 by Yohei Tanaka, MD, PhD; Toru Aso, MD, PhD; Jumpei Ono, MD; Ryu Hosoi, MD; and Takuto Kaneko, MD
by Yohei Tanaka, MD, PhD; Toru Aso, MD, PhD; Jumpei Ono, MD; Ryu Hosoi, MD; and Takuto Kaneko, MD
Dr. Tanaka is with Clinica Tanaka Plastic, Reconstructive Surgery and Anti-aging Center in Matsumoto, Nagano, Japan, and the AGA Skin Clinic in Tokyo, Japan. Drs. Aso, Ono, Hosoi, and Kaneko are also with the AGA Skin Clinic in Tokyo, Japan.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Background. Androgenetic alopecia (AGA) is a common form of hair loss in Asian men. Although AGA is often regarded as a relatively minor dermatological condition, hair loss can impact self-image and is a main cause for anxiety and depression in some men. We have treated patients with AGA for seven years. Objective. The goal of this study was to evaluate the effectiveness of our combination therapy in Asian men with AGA. Participants. Between the years 2011 and 2017, 18,918 male patients were treated in our center. Our combination therapy consists of oral finasteride once daily, oral and topical minoxidil twice daily, and an injectable treatment of lidocaine and an AGA treatment solution comprising minoxidil, arginine, aspartic acid, caffeine, copper tripeptide, lysine, niacin, panthenol, propanediol, propylen glycol, retinyl palmitate, pyridoxine, sodium hyaluronate, and ubiquinone once monthly for more than six months. Measurements. Digital photographs were taken pre- and post-treatment, and patient assessments were recorded after six and 12 months post-treatment. Results. Significant improvement was observed in all patients in the digital photographs. Ninety-six and 80 percent of the patients reported satisfaction with the results of the treatment after six and 12 months post-treatment. Minor complications were observed in 802 (4.2%) patients, characterized by slight pain and bleeding due to injection, swelling, dizziness, itching, and erythema of the scalp. Slight pain was reported in 651 patients (3.4%), and slight bleeding was reported in 56 patients (0.3%). Sexual dysfunctions were uncommon. These minor complications resolved spontaneously. No treatment-related adverse events were observed. Conclusion. A combination of these therapeutic options offers safe and highly efficacious treatment for AGA with minimal complications.
Keywords: Androgenetic alopecia (AGA), Combination therapy, Finasteride, Hair loss, Minoxidil
J Clin Aesthet Dermatol. 2018;11(7):32–35
Introduction
Androgenetic alopecia (AGA) is the most common type of hair loss in Asian men.1,2 AGA is characterized by follicular miniaturization in a patterned hair loss occurring due to systemic androgen and genetic factors.1,3–5 The age of onset is usually the third and fourth decades, but the hair loss starts immediately after puberty and continues progressively.1,6 AGA can affect a variety of psychological and social experiences, as well as the individual’s quality of life.7–9 Different results have been reported regarding the prevalence of AGA, depending on ethnic groups.1,6,10–12 According to Hamilton’s study, by the age of 30 years, the mean prevalence of AGA is 30 percent, and this rate rises to 50 percent by the age of 50 years.12 There are racial as well as age-related differences in the incidence and pattern of hair loss in AGA,13 and both prevalence and severity of AGA were reported to be lower in Asian and black men than in Caucasians.1,4,10,11 The onset of AGA in Japanese men occurs one decade later than in Caucasian men.14 A familial tendency to AGA and racial variation in the prevalence is well recognized, with heredity accounting for approximately 80 percent of predisposition.15 Normal levels of androgens are sufficient to cause hair loss in genetically susceptible individuals.15 AGA has various psychosocial impacts on the affected individual and can cause emotional distress.
Alteration in the hair cycle development, follicular miniaturization, and inflammation are the key pathophysiological features of AGA.15 The anagen phase decreases with each cycle, and the length of telogen remains constant or is prolonged in patients with AGA.15 Anagen duration becomes so short that the growing hair fails to achieve sufficient length to reach the surface of the skin, leaving an empty follicular pore. Hair follicle miniaturization is the histological hallmark of AGA.15 Once the arrector pili muscle, which attaches circumferentially around the primary follicle, has detached from all secondary follicles and primary follicles have undergone miniaturization and detachment, hair loss is likely irreversible.15 Inflammation around the upper part of the hair follicles has been well described by many researchers.16–18
We have experienced clinically satisfactory results in some patients treated by an injectable treatment without oral and topical treatments, as well as patients treated by oral and topical treatments. However, in our experience, some patients decide not to continue the treatments any further due to the length of the treatment period, which is at least six months. Therefore, we hypothesized that a combination therapy including oral, topical, and injectable treatments would provide rapid and effective stimulation of hair regrowth, which is beneficial for treating AGA. To test this hypothesis, we evaluated the efficacy of our combination therapy for AGA not only subjectively but also objectively. We sought to quantitatively demonstrate the results of our combination therapy for AGA by retrospectively reviewing and describing our cumulative experience over the past seven years.
