 by Philip R. Cohen, MD
by Philip R. Cohen, MD
Dr. Cohen is from the Department of Dermatology at the University of California San Diego, La Jolla, California.
Funding: No funding was provided for this study.
Disclosures: The author has no financial conflicts relevant to the content of this article.
Abstract: Background. Fixed drug eruption is a cutaneous reaction to a systemic agent that typically presents as an annular or oval erythematous patch or blister and subsequently resolves with postinflammatory hyperpigmentation at the site. Ginkgo biloba leaf extract and vinpocetine are nutritional supplements used to enhance memory in patients with dementia and age-related memory impairment conditions such as Alzheimer’s disease.
Purpose. To describe a fixed drug eruption in a man who repeatedly developed pruritus and macular erythema on his distal penile shaft after ingesting a natural product containing Ginkgo biloba and vinpocetine.
Methods. The medical literature was retrospectively reviewed using PubMed, searching specifically for the terms cutaneous/skin adverse/side effects, fixed drug eruption, Ginkgo biloba, and vinpocetine. Patient reports and previous reviews of the subject were critically assessed, and the salient features of cutaneous adverse effects in patients receiving either Ginkgo biloba or vinpocetine are presented.
Results. Cutaneous adverse effects from Ginkgo biloba and vinpocetine are infrequent. Ginkgo biloba fruit can result in contact dermatitis (following topical exposure) and mucosal symptoms of the mouth and anus (following oral exposure); in addition, an erythematous maculopapular generalized eruption or possibly Steven-Johnson syndrome can occur after oral ingestion of the Ginkgo biloba leaf extract. Facial erythema has been associated with vinpocetine ingestion. Pruritus and an annular erythema localized to the distal penile shaft developed after initial and repeat ingestion of a Ginkgo biloba/vinpocetine product.
Conclusion. Ginkgo biloba and vinpocetine should be added to the agents that can potentially cause a fixed drug eruption.
Keywords: Fixed drug eruption, genitalia, Ginkgo biloba, penis, vinpocetine
J Clin Aesthet Dermatol. 2017;10(10):44–47
Ginkgo biloba is also referred to as either “the silver apricot,” describing its yellow drupe-like fruit, or “the maidenhair tree,” since its leaves resemble some of the pinnae of the maidenhair fern. Ginkgo leaf extract has demonstrated efficacy in the treatment of Alzheimer’s disease, dementia, intermittent claudication, schizophrenia, tinnitus, and vertigo. It has also been reported to have anticancer, cardioprotective, neuroprotective, memory enhancing, and stress alleviating effects.1–11
Vinpocetine is also known as ethyl apovincaminate. It is a synthetic-derived vinca alkaloid from the leaves of the lesser periwinkle plant (Vinca minor). The agent has been used in the management of age-related memory impairment and cerebrovascular disorders. It is reported to increase cerebral blood flow.12-16
Fixed drug eruption describes a cutaneous reaction to a systemic agent that typically presents as an annular or oval erythematous patch or blister and subsequently resolves with postinflammatory hyperpigmentation at the site. Repeat exposure to the same agent typically results in a new skin lesion developing at the same location that was previously affected; in addition, new lesions might also develop. Common locations for fixed drug eruptions include the lips, hands, and genitalia.17-21
A man developed pruritus and macular erythema on his distal penile shaft after ingesting a natural product containing Ginkgo biloba and vinpocetine. Both the pruritus and erythema resolved after he discontinued the supplement. However, the same local symptom and skin lesion occurred after he restarted the product, confirming the suspected clinical diagnosis of a fixed drug eruption to the Ginkgo biloba/vinpocetine product.
