, 90.5 percent of patients were diagnosed both clinically and trichoscopically as TE associated with other hair loss disorders (56.0% had TE/AGA, 6.5% had TE/TA, 28.0% had TE/AGA/TA) while only 9.5 percent of patients had TE alone.
The results are in agreement with a study conducted by Brenner and Oldoni,13 which found that 75.0 percent of postpartum patients experiencing TE were diagnosed with FAGA after one year of follow-up, which may suggest PPTE as a possible presenting sign of FAGA. The study by Brenner and Oldoni,13 included only 16 patients, while our study included 200 patients and other forms of hair loss. Our results are also consistent with Samrao and Mirmirani9 in their case series that reported three patients with PPTE, which revealed the presence of underlying traction alopecia.
We reported that 44.0 percent of our patients were experiencing trichodynia. This agrees with the result of early studies by Rebora et al,15 Grimalt et al,16 and Kivanç-Altunay et al,17 who observed trichodynia in 50.7%, 89%, and 36.6%, respectively, of female patients experiencing hair loss as a result of either TE or AGA.
Trichodynia refers to pain, discomfort, and/or paresthesia in the skin of the scalp. Mostly, trichodynia is associated with psychological comorbidity.18 Both TE and AGA may be influenced by psychological stress and may be the cause of secondary stress. Psychological stress acts as a primary inducer of TE and as an aggravating factor in AGA as it is hair follicle androgen receptor co-activator.19,21 This interprets the higher statistically significant relation between TE with AGA and trichodynia.
In our study, there was a higher statistically significant relation between TE with AGA and older age, increased number of pregnancies, and breast feeding (P-value<0.05). This higher statistically significant relation between TE with AGA and older age may be explained by a protective role of estrogen against FAGA, which decreases with age.21 Ramos and Miot,22 also reported that the frequency of FAGA increased with age in their patients. In contrast to our study, Ebrahimzadeh et al,23 found that there was no statistically significant relationship between postpartum hair loss and number of pregnancies.However, our study agrees with Ebrahimzadeh et al’s finding that there was a statistically significant relationship between postpartum hair loss and breastfeeding.23
In contrast to our study, Gizlenti and Ekmekci,24 found that there was no statistically significant relationship between PPTE and breastfeeding. This might be due to the low number of patients in this study compared to our study, as it was conducted on only 58 postpartum women, while our study included 200 patients.
In our study, there was no statistically significant relationship between diagnosis and socioeconomic status, special habits, post labor complications, type of labor (CS or vaginal), acne, hirsutism, poly cystic ovaries (PCOS), and menstrual disturbance, with a p-value>0.05. Our study is in agreement with Ebrahimzadeh et al,23 who found that there was no statistically significant relationship between postpartum hair loss and type of delivery (vaginal or CS).
In our study, there was a high statistically significant relationship between family history of AGA and TE/AGA than TE/AGA/TA than other groups (P<0.001). This agrees with Nyholt et al,25 who reported that genetic factors have a main role in etiopathogenesis of FAGA. Our current study might agree with the result of a retrospective study by Siah et al,26 who found that nearly 85 percent of FAGA patients had a family history of AGA.
We conducted our study on postpartum female patients 6 to 8 weeks after delivery, as it is the period of the peak of postpartum hair loss as mentioned by Lynfield,27 Ebrahimzadeh et al,23 and Gizlenti and Ekmekci.24
In our study, there was a statistically significant relation between the TE/AGA/TA group and duration of PPTE than other groups (p=0.008). With regard to pattern of hair loss; 16.0 percent of patients experienced shedding and 84.0 percent of patients experienced shedding and thinning. There was a statistically significant higher frequency of shedding in the TE group and TE/TA group, while there was a higher frequency of shedding and thinning in the TE/AGA group and TE/AGA/TA group (p<0.001).This is in close agreement with a study of Kasumagic-Halilovic,28 who found that FAGA is characterized by diffuse thinning of the crown area with an intact frontal hairline seen in 100 percent of patients in the study.