Methods
Japanese patients. In this study, 18,918 male patients aged 18 to 81 years (mean age, 32.0±9.92 years) were enrolled. Patients underwent our AGA combination therapy between 2011 and 2017. All of the patients had visited the AGA Skin Clinic to treat AGA. All patients gave their informed consent to the operation after being fully informed about the treatment risks. To avoid underestimation of prostatic cancer risk, serum prostate specific antigen (PSA) levels were tested by blood serum before treatment in all patients. Patients whose pretreatment PSA levels were over 4.00ng/mL were excluded from this study. Also, since oral minoxidil could decrease blood pressure, patients under oral treatment for hypertension were also excluded from this study. None of the patients had a history of any type of skin disease or other hair restoration procedure.
Combination therapy. Our combination therapy consists of 1mg oral finasteride (5 alpha reductase type II inhibitor) once daily, 2.5mg oral and 5% solution topical minoxidil twice daily, and an injection once monthly for more than six months. The injectable treatment (4 mL per treatment) comprised 2mL of 1% lidocaine and 2mL of a solution containing minoxidil, arginine, aspartic acid, caffeine, copper tripeptide, lysine, niacin, panthenol, propanediol, propylen glycol, retinyl palmitate, pyridoxine, sodium hyaluronate, and ubiquinone—all of which was injected directly into areas where more hair was desired. Postoperative follow-up was scheduled after six and 12 months.
Subjective patients’ assessments. Subjective assessments were performed using questionnaire data collected after six and 12 months post-treatment. Patients rated their degree of satisfaction with improvement of the treated area. Scores were based on a five-point scale ranging from 0 to 4 (0=worse; 1=little satisfaction or not satisfied; 2=fairly satisfied; 3=satisfied; and 4=very satisfied).
Results
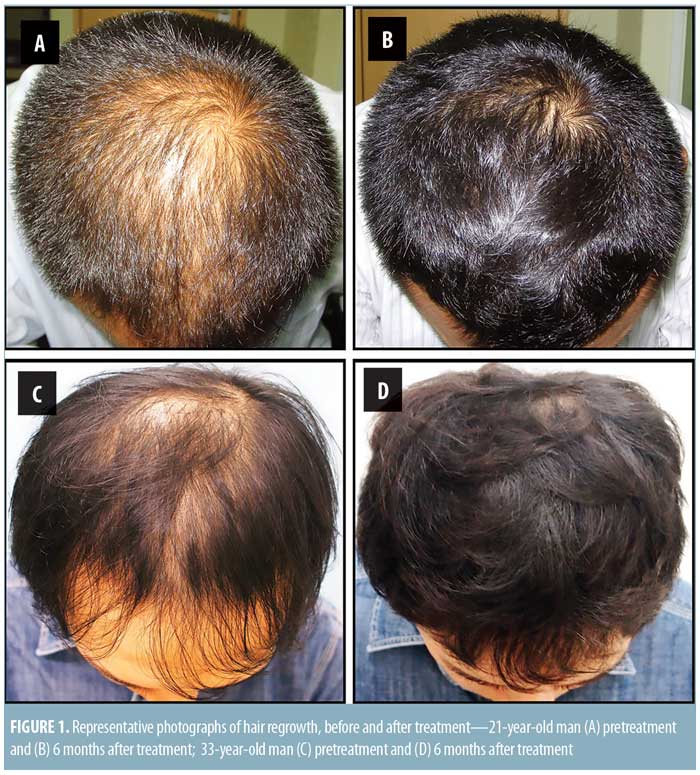
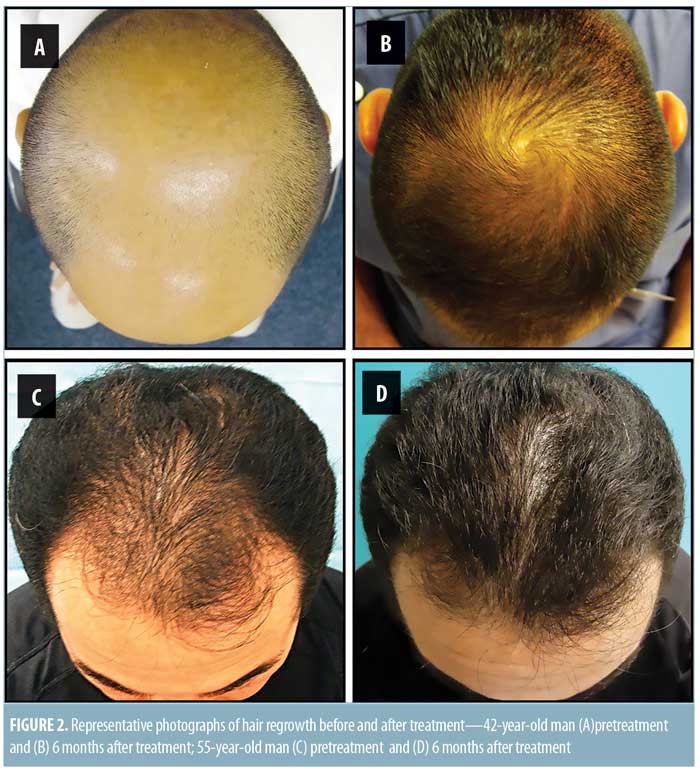
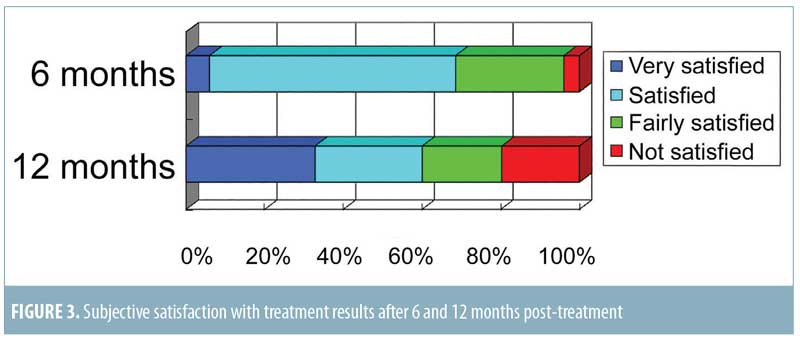
Significant improvement was observed in digital photographs compared to the pre-treatment areas for all patients (Figures 1 and 2). Ninety-six and 80 percent of patients reported satisfaction with the results of the treatment after six and 12 months post-treatment, respectively (Figure 3).
