 by Zoe Diana Draelos, MD
by Zoe Diana Draelos, MD
Dr. Draelos is with Dermatology Consulting Services, PLLC in High Point, North Carolina.
FUNDING: Lumity Life Ltd. provided a grant for this study.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
ABSTRACT: The relationship between good nutrition and skin health has been difficult to scientifically document. While the minimum daily allowances of nutrients to prevent disease have been established, the optimal nutritional state for skin health is unknown. This single-site, double-blind, placebo-controlled study enrolled 50 female subjects, aged 35 to 65 years, with mild-to-moderate photoaging and stable prescription medication usage for three months prior to study enrollment. All nutritional supplements were stopped at two weeks prior to study entry. Subjects took three morning and three evening supplement tablets or an equal number of placebo sugar pills to maintain the blinding. The blinded investigator rated a statistically significant improvement at Week 8 compared to baseline, which continued into Week 12, for the active supplement over the placebo in terms of firmness (p=0.035), hydration (p<0.001), dullness (p<0.001), and overall skin appearance (p=0.010). The subjects noted a statistically significant improvement in healing (p=0.042), hydration (p=0.004), dullness (p=0.003), roughness (p=0.015), pigmentation (p=0.025), and overall appearance (p=0.005) at Week 8 that also continued into Week 12. In addition, the subjects completed a quality of life evaluation, which split statistically from placebo in all attributes at Week 12. Finally, there was a statistically significant reduction in wrinkle breadth in the supplement group compared to the placebo group (p=0.018), as analyzed from laser-scanned silicone replicas from the left orbit when comparing baseline to Week 12. These findings support the value of the study supplement in improving skin appearance.
KEYWORDS: Aging skin, nutrition, supplements, quality of life
J Clin Aesthet Dermatol. 2019;12(7):13–16
The nutrition–skin connection has been difficult to substantiate in dermatology. It is the popular consumer belief that a healthy diet contributes to beautiful skin. Indeed, this seems to make common sense, since the body requires the proper nutritional building blocks to build and repair body tissues, especially those with a high metabolic turnover rate such as skin, hair, and nails.1 For example, a source of protein is needed to repair skin, vitamin C is required for collagen production, and vitamin E is necessary to function as a primary antioxidant.2,3 Thus, the nutritional requirements for minimal skin functioning to prevent the occurrence of disease is well understood; however, understanding of nutritional requirements for optimal skin functioning and appearance maximization is less robust.4 Specifically, we have minimum daily nutrition requirements, but we do not have optimal daily nutrition recommendations. The present research was undertaken to gain a better understanding of how a commercially available nutritional supplement might improve skin function and appearance as defined by increased hydration and reduction of wrinkles. In addition, the ability of the nutritional supplement to improve quality of life (QOL), including energy levels and the ability to sleep, was also investigated.
Methods
This single-site, double-blind, placebo-controlled study enrolled 50 female subjects, 35 to 65 years of age, with Fitzpatrick Skin Types I to VI and no known medical conditions or facial skin disease that might interfere with study participation. Enrolled participants provided informed and photo consent (Concordia Clinical Research, Cedar Knolls, New Jersey). Twenty subjects were in menopause. Enrollees had to possess mild-to-moderate photoaging with stable prescription medication usage for three months prior to study enrollment. All nutritional supplements had to be stopped at two weeks prior to study entry. Subjects taking oral corticosteroids, immunosuppressive drugs, antihistamines, insulin, antibiotics, or facial topical drugs were not enrolled. Subjects with uncontrolled metabolic diseases, such as diabetes (Types I and II), hypertension, hyperthyroidism, hypothyroidism, severe chronic asthma, immunological disorders (e.g., human immunodeficiency virus [HIV]), systemic lupus erythematosus, or mastectomy with lymph node removal were also deemed ineligible for enrollment. Subjects had to discontinue topical hydroxy acids for two weeks, topical prescription or over-the-counter retinoids for one month, and oral retinoids for one year prior to study entry. Use of chemical peels or dermabrasion within six months and tanning booth use within one year of study enrollment were not allowed. Subjects who were pregnant, nursing, or planning to become pregnant were not included. Subjects continued with their own self-selected cleanser, skin care products, sunscreens, and cosmetics in an unchanged manner throughout the study.
Subjects deemed eligible for study enrollment underwent a dermatologist investigator assessment and self-assessment for facial efficacy (5-point ordinal scale: 0=none, 1=minimal, 2=mild, 3=moderate, 4=severe) with respect to lines/wrinkles, firmness, healing ability, hydration, dullness, skin texture/roughness, mottled pigmentation, and overall appearance. The investigator queried the subjects and the subjects also evaluated the supplement regarding tolerability on the same five-point ordinal scale with respect to flatulence, burping, and indigestion.