In our study, higher grades (Grade II, III) of Sinclair scale grades were presented in the TE/AGA/TA group and TE/AGA group, while low grade (Grade I) were presented in the TE group and TE/TA group. This is in agreement with the results of a study by Messenger and Sinclair,11 who reported that Sinclair Scale grades increase with the severity of hair loss in FAGA.
In our study, there was a highly statistically significant positive correlation between duration of PPTE and Sinclair scale (r=0.283 and p<0.001). Also, there was a statistically significant positive correlation between age “years” and number of pregnancies with Sinclair scale (r=0.197, r=0.283 and p=0.005, p<0.05, respectively).
All these findings are similar to the findings by Zhang et al,29 and Bains et al,30 in which the Sinclair scale was positively correlated with duration of hair loss and age of patients with (r=0.531, p<0.001; r=0.278, p=0.042; r=0.42, p=0.01; r=0.63, p=0.001) respectively.
In our study, regarding trichoscopic findings in the central area, there was no statistically significant relation between the diagnostic groups and number of terminal and vellus hairs (p>0.05). In spite of this, there was a decrease in the number of terminal hair and increase in number of vellus hair in the TE/AGA group and the TE/AGA/TA group without significant difference. This agrees with Rushton et al,31 and Kasumagic-Halilovic,28 who found that trichoscopy of AGA shows an increased proportion of vellus hair and this is presumed to reflect miniaturization of terminal into vellus follicles.
Our study showed that all patients (100%) demonstrated upright regrowing hair, as it is the most sensitive trichoscopic finding in TE.32 We also found that in patients diagnosed with TE (n=19, 9.5%), the most frequent trichoscopic findings in the central area were upright regrowing hair (100%) and single pilosebaceous unit (94.7%), followed by hair diameter diversity greater than 10 percent (78.9%) and perifollicular scales (47.4%) while the less frequent trichoscopic findings were peripilar sign and perifollicular erythema, with the same percentage of 26.3%.
Regarding patients with TE /TA (n=13, 6.5%), the most frequent trichoscopic findings in the central area were upright regrowing hair (100%) and perifollicular scales (76.9%), followed by single pilosebaceous unit (69.2%), hair diameter diversity greater than 10 percent (61.5%) than the less frequent trichoscopic findings were peripilar sign (30.8%) and perifollicular erythema (23.1%). We noticed that the findings in the two groups are nearly close to each other as traction alopecia has no effect on the central area, but its effect is usually along the marginal hair line (frontal, temporal, or occipital).33 In accordance with our study, Bains et al,30 found the most frequent trichoscopic findings in TE patients were single pilosebaceous unit, perifollicular scaling, and hair diameter diversity greater than 10 percent and peripilar sign.
Studies conducted by De Lacharrière et al,34 Ross et al,35 and Rudnicka et al,7 have established that there are no specific trichoscopic features in telogen effluvium. However, the presence of upright regrowing hair and single pilosebaceous unit may suggest telogen effluvium in absence of features characteristic for other causes of hair loss. Therefore, based on the current knowledge, telogen effluvium is considered to be a diagnosis of exclusion.