Minor complications were observed in a total of 802 (4.2%) patients out of 18,918. Slight pain due to injection was found in 651 patients (3.4%), and slight bleeding was found in 56 patients (0.3%). Swelling and dizziness due to oral minoxidil and itching and erythema of the scalp due to topical minoxidil were found in 42 patients (0.22%), 28 patients (0.15%), 7 patients (0.04%), and 4 patients (0.02%), respectively. Sexual dysfunctions (erectile dysfunction and low libido) were observed in 14 patients (0.07%). These minor complications resolved spontaneously. No treatment-related adverse events were observed.
Discussion
During this seven-year period, over 18,000 patients were successfully treated using our combination therapy. Overall, there were no severe complications, with an overall minor complication rate of 4.2 percent, characterized by slight pain and bleeding due to injection, swelling, dizziness, itching and/or erythema of the scalp, and sexual dysfunctions. Overall, this efficacious combination therapy, consisting of a daily oral finasteride, oral and topical minoxidil, and a monthly injectable treatment has been well tolerated with minimal complications.
Finasteride is a potent and highly selective antagonist of type II 5 alpha reductase and is not an anti-androgen. Five alpha reductase converts testosterone into dihydrotestosterone (DHT). DHT, binding to the scalp hair follicle androgen receptors, produces AGA. Finasteride reduces DHT production and limits its action on scalp hair follicles. Finasteride has been reported to slow the progression of male androgenetic alopecia (MAA) and to produce partial regrowth.19 A study measuring hair counts using macrophotographs found that both total and anagen hair counts increase with treatment of finasteride.20 Finasteride reverses the miniaturization process, producing hair of greater length and thickness, and possibly with a greater growth rate.15 Adverse effects, including sexual dysfunction (e.g., erectile dysfunction, low libido, anorgasmia), are uncommon and most resolve spontaneously, even without discontinuing treatment.21,22 However, sexual adverse effects reported on social media and internet forums could keep potential patients away from AGA treatment.