Case Report
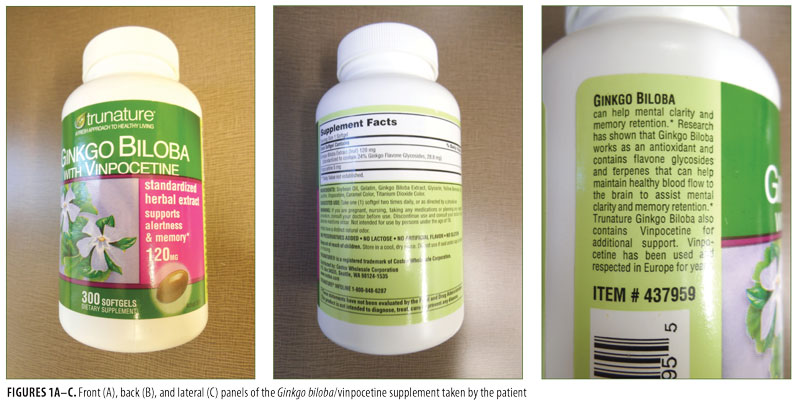
A 36-year-old man developed pruritus and redness localized to his distal penile shaft. Additional inquiry revealed that he had begun daily ingestion of a preparation containing 120mg of Ginkgo biloba leaf extract (standardized to contain 24% Ginkgo flavone glycerides, 28.8g) and 5mg of vinpocetine [manufactured by Trunature (item #437959)] to increase cerebral blood flow and memory (Figure 1). Within the first day of treatment, he noticed that the affected area of his penis was itching; the itching persisted and the location became red during the next 3 to 4 days. He also noted that when he discontinued the Ginkgo biloba/vinpocetine supplement, the symptoms stopped immediately and the redness resolved over the next two weeks.
The patient wanted to confirm the suspected diagnosis of fixed drug eruption to his nutritional supplement. He restarted the daily Ginkgo biloba/vinpocetine supplement two days prior to his follow-up appointment. After two doses, he again noted pruritus and macular erythema on the same area of his distal penis. In addition, similar to his initial exposure to the supplement, he commented that he felt smarter and that his libido was increased. He also noted that his heart rate was increased and that he had a stomach ache, similar to the first time he took the supplement. However, he had not shared this information prior to his repeat exposure to the agent.
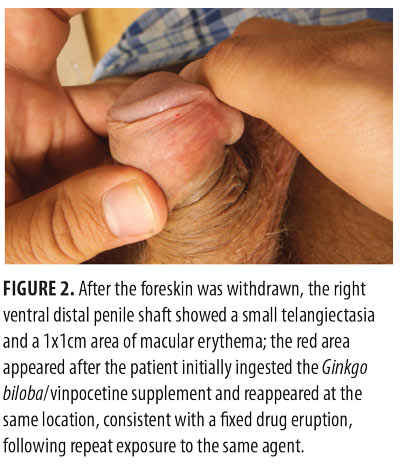
Cutaneous examination of his uncircumcised penis, after the foreskin had been pulled back, showed a 1mm red macule consistent with a telangiectasia. The right ventral distal penile shaft proximal to the corona also showed a 1x1cm area of mild erythema at the same location at which he had previously noted the skin lesion following his initial exposure to the Ginkgo biloba/vinpocetine agent (Figure 2).
Discussion
Ginkgo biloba is the national tree of China and the official tree of Tokyo, the capital of Japan. It is a dioecious tree, divided into male and female species. The allogenic ginkgolic acid is present in the outer fleshy layer of the seeds within the ovules of the female trees. Therefore, when Ginkgo biloba trees are planted along streets for ornamental reasons, urban developers often recommend only planting the male trees
(Figure 3).1–3

The Ginkgo biloba leaf is the symbol of Tokyo. The leaf is fan-shaped with dichotomous venation—the veins radiate out into the leaf blade, sometimes splitting into two, but never anastomosing. The leaves are green and often notched or lobed; however, prior to falling, the leaves turn yellow during autumn (Figure 4).