Noninvasive assessments were also incorporated into the study design to assess skin hydration through pin probe corneometry (Dermalab; Cortex Technologies, Hadsund, Denmark) on a facial target site, and skin replicas to assess facial wrinkle architecture were obtained at baseline and Week 12. Replicas were made at the corner of the left eye from silicone to capture the crow’s feet area. Digital JPEG photographs (Nikon D90; Nikon Corp., Toyko, Japan with Canfield three-point head mount; Canfield Scientific, Fairfield, NJ, USA) were taken of the front, right, and left facial areas with a standardized F-stop, magnification, and flash to insure reproducibility.
All subjects were given a one-month supply of the study product (Lumity; Lumity Life Ltd., London, United Kingdom) in a generic white bottle or the placebo (sucrose tablets) in an identical generic white bottle. Subjects were instructed to ingest three tablets of the designated morning supplement in the morning and three tablets of the designated evening supplement in the evening. It was recommended that subjects place the study supplement by their toothbrush to facilitate adherence. The bottles were labeled identically, and the study coordinator maintained the blinding throughout the study. Half of the study subjects were randomized to receive the study product and the other half received the placebo. An adherence diary was also provided to subjects. Subjects returned to the research center at Weeks 4, 8, and 12 for all previously discussed study activities and redispensing as needed.
QOL assessments were obtained at Weeks 4, 8, and 12 to determine if the supplement improved attributes associated with life performance based on the following statements: “my energy has improved,” “my sleep has improved,” “it is easier for me to fall asleep,” “I wake up less frequently during the night,” “it is easier to wake up in the mornings,” “I experience fewer energy slumps during the day,” “I have fewer coughs or colds,” “I am more mentally alert,” “I feel more emotionally balanced,” “I have experienced improvement in my monthly cycle or with menopause,” “my strength and endurance have improved,” “the quality and growth of my hair have improved,” “my nails are stronger and healthier,” “my specific skin conditions have improved,” “my skin is more hydrated and supple,” and “I am more positive about the way I look.” The following assessment scale was used: 0=agree strongly, 1=agree, 2=neutral, 3=disagree, and 4=disagree strongly. Thus, a lower score was a more favorable score.
Significance was defined at a p-value of less than or equal to 0.05. A paired Mann–Whitney test for nonparametric data were used to analyze the investigator and subject ordinal assessment outcomes. A Student’s t-test was used to analyze the findings of the noninvasive assessments (e.g., replicas, corneometry).
Results