Minoxidil induces cutaneous blood flow due to vasodilatory properties23 and up-regulates vascular endothelial growth factor (VEGF), which helps in maintaining dermal papilla vasculature and hair growth.24 Minoxidil also promotes hair regrowth through its action to open potassium channels.25 The effect on the cell cycle is to initiate the onset of anagen (and thereby shorten telogen duration) and to prolong the duration of anagen by delaying initiation of catagen.15
Several studies have shown the effect of topical minoxidil in promoting hair growth.26–28 A five-year follow-up study with topical minoxidil demonstrated the sustained effect of minoxidil with long term use.29 Minoxidil works as a nonspecific promoter of hair growth, but the slow miniaturization of hair follicles induced by androgens continues.15 This would mean that patients using minoxidil as monotherapy for MAA continue to go bald despite treatment. A meta-analysis showed the efficacy of minoxidil versus placebo in the treatment of AGA.30
Minoxidil might cause a surge in the growth of miniaturized hairs and induction of anagen from resting hair follicles, which can produce a rapid hair shedding of previous telogen hairs 2 to 8 weeks after treatment initiation.15 This temporary hair shedding resolved within several weeks during treatment in all of our patients.
Both finasteride and minoxidil arrest progression of hair loss and stimulate partial regrowth of hair. They are effective but require continuous use to maintain the effect.31
An open, randomized, parallel-group study comparing the efficiency of available medications as monotherapy or combined therapy (finasteride alone, finasteride and topical minoxidil, topical minoxidil alone and finasteride and ketoconazole shampoo) showed that hair growth was observed in all groups, with best results reported using a combination of finasteride and minoxidil, followed by finasteride and ketoconazole shampoo, finasteride alone, topical minoxidil alone.32
Previously, some of our patients could not continue the therapy due to the long treatment time (?6 months). We wanted to shorten the treatment period, and postulated that a combination therapy including oral, topical and injectable treatments would provide more rapid therapeutic effects. We performed the injectable treatment, as described, to deliver the effective ingredients to the scalp directly. Slight pain and bleeding during injection were reported in some patients, but these adverse effects were not severe enough to cause any of our patients to discontinue treatment.
Some of our patients were not satisfied with the results, even though we observed varying degrees of improvements in every patient. In the first six months, satisfaction rate was high, likely due to patients the drastic improvements often seen during this period. Though hair regrowth was still observed at the 12-month follow-up, it was less dramatic, and this might account for the decrease in patient satisfaction. The combination of medications that have different mechanisms of action enhances the efficacy of AGA treatments.15,32 Topical antiandrogens, prostaglandin analogues, topical antifungals, growth factors, laser treatment, and hair transplantation would be hopeful additional medical treatments for AGA.
Limitations. Lack of a control group and lack of a comparison between dosage strengths and frequencies limit the significance of our findings.
Conclusion
Our combination therapy offers safe and efficacious treatment of AGA among Asian men with minimal complications. Further studies are necessary to determine if a higher dose or increased frequency of injectable treatments could enhance the effects of the combination therapy. Additionally, studies that compare our combination therapy to other treatment methods are warranted.
References
- Jang WS, Son IP, Yeo IK, et al. The annual changes of clinical manifestation of androgenetic alopecia clinic in Korean males and females: a outpatient-based study. Ann Dermatol. 2013;25:181–188.
- Choi J, Jun M, Lee S, et al. The association between exercise and androgenetic alopecia: a survey-based study. Ann Dermatol. 2017;29:513–516.
- Yang CC, Hsieh FN, Lin LY, et al. Higher body mass index is associated with greater severity of alopecia in men with male-pattern androgenetic alopecia in Taiwan: a cross-sectional study. J Am Acad Dermatol. 2014;70:297–302.e1.
- Sinclair R, Torkamani N, Jones L. Androgenetic alopecia: new insights into the pathogenesis and mechanism of hair loss. Version 1. F1000Res. 2015; 4(F1000 Faculty Rev): 585. Published online 2015 Aug 19.
- Di Loreto C, La Marra F, Mazzon G, et al. Immunohistochemical evaluation of androgen receptor and nerve structure density in human prepuce from patients with persistent sexual side effects after finasteride use for androgenetic alopecia. PLoS One. 2014;9:e100237.
