 by Mario Tagliagambe, MD; Tuan A. Elstrom, BS; and Daniel B. Ward, Jr, MD, FAAD
by Mario Tagliagambe, MD; Tuan A. Elstrom, BS; and Daniel B. Ward, Jr, MD, FAAD
Dr. Tagliagambe is a primary care physician at Adirondack Health in Saranac Lake, New York. Mr. Elstrom was an employee of Cipher Pharmaceuticals US, LLC at the time of this research. Dr. Ward is a practicing dermatologist in Charleston, South Carolina, and was an employee of Cipher Pharmaceuticals US, LLC at the time of this research.
Funding: No funding was received.
Disclosures: Dr. Tagliabambe reports no financial conflicts relevant to the content of this article. Mr. Elstrom reports that he was an employee of Cipher Pharmaceuticals, LLC at the time of this research. Dr. Ward reports that he was an employee of Cipher Pharmaceuticals, LLC at the time of this research.
Abstract: Venous leg ulcers can lead to debilitation and a decrease in quality of life and can require costly treatments. Compression therapy remains the foundation of conservative treatment. However, some ulcers become indolent, chronic, and unresolved for years, even with adherence to standard of care. Here, the authors describe the case of a 56-year-old male patient with a recalcitrant recurrent distal leg ulcer. The ulcer was treated initially with debridement and compression therapy, respectively, and then subsequently with hyaluronic acid sodium salt 0.2% gel, which prompted complete wound closure. Hyaluronic acid is known to stimulate angiogenesis and exert fibrogenic action within inflamed and impaired healing tissues. Only a limited number of studies have been conducted to evaluate the clinical use of hyaluronic acid for treating venous leg ulcers. Success obtained with this patient should spur future clinical studies to fully evaluate this modality as a safe, efficacious, expeditious, and cost-effective option for the management of recalcitrant chronic ulcers.
Keywords: Venous leg ulcer, hyaluronic acid, topical, gel, wound healing
J Clin Aesthet Dermatol. 2017;10(11):49–51
Introduction
Venous leg ulcers (VLUs), often resulting from chronic venous insufficiency and hypertension,1 can lead to debilitation2,3 and a decrease in quality of life.2,4,5 Ulcer formation is linked with a cascade of complex cellular and humoral mechanisms.6,7 Many VLUs require protracted therapy, lasting longer than a year, and recur at a rate up to 70 percent,8,9 with an overall annual healthcare payer burden of $14.9 billion.10 There is a need for a safe, efficient, and cost-effective treatment option for patients suffering from VLUs.
Hyaluronic acid (HA), present through all the steps of wound healing, is a sentinel for tissue damage and a driver of the reparative process, and has been rediscovered as a vital component in wound resolution.19 However, limited evidence exists to guide decisions regarding the use of HA in recalcitrant ulcers, such as arterial and venous ulcers, diabetic foot ulcers, and VLUs.20
Here, we report the successful healing of a recalcitrant VLU using an HA sodium salt 0.2% gel (Bionect®; Cipher Pharmaceuticals, Charleston, South Carolina), which is indicated for the management of partial to full thickness dermal ulcers and wounds. The safety and efficacy of this device has been previously evaluated as a topical anti-inflammatory.17,18 The excellent adherence and tolerance achieved suggest that this therapy could potentially address chronic indolent or recalcitrant venous ulcers.
Case Report
A 56-year-old male was referred to our Wound Center for a chronic, recurrent, nonhealing, partial-thickness ulcer overlying the right medial malleolus that had been previously treated with wet-to-dry dressings over several weeks. The patient had a history of a right ankle fracture that occurred decades prior and that had been treated with open reduction and internal fixation. He subsequently developed recurring ulcers throughout the years overlying the surgical site. Medical history included alcohol-related seizures and degenerative joint disease, but no hypertension, diabetes, heart disease, or endocrine disorders. The history included oral indomethacin 50mg three times daily as needed and an allergy to nickel. On exam, there were chronic venous stasis changes of the right distal leg, but no cyanosis or edema, and pulses were 2+ bilaterally. Sensory testing was normal for both feet. A partial thickness ulcer was measured at 1.3cm x 0.5cm x 0.2cm, overlying the right medial malleolus. There was pink hypergranulation, and the margins were distinct. There was no necrosis, tunneling, undermining, or exposed structures. Moderate serous exudate was present, but no foul odor.