The investigator assessments for efficacy were evaluated as change from baseline for the active supplement compared to the placebo groups. No statistically significant findings were observed at Week 4, but improvements in firmness (p=0.035), hydration (p<0.001), dullness (p<0.001), and overall skin appearance (p=0.010) were statistically significantly better in the active supplement group at Week 8 compared to the placebo group. These same findings continued into Week 12, demonstrating superiority of the supplement over the placebo (Figure 1). No tolerability issues were noted by the subjects or the investigator.
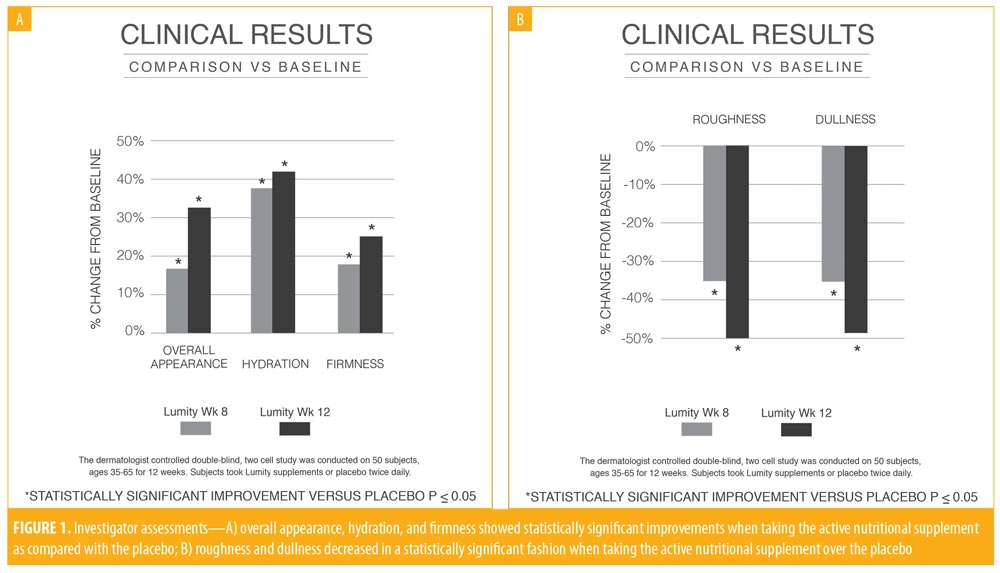
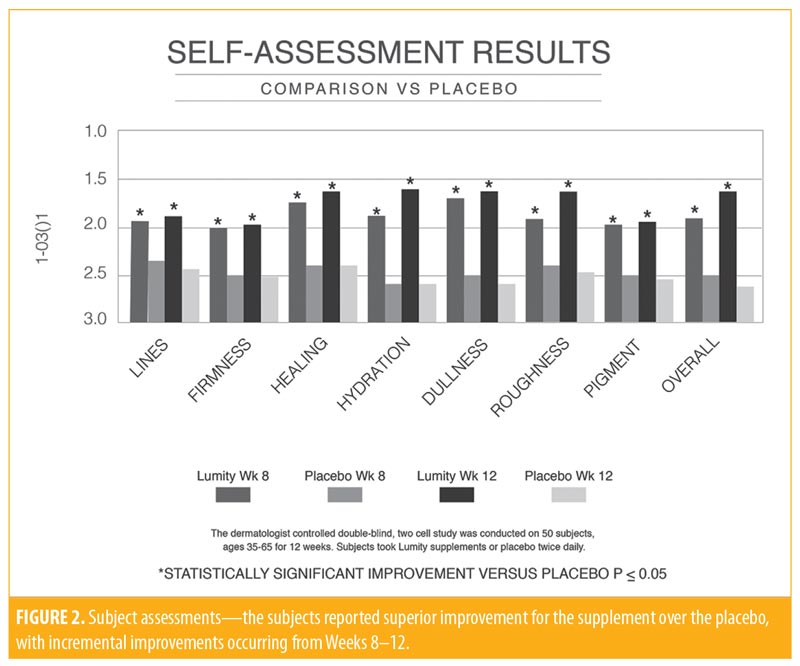
The subject efficacy data were examined according to change in the ordinal scores from baseline and demonstrated statistically significant improvements in firmness (p=0.036), pigmentation (p=0.011), and overall appearance (p=0.007) at Week 4 with the active supplement compared to the placebo. By Week 8, more improvements became statistically significant, including healing (p=0.042), hydration (p=0.004), dullness (p=0.003), roughness (p=0.015), pigmentation (p=0.025), and overall appearance (p=0.005). The improvement in these parameters continued into Week 12 (Figure 2). No statistically significant difference was noted between the active and placebo groups in terms of corneometry. Both groups experienced improvement, probably due to environmental factors.
The QOL questionnaire data were analyzed by comparing the mean rating for each question between the placebo and active groups. At Week 4, there was a statistically significant preference for the supplement over the placebo in terms of waking up less (p=0.005), fewer colds (p=0.36), increased mental alertness (p=0.020), increased emotional balance (p=0.030), enhanced endurance (p=0.003), improved hair quality (p=0.018), stronger and healthier nails (p=0.031), better skin (p=0.005), and increased skin hydration (p=0.007). Thus, the subjects were able to differentiate between the benefits of the supplement over placebo as early as at Week 4. This cumulative improvement continued into Week 8 with the same statistically significant findings and additional benefits noted with the supplement over placebo in terms of increased energy (p=0.020), improved sleep (p=0.010), easier to wake up in morning (p=0.029), fewer energy slumps (p=0.018), improved menses (p=0.010), and positive outlook (p=0.010). By Week 12, all of the QOL attributes broke statistically from the findings associated with the placebo.
The silicone lateral orbital replicas were analyzed with a scanning laser (BioNET Inc., Dallas, Texas), utilizing normal and perpendicular lighting to analyze horizontal and vertical lines at the lateral left orbit and comparing baseline to Week 12. At Week 12, there was a statistically significant reduction in wrinkle breadth in the supplement group compared with in the placebo group (p=0.018). This finding would be appreciated visually as a reduction in the wrinkled appearance of the skin. Representative before and after photographs following the completion of 12 weeks of oral supplementation are presented in Figure 3.