- Wang TL, Zhou C, Shen YW, et al. Prevalence of androgenetic alopecia in China: a community-based study in six cities. Br J Dermatol. 2010;162:843–847.
- Monselise A, Bar-On R, Chan L, et al. Examining the relationship between alopecia areata, androgenetic alopecia, and emotional intelligence. J Cutan Med Surg. 2013;17:46–51.
- Han SH, Byun JW, Lee WS. Quality of life assessment in male patients with androgenetic alopecia: results of a prospective, multicenter study. Ann Dermatol. 2012;24:311–318.
- Schmidt S, Fischer TW, Chren MM, et al. Strategies of coping and quality of life in women with alopecia. Br J Dermatol. 2001;144:1038–1043.
- Severi G, Sinclair R, Hopper JL, et al. Epidemiology and health services research. Androgenetic alopecia in men aged 40–69 years: prevalence and risk factors. Br J Dermatol. 2003;149:1207–1213.
- Krupa Shankar D, Chakravarthi M, Shilpakar R. Male androgenetic alopecia: Population-based study in 1,005 subjects. Int J Trichology. 2009;1:131–133.
- Hamilton JB. Patterned long hair in man: types and incidence. Ann N Y Acad Sci. 1951;53: 708–728.
- Olsen EA. Disorders of Hair Growth: Diagnosis and Treatment. New York: McGraw-Hill Inc. 2003
- Ishino A, Uzuka M, Tsuji Y, et al. Progressive decrease in hair diameter in Japanese with male pattern baldness. J Dermatol. 1997;24:758–764.
- Cranwell W, Sinclair R. Male Androgenetic Alopecia. De Groot LJ, Chrousos G, Dungan K, et al., editors. South Dartmouth (MA): MDText.com, Inc.; 2000–.
- Pinkus H. Differential patterns of elastic fibers in scarring and non-scarring alopecias. J Cutan Pathol. 1978;5:93–104.
- Sueki H, Stoudemayer T, Kligman AM, Murphy GF. Quantitative and ultrastructural analysis of inflammatory infiltrates in male pattern alopecia. Acta Derm Venereol. 1999;79: 347–350.
- Kligman AM. The comparative histopathology of male-pattern baldness and senescent baldness. Clin Dermatol. 1988;6:108–118.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39:578–589.
- Van Neste D, Fuh V, Sanchez-Pedreno P, et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol. 2000;143:804–810.
- Tosti A, Piraccini BM, Soli M. Evaluation of sexual function in subjects taking finasteride for the treatment of androgenetic alopecia. J Eur Acad Dermatol Venereol. 2001;15:418–421.
- Yim E, Baquerizo Nole KL, Tosti A. 5a-Reductase inhibitors in androgenetic alopecia. Curr Opin Endocrinol Diabetes Obes. 2014;21:493–498.
- Wester RC, Maibach HI, Guy RH, Novak E. Minoxidil stimulates cutaneous blood flow in human balding scalps: pharmacodynamics measured by laser Doppler velocimetry and photopulse plethysmography. J Invest Dermatol. 1984;82:515–517.
- Lachgar S, Charveron M, Gall Y, Bonafe JL. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br J Dermatol. 1998;138:407–411.
- Buhl AE, Conrad SJ, Waldon DJ, Brunden MN. Potassium channel conductance as a control mechanism in hair follicles. J Invest Dermatol. 1993;101:148S–152S.
- Katz HI, Hien NT, Prawer SE, Goldman SJ. Long-term efficacy of topical minoxidil in male pattern baldness. J Am Acad Dermatol. 1987;16:711–18.
- Rietschel RL, Duncan SH. Safety and efficacy of topical minoxidil in the management of androgenetic alopecia. J Am Acad Dermatol. 1987;16:677–685.
- Price VH, Menefee E, Strauss PC. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J Am Acad Dermatol. 1999;41: 717–721.
- Olsen EA, Weiner MS, Amara IA, DeLong ER. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:643–646.
- 30. Gupta AK, Charrette A. Topical minoxidil: Systematic review and meta-analysis of its efficacy in androgenetic alopecia. Skinmed. 2015;13:185–189.
- Olsen EA, Weiner MS. Topical minoxidil in male pattern baldness: effects of discontinuation of treatment. J Am Acad Dermatol. 1987;17: 97–101.
- Khandpur S, Suman M, Reddy BS. Comparative efficacy of various treatment regimens for androgenetic alopecia in men. J Dermatol. 2002;29:489–498.