Upon each visit, selective debridement was performed with curettage, and the wound was cleansed with normal saline. Puracol® MicroScaffold™ collagen dressing (Medline, Vernon Hills, Illinois) was then placed onto the wound bed, covered with an adaptic dressing, and secured in place with Steri-Strips™ (3M, St. Paul, Minnesota).
The patient was instructed to return to the center in two weeks (Figure 1) and was then treated weekly until wound closure.
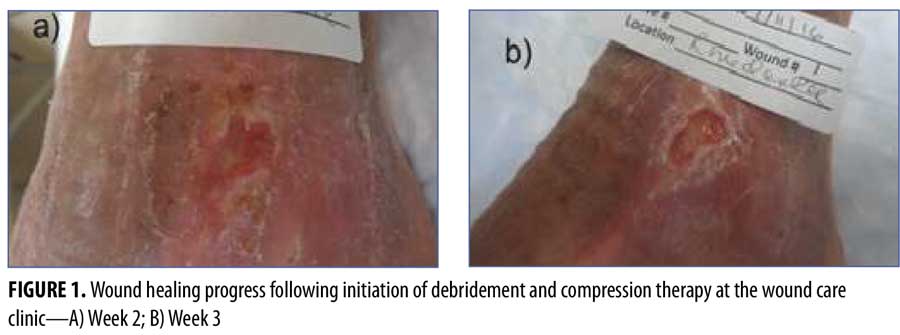
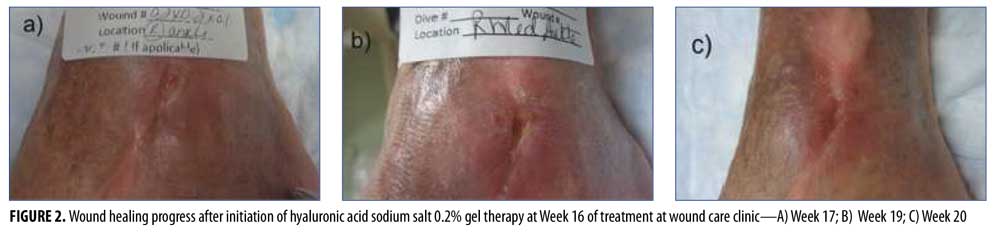
Despite ongoing wound care and compression therapy initiated at Week 9, there was a plateau in the progress of closure at Week 15, with a persistent area of 0.8cm x 0.3cm x 0.2cm. Therefore, HA sodium salt 0.2% gel was initiated twice daily and covered with foam and gauze wrap.
Wound closure (Figure 2) was achieved four weeks later, at Week 20. The patient was discharged and instructed to continue applications of HA sodium salt 0.2% gel to the epithelialized area, to cover it with foam and gauze for one additional week, and to follow up with the Center as necessary.
Discussion
VLUs are painful, debilitating, can take weeks to months to heal,21 and have high recurrence rates, even with adherence to standard of care; thus, correcting impaired venous returns is critical when treating VLUs.11,12 The treatment of this ulcer with HA sodium salt 0.2% gel resulted in prompt healing in four weeks as compared with the initial progress the previous 14 weeks.
HA stimulates angiogenesis, moistens wounds, reduces exudation, is vasoprotective, and exerts fibrogenic action in inflamed and impaired healing tissues.22 However, only a very small number of studies have been conducted to evaluate the safety and efficacy of using HA in VLUs.20,22 One controlled study demonstrated that HA-treated ulcers decreased in size by 48 percent after only seven days and also demonstrated reduced edema.22 HA is a basic extracellular matrix (ECM) component, and the replacement of the ECM in combination with compression has demonstrated success in promoting wound healing.23,24
HA appears to promote wound healing via increasing ZO-1, a specific epithelia tight junction protein with an important role in epidermal barrier function and regeneration during wound healing.25 HA also stimulates cell proliferation and wound healing as evidenced by the activation of the P2X7 receptor, which is known to stimulate cell proliferation and increase the expression of tight junctions, a sign of the restoration of barrier functional properties.26 In addition, HA has been shown to stimulate beta-defensin-2,27 a peptide that exerts strong antimicrobial activity against Gram-negative bacteria and Candida albicans as well as bacteriostatic activity against Gram-positive bacteria.28 It is plausible that the combined mechanistic effects and the stimulatory and indirect antimicrobial activity of HA sodium salt 0.2% gel promoted tissue repair and resolution of the recalcitrant leg ulcer in our patient.