Discussion
This study examined the efficacy of a nutritional supplement in improving skin appearance and QOL compared to a placebo. The supplement was structured with both morning and night formulations, uniquely designed to meet the needs of the body at these two distinct time points.5 The morning formulation contained vitamin A (1,500 U), vitamin C (12mg), and vitamin E (9IU), which are important systemic and skin antioxidant vitamins.6 Vitamin D (600 IU) was also present for bone health and emotional well-being. The morning supplement also contained other trace nutrients, including iodine for thyroid health (225mcg), the antioxidant ingredient selenium (24.75mcg), and magnesium (60mg) and zinc (3mg), which are found in matrix metalloproteinases.7 Flaxseed oil (1gm) was included as a phytoestrogen and a source of essential fatty acids (omega-3 and omega-6).8 Other antioxidant nutrients included l-cysteine (682 mg) and tumeric extract (30 mg). Finally, the morning contained acetyl-l-carnitine and coenzyme Q10, which are important in cellular energy production.9 Acetyl-l-carnitine and coenzyme Q10 have been used in mature adults to decrease physical fatigue.10
The night supplement was a different formulation designed to meet the needs of a body at rest. It contained additional flaxseed oil (933mg), a phytoestrogen helpful in menopause-induced hot flashes, and l-cysteine (634mg). A variety of amino acids, such as lysine hydrochloride, l-glutamine, and l-arginine were also included.11 This combination of amino acids is purported to stimulate the pituitary gland to produce human growth hormone. Finally, alanine was incorporated for its ability to increase carnosine levels, which are important in glycation interference.12
The effects of three morning softgels and three evening softgels were compared to effects in subjects who took an equivalent number of placebo tablets in the morning and evening, both of which were well-tolerated.The studied supplement was not intended to correct nutritional deficiencies, but rather to provide targeted support through a carefully constructed set of nutrients, including amino acids, vitamins, and minerals. For example, carnitine, iodine, magnesium, and coenzyme Q10 were intended to increase cellular energy production.
The morning and evening supplements were delivered via a softgel. Softgel capsules were made from gelatin, water, an opacifier, and a plasticizer. The supplements were manufactured via a process known as encapsulation, which involves making two flat ribbons of shell material that are heated to form a seal, followed by an injection pump filling in the liquid contents. Heating seals the injection opening and filling in a die gives rise to the three-dimensional shape of the softgel. The softgel is smooth, which can make it easier to swallow, while ensuring the stability of the supplement ingredients due to the airtight seal. The opaque softgel provides protection from light, ultraviolet radiation, and oxidation, which can damage supplement ingredients.
Data were collected to evaluate the effects of the nutritional supplement assessed by the blinded dermatologist investigator and blinded subjects regarding both appearance and QOL. Both the investigator and subjects noted statistically significant improvements in hydration, dullness, overall appearance, and QOL measures when using the supplement compared to the placebo. The improvement in appearance in particular was supported by the replica analysis demonstrating a statistically significant reduction in wrinkle breadth with supplement usage.
Conclusion
This double-blind, placebo-controlled study demonstrated appearance and functional benefits from a supplement containing antioxidant vitamins, trace minerals, and a variety of amino acids and essential fatty acids.
References
- Finner AM. Nutrition and Hair. Dermatol Clin. 2013;31:167–172.
- Bailey DM, Williams C, Betts JA, Thompson D. Oxidative stress, inflammation and recovery of muscle function after damaging exercise: effect of 6-week mixed antioxidant supplementation. Eur J Appl Physiol. 2011;111(6):925–936.
- Morrison D, Hughes J, Della Gatta PA, et al. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med. 2015;89:852–862.
- Miquel J. An update on the mitochondrial – DNA mutation hypothesis of cell aging. Mutat Res. 1992;275(3–6):209–216.
- Ames BN. Micronutrient deficiencies: a major cause of DNA damage. Ann NY Acad Sci. 2000;889(1):87–106.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25(9):1006–1012.
- Boelsma E, Hendriks HF. Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. Am J Clin Nutr. 2001;73(5):853–864.
- De Spirt S, Stahl W, Tronnier H, et al. Intervention with flaxseed and borage oil supplements modulates skin condition in women. Br J Nutr. 2009;101(3):440–445.
- Cherniack EP. Ergogenic dietary aids for the elderly. Nutrition. 2002;28(3):225–229.
- Overvad K, Diamant B, Holm L, et al. Coenzyme Q10 in health and disease. Eur J Clin Nutr. 1999;53(10):764–770.
- Mohamed Z. Gad. Anti-aging effects of l-arginine. J Adv Res. 2010;1(3):169–177.
- Hoffman J, Ratamess RA, Ross R, et al. Beta-alanine and the hormonal response to exercise. Int J Sports Med. 2008;29(12):952–958.