In patients diagnosed with TE/AGA (n=112, 56.0%), upright regrowing hair, single pilosebaceous unit and hair diameter diversity greater than 20 percent were detected in all patients in this group, followed by peripilar sign (89.3%), perifollicular scales (50.9%), perifollicular erythema (40.2%), yellow dots (40.2%), pig tail hair (39.3%), pinpoint white dots (35.7%), black dots (17.0%), and circular hair (16.1%). The trichoscopic findings of the patients with TE/AGA/TA (n=56, 28.0%) in the central area were almost similar to that of the TE/AGA as traction alopecia has no effect on the central area and the most prominent findings in the central area are that of AGA.36
In our study, in the TE group and TE/TA group, hair diameter diversity was greater than 10 percent while in other groups (TE/AGA and TE/AGA/TA) hair diameter diversity became greater than 20 percent as PPTE showed the underlying female pattern AGA which increases hair diameter diversity. This agrees with various studies such as Inui et al,37 Kibar et al,36 Rakowska et al,38 Tawfik et al,39 Nagar and Dhudshia,40 and Bains et al,30 which considered hair diameter diversity of more than 20 percent as a hallmark of AGA. Hair diameter diversity is due to miniaturization of hair follicles in the genetically predisposed scalp regions due to androgen effect and modulated through DHT as explained by De Lacharrière et al.34
Regarding single pilosebaceous unit, our results are similar to Rakowska et al38 and Tawfik et al,39 who reported a high percentage of single pilosebaceous unit on the frontal scalp of AGA patients.
In our study, we found peripilar sign in TE/AGA and TE/ AGA/TA groups with a percentage of more than 89 percent, and a lower percentage in other groups, as it is linked to superficial perifollicular infiltrates mainly composed of lymphocytes denoting micro-inflammation. This inflammation occurs early in the course of AGA and it is believed to facilitate the progressive miniaturization of hair follicles.41
This is similar to studies by Hu et al,42 Köse and Güleç.,43 Ramos et al,44 and Tawfik et al,39 in which peripilar sign was observed in the range of 20 to 60 percent of AGA patients.
We suggest that percentage of our study is more than other studies as our patients are already experiencing TE associated with AGA while other studies focused on AGA alone. The presence of underlying TE contributes to decreased hair density, which makes the peripilar sign more noticeable.
We observed yellow dots in 40.0 percent of patients. Previous studies reported different percentages in yellow dots. Hu et al,42 Kibar et al,36 and Tawfik et al,39 found that yellow dots range from 15 to 26 percent. Other studies as Rakowska et al,38 reported higher percentages (63%) than our study. Lima et al,45 suggested that yellow dots are seen in more advanced stages of the disease and indicate the chronicity of AGA.
In our study, the less frequent trichoscopic findings in the central area were scalp discoloration, arborizing red lines, pig tail hair, pinpoint white dots, black dots, and circular hair. Compatible to our study, Bains et al,30 found that black dots, scalp discoloration, scalp erythema, pig tail hair, and arborizing red lines were rare findings either in TE or AGA. In our study, increased hair diameter diversity made scalp honeycomb pigmentation and arborizing red lines appear more clearly.
Also in our study, the trichoscopic findings in area of traction in patients with hair traction (n=69, 34.5%) showed no statistically significant difference between the TE/TA group (n=13, 6.5%) and the TE/AGA/TA group (n=56, 28.0%) (p>0.05). As the site of hair traction is away from the area affected by AGA which is the crown region with maintenance of the frontal hairline.36
The most common trichoscopic findings were reduction in hair density, hair diameter diversity, empty follicles and vellus hair (100%), followed by perifollicular erythema (88.4%) and yellow dots (53.6%) then the less frequent trichoscopic findings were broken hair, loss of follicular openings, black dots, hair cast, comma hairs and circle hairs. These findings are in line with those of Polat,46 who reported that reduction in hair density, hair diameter diversity, empty follicles, and vellus hairs were observed in all patients (100%). In addition, the following were observed: loss of follicular openings (76%), yellow dots and broken hairs (68%), black dots (48%), hair casts (28%), and circular hairs (20%).
Conclusion
The present study highlights a novel and important association between postpartum TE and other underlying hair loss disorders. TE can unmask underlying FAGA and TA. Awareness of this phenomenon is important for appropriate diagnosis and treatment. The present study also supports the trichoscopic evaluation of hair loss disorders which provides a noninvasive diagnostic tool that can differentiate between different causes of hair loss. Proper dermatological care and counseling of postpartum female patients is a necessity to explore any underlying hair loss disorder.