Conclusion
In extreme cases, VLUs can remain indolent and unresolved for long periods of up to years. Here, we described the case of a patient with closure of a recalcitrant VLU, most likely caused by venous insufficiency, treated initially with debridement and compression therapy for 16 weeks, and then treated with HA sodium salt 0.2% gel. After the initiation of the HA therapy, the patient achieved complete wound closure in four weeks of treatment. This result suggests that further studies should assess the role of HA sodium salt 0.2% gel in the management of VLUs. Future studies might further support use of this therapy as a safe, efficacious, expeditious, and cost-effective option for the treatment of recalcitrant ulcers.
References
- Andersen CA, Aung BJ, Chandy RM et al. Improving the standard of care for treating venous leg ulcers within the Veterans Administration. 2012.
- Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44(3):
401–421. - Eberhardt RT, Raffetto JD. Chronic venous insufficiency. 2005;111(18):
2398–2409. - Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol. 1994;31(1):49–53.
- Green J, Jester R. Health-related quality of life and chronic venous leg ulceration: part 1. Br J Community Nurs. 2009;14(12):S12, S14, S16–S17.
- Comerota A, Lurie F. Pathogenesis of venous ulcer. Semin Vasc Surg. 2015;28(1):6–14.
- Augustin M, Vanscheidt W. Chronic venous leg ulcers: the future of cell-based therapies. 2012;380(9846):953–955.
- Barwell J, Davies C, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomized controlled trial. 2004;363(9424):1854–1859.
- Abbade LPF, Lastoria S. Venous ulcer: epidemiology, physiology, diagnosis and treatment. Int J Dermatol. 2005;44(6):449–456.
- Rice JB, Desai U, Cummings AK, et al. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17(5):347–356.
- O’Meara SO, Cullum N, Nelson AE, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;11:CD000265.
- Alavi A, Sibbald RG, Phillips TJ, et al. What’s new: management of venous leg ulcers: approach to venous leg ulcers. J Am Acad Dermatol. 2016;74(4):627–640.
- Charles CA, Tomic-Canic M, Vincek V, et al. A gene signature of non healing venous ulcers: potential diagnostic markers. J Am Acad Dermatol. 2008;59(5):758–771.
- Tang JC, Marston WA, Kirsner RS. Wound Healing Society (WHS) venous ulcer treatment guidelines: What’s new in five years?. Wound Repair Regen. 2012;20(5):619–637.
- Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24(2):290–295.
- Hankin CS, Knispel J, Lopes M, et al. Clinical and cost efficacy of advanced wound care matrices for venous ulcers. J Manag Care Pharm. 2012;8(5):375–384.
- Schlesinger T, Rowland Powell C. Efficacy and safety of a low-molecular weight hyaluronic Acid topical gel in the treatment of facial seborrheic dermatitis. J Clin Aesthet Dermatol. 2012;5(10):20-23.
- Schlesinger T, Rowland Powell C. Efficacy and safety of a low-molecular weight hyaluronic Acid topical gel in the treatment of facial seborrheic dermatitis final report. J Clin Aesthet Dermatol. 2014;7(5):15–18.
- Aya KL, Stern R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014;22(5):579–593.
- Shaharudin A, Aziz Z. Effectiveness of hyaluronic acid and its derivatives on chronic wounds: a systematic review. J Wound Care. 2016;25(10):585–592.
- Vowden KR, Vowden P. Preventing venous ulcer recurrence: a review. Int Wound J. 2006;3(1):11–21.
- Ortonne JP. A controlled study of the activity of hyaluronic acid in the treatment of venous leg ulcers. J Dermatolog Treat. 1006;7(2):75–81.
- Rando T. Use of a biological extracellular matrix wound therapy to heal complex, chronic wounds. J Wound Care. 2009;18(2):70–74.
- Liden BA, May BCH. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care 26(4):164-167, 2013.
- Brandner JM, Kief S, Grund C, et al. Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol. 2002;81(5):253–263.
- Ghazi K, Deng-Pichon U, Warnet JM, Rat P. Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2X7 purinoreceptor basal activation: role of molecular weight. PLoS ONE. 2012;7(11):e48351.
- Gariboldi S, Palazzo M, Zanobbio L, et al. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. J Immunol. 2008;181(3):2103–2110.
- Schröder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31(6):645–651.