Ginkgo biloba leaves are usually collected when they are still green, between the months of July and September. The extract of the Ginkgo biloba leaf has been standardized (EGb 761) and contains 24% flavonoid glycosides, 6% terpene lactones, and less than five parts per million ginkgolic acid. The content of flavonoids (also referred to as phenylbenzopyrones or phenylchromones) varies between and within the seasons; greater amounts are present in the fall than in the spring. Ginkgolides and bilobalide are the two types of terpenoids.1–3
The recommended dose of Ginkgo biloba extract to achieve therapeutic effectiveness has not been definitively determined; recommended doses range from 40mg to 60mg, three to four times daily for a possible minimum of eight weeks. Ginkgo biloba inhibits platelet-activating factor; therefore, caution should be used when considering this agent in patients taking warfarin, aspirin, or other antiplatelet medications. Conservative recommendations suggest discontinuation of Ginkgo biloba between 36 hours to 14 days before surgery. Other potential side effects of Ginkgo biloba include diarrhea, dizziness, headaches, nausea, vomiting, palpitations, restlessness, and weakness.1–3
Mucocutaneous side effects from Ginkgo biloba have occurred following topical exposure to the fruit4–6 or systemic exposure to the fruit7 or leaf extract.8,9 Both allergic and irritant contact dermatitis have been demonstrated following topical exposure to fruit; although Sower et al4 emphasized that the dermatitis required the fruit to be ruptured and subsequent contact with the exposed juicy pulp,4–6 other individuals also developed dermatitis after handling the intact fruit.5 These patients presented with erythematous papules, vesicles and edema.4–6
One man ate the outer fruit and discarded the edible seed. Three days later, he initially presented with burning, tingling, and soreness of the mouth; a sensation of thickness of his tongue; perioral erythema and swelling; and erythema of his buccal mucosa, tongue, and tonisillar pillars. The next day, he had developed tenesmus, perirectal burning, and pruritus.7
Skin and mucosal reactions to oral Ginkgo biloba leaf extract include a diffuse morbilliform eruption8 and possibly Stevens-Johnson syndrome that evolves into toxic epidermal necrolysis.9 A 66-year-old woman had ingested two 60mg doses of Trader Joe’s Ginkgo biloba supplement about a week before developing a progressive erythematous eruption of macules and papules that began on her abdomen and progressed to cover most of her skin, including her face, neck, trunk, and extremities.8 Yuste et al9 suggested that Stevens-Johnson syndrome occurring in a 75-year-old man with arterial hypertension, hypercholesterolemia, and glaucoma was “probably because of the ingestion of Ginkgo biloba extract”; however, the authors also mentioned that the patient had been on doxazosin for years and that they did not know when the patient actually began taking the Ginkgo biloba extract (to improve blood supply) with regard to the onset of his skin condition.
Vinpocetine is a synthetic ethyl ester of apovicamine. Vinpocetine has been used for the treatment of acute stroke, cerebrovascular disorders, cognitive decline, and dementia. Vinpocetine has also been used for preventing motion sickness and for treating chronic fatigue syndrome, menopause symptoms, and seizure disorders. The daily dose of vinpocetine varies between 15mg and 60mg; most patients receive between 30mg to 60mg each day for periods ranging from 12 to 16 weeks to up to one year.12–15
Vinpocetine is considered to be safe for most people. In a study of patients with Alzheimer’s disease receiving 60mg of vinpocetine daily and a placebo group, no significant difference regarding adverse effects was observed after one year of treatment.12–13 Described side effects in some patients who have taken the agent include dizziness, headache, nausea, nervousness, sleep disturbances, and stomach pain. With the exception of facial flushing, cutaneous adverse events in patients taking vinpocetine have not been described. Similar to Ginkgo biloba, vinpocetine may slow clotting; therefore, it should not be used in patients with clotting disorders, should not taken with other anticoagulants, and should be discontinued before surgery.16
The most frequent causative agents associated with fixed drug eruptions are analgesics (naproxen), antibiotics (such as tetracycline and trimethoprim-sulfamethoxazole), anticonvulsants, muscle relaxants, phenolphthalein (in stool softeners), and sedatives. Fixed drug eruptions are classified as delayed Type IV immune reactions in which intraepidermal effector-memory CD8+ T cells at the lesional site of the fixed drug eruption play a role in the subsequent development of localized tissue damage. To date, a fixed drug eruption has not been described in patients receiving either Ginkgo biloba or vinpocetine.17-21
The reported patient not only developed pruritus and an annular erythematous patch on his distal penile shaft following initial daily ingestion of the Ginkgo biloba/vinpocetine product, but also systemic symptoms (tachycardia and gastrointestinal pain) within four days. Subsequent repeat daily challenge with the same agent produced the same cutaneous symptom (pruritus) and skin lesion (macular erythema) at the identical location, in addition to tachycardia and gastrointestinal pain, within two days. Additional oral challenge or patch testing to the Ginkgo biloba/vinpocetine product or the individual agents was not recommended because of the systemic symptoms that he had experienced.