References
- Phillips TG, Slomiany WP, and Robert Allison II. Hair loss: common causes and treatment. American family physician. 96(6), 371–378.
- Rebora A. Proposing a Simpler Classification of Telogen Effluvium. Skin Appendage Disord. 2(1-2):35–38.
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: A review of the literature. Cureus. 2020 May 27;12(5):e8320.
- Sahin G, Pancar GS, and Kalkan G. New pattern hair loss in young Turkish women; What’s wrong in their daily life? Skin Research and Technology. 2019 May;25(3):367–374.
- Udompanich S, Chanprapaph K, and Suchonwanit, P. Hair and scalp changes in cutaneous and systemic lupus erythematosus. Am J Clin Dermatol. 2018 Oct;19(5):679–694.
- Grymowicz M, Rudnicka E, Podfigurna A, et al. Hormonal effects on hair follicles. Int J Mol Sci. 2020 Aug; 21(15): 5342.
- Rudnicka L, Olszewska M, Rakowska A. Trichoscopy update 2011. J Dermatol Case Rep. 2011 Dec 12;5(4):82–88.
- Lopez-Gonzalez DM and Kopparapu AK. Postpartum care of the new mother. Study Guide from. StatPearls Publishing, Treasure Island (FL).
- Samrao A and Mirmirani P. Postpartum Telogen Effluvium Unmasking Traction Alopecia. Skin Appendage Disorders. 2022 Jul; 8(4): 328–332.
- Grover C and Khurana A. Telogen effluvium. Indian J Dermatol Venereol Leprol. 79:591–603
- Messenger AG and Sinclair R. Follicular miniaturization in female pattern hair loss: clinicopathological correlations. British Journal of Dermatology. 2006 Nov;155(5):926–930.
- Mirallas O and Grimalt R. The postpartum telogen effluvium fallacy. Skin Appendage Disord. 2016 May;1(4):198–201.
- Brenner FM and Oldoni C. Telogen effluvium x female pattern hair loss: is there correlation? An Bras Dermatol. 2019 Jul-Aug; 94(4): 486–487.
- Mirowsky J. Parenthood and health: The pivotal and optimal age at first birth. Social Forces. 81(1), 315–349.
- Rebora A, Semino MT and Guarrera, M. Trichodynia. Dermatology. 192(3), 292–293.
- Grimalt R, Ferrando J and Grimalt F. Trichodynia. Dermatology (Basel, Switzerland). 196(3), 374–374.
- Kivanç-Altunay İ, Savaş C, Gökdemir G, et al. The presence of trichodynia in patients with telogen effluvium and androgenetic alopecia. Int J Dermatol. 2003 Sep;42(9):691–693.
- Trüeb RM. Telogen effluvium and trichodynia. Dermatology (Basel, Switzerland).196(3), 374–375.
- Tellez-Segura R. Involvement of mechanical stress in androgenetic alopecia. Int J Trichology. 2015 Jul-Sep; 7(3):95–99.
- Hadshiew IM, Foitzik K, Arck PC, et al. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004 Sep;123(3):455–457.
- Sawaya ME and Price VH. Different levels of 5α-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997 Sep;109(3):296–300.
- Ramos PM and Miot HA. Female pattern hair loss: a clinical and pathophysiological review. An Bras Dermatol. 2015 Jul-Aug; 90(4): 529–543.
- Ebrahimzadeh-Ardakani M, Ansari K, Pourgholamali H, et al. Investigating the prevalence of postpartum hair loss and its associated risk factors: a cross-sectional study. Iranian Journal of Dermatology. 2021; 24(4), 295–299.
- Gizlenti S and Ekmekci TR. The changes in the hair cycle during gestation and the post-partum period. J Eur Acad Dermatol Venereol. 2014 Jul;28(7):878–881.
- Nyholt DR, Gillespie NA, Heath AC, et al. Genetic basis of male pattern baldness. Journal of Investigative Dermatology. 2016;121(6),1561–1564.
- Siah TW, Muir-Green L and Shapiro J. Female pattern hair loss: a retrospective study in a tertiary referral center. Int J Trichology. 2016 Apr-Jun; 8(2):57–61.
- Lynfield YL. Effect of pregnancy on the human hair cycle. J Invest Dermatol. 1960 Dec:35:323–327.
- Kasumagic-Halilovic E. Trichoscopic Findings in Androgenetic Alopecia. Medical Archives. 2021 75(2),109.
- Zhang X, Caulloo S, Zhao Y, et al. Female pattern hair loss: clinico-laboratory findings and trichoscopy depending on disease severity. Int J Trichology. 2012 Jan;4(1):23–28.
- Bains P, Kaur S, and Kaur K. Comparison of Dermoscopic Findings in Female Androgenetic Alopecia and Telogen Effluvium and Female Controls in a Tertiary Care Center. J Clin Aesthet Dermatol. 2022 May; 15(5): 29–34.
- Rushton DH, Norris MJ and Van Neste D. Hair regrowth in male and female pattern hair loss does not involve the conversion of vellus hair to terminal hair. Exp Dermatol. 2016 Jun;25(6):482–484.
- Park J, Kim JI, Kim HU, et al. Trichoscopic findings of hair loss in Koreans. Ann Dermatol. 2015 Oct;27(5):539–550.
- Pulickalm JK and Kaliyadan F. Traction Alopecia. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
- De Lacharrière O, Deloche C, Misciali C, et al. Hair diameter diversity: A clinical sign reflecting the follicle miniaturization. Arch Dermatol. 2001 May;137(5):641–646.
- Ross EK, Vincenzi C, and Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006 Nov;55(5):799–806.
- Kibar M, Aktan Ş, Bilgin M, et al. Scalp dermatoscopic findings in androgenetic alopecia and their relations with disease severity. Ann Dermatol. 2014 Aug; 26(4): 478–484.
- Inui S, Nakajima T, and Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009 Feb;36(2):82–85.
- Rakowska A, Waśkiel A, Sikora M, et al. Two different trichoscopic patterns of mid-frontal scalp in patients with frontal fibrosing alopecia and clinical features of androgenetic alopecia. Przegl Dermatol. 2017,104,9–15.
- Tawfik SS, Sorour OA, Alariny AF, et al. White and yellow dots as new trichoscopic signs of severe female androgenetic alopecia in dark skin phototypes. Int J Dermatol. 2018 Oct;57(10):1221–1228.
- Nagar R and Dhudshia R. Utility of trichoscopy to diagnose early female pattern hair loss in resource-poor setting: a cross-sectional study. Indian J Dermatol Venereol Leprol. 2019 Nov-Dec;85(6):681.
- Deloche C, de Lacharrière O, Misciali C, et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res. 2004 Mar;295(10):422–428.
- Hu R, Xu F, Han Y, et al. Trichoscopic findings of androgenetic alopecia and their association with disease severity. J Dermatol. 2015 Jun;42(6):602–607.
- Köse ÖK, and Güleç AT. Clinical evaluation of alopecias using a handheld dermatoscope. J Am Acad Dermatol. 2012 Aug;67(2):206–214.
- Ramos LD, Santili MCN, Bezerra FC, et al. Dermoscopic findings in female androgenetic alopecia. An Bras Dermatol. 2012 Sep-Oct;87(5):691–694.
- Lima CDS, Lemes LR, and Melo DF. Yellow dots in trichoscopy: relevance, clinical significance and peculiarities. An Bras Dermatol. 2017 Sep-Oct;92(5):724–726.
- Polat M. Evaluation of clinical signs and early and late trichoscopy findings in traction alopecia patients with Fitzpatrick skin type II and III: a single-center, clinical study. Int J Dermatol. 2017 Aug;56(8):850–855.