Conclusion
Ginkgo biloba and vinpocetine are natural products that have been used for several purposes, including to enhance memory in patients with dementia and/or age-related memory impairment conditions such as Alzheimer’s disease. Both Ginkgo biloba and vinpocetine can inhibit clotting; however, systemic side effects from these agents are uncommon. Cutaneous adverse effects from these agents are infrequent. Topical exposure to Ginkgo biloba fruit can result in contact dermatitis, and oral exposure to the fruit, in one individual, resulted in mucosal symptoms of the mouth and anus. In addition, individual case reports describe either an erythematous maculopapular generalized eruption, or possibly Steven-Johnson syndrome after oral ingestion of the Ginkgo biloba leaf extract. Facial erythema has been associated with vinpocetine ingestion. A man with pruritus and annular erythema localized to the distal penile shaft who experienced the identical symptoms and lesion after repeat challenge with a Ginkgo biloba/vinpocetine product is described here. Ginkgo biloba and vinpocetine should be added to the agents that can potentially cause a fixed drug eruption.
References
- Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician. 2003;68(5):923–926.
- Mahadevan S, Park Y. Multifaeted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008;73(1):R14–R19.
- Sasseville D: Clinical patterns of phytodermatitis. Dermatol Clin. 2009;27:299–308.
- Sowers WF, Weary PE, Collins GD, Cawley EP. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452–456.
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19(4):281–283.
- Bolus M. Dermatitis venenata due to Ginkgo berries [minor notes]. Arch Dermatol Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231(11):1162–1163.
- Chiu AE, Lane AT. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement [letter]. J Am Acad Dermatol. 2002;46(1):145–146.
- Yuste M, Sanchez-Estella J, Santos JC, et al. [Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins]. Actas Dermosifiliogr. 2005;96(9):589–592.
- Lepoittevin JP, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281(4):227–230.
- Micozzi MS, Pribitkin EA. Common herbal remedies, adverse reactions, and dermatologic effects. Skinmed. 2010;8(1):30–36.
- Szatmari SZ, Whitehouse PJ. Vinpocetine for cognitive impairment and dementia. Cochrane Database Syst Rev. 2003;(1):CD003119.
- Fenzl E, Apecechea M, Schaltenbrand R, Friedel R. Long-term study concerning tolerance and efficacy of vinpocetine in elderly patients suffering from a mild to moderate organic psychosyndrome. In: Bes A, Cahn J, Cahn R, et al (eds). Senile dementias: early detection. London, Paris: John Libby Eurotext; 1986:580–585.
- Bonoczk P, Gulyas B, Adam-Vizi V, et al. Role of sodium channel inhibition in neuroprotection: effect of vinpocetine. Brain Res Bull. 2000;53(3):245–254.
- Erdo SL, Ning-sheng C, Wolff JR, Kiss B. Vinpocetine protects against excitotoxic cell death in primary cultures of rat cerebral cortex. Eur J Pharmacol. 1990;187(3):551–553.
- Vinpocetine: uses, side effects, interactions and warnings. WebMD site. Available at: http://www.webmd.com/vitamins-supplements/ingredientmono-175-vinpocetine.aspx? activeingredientld=175&activeingredient
Name=vinpocetine. Accessed August 10, 2013. - Ozkaya E. Fixed drug eruption: state of the art. J Dtsch Dermatol Ges. 20098;6(3):181–188.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17(3):201–208.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9(4):316–321.
- Mizukawa Y, Shiohara T. Fixed drug eruption: a prototypic disorder mediated by effector memory T cells. Curr Allergy Asthma Rep. 2009;9(1):71–77.
- Andrade P, Brinca A, Goncalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65(4):195–201.

