J Clin Aesthet Dermatol. 2020;13(2):47–52
 by Jagdish Sakhiya, MD; Dhruv Sakhiya, MBBS; Ravi Khambhati, MBBS, DVD; Mehul Patel, DNB, DVL; Piyush Darji, mD; Trusha Gajjar, DVD; Feral Daruwala, M. Pharm; and Nimish Dudhatra, MSc, CR
by Jagdish Sakhiya, MD; Dhruv Sakhiya, MBBS; Ravi Khambhati, MBBS, DVD; Mehul Patel, DNB, DVL; Piyush Darji, mD; Trusha Gajjar, DVD; Feral Daruwala, M. Pharm; and Nimish Dudhatra, MSc, CR
Dr. J. Sakhiya and Drs. Khambhati, Patel, Darji, and Gajjar are with the Sakhiya Skin Clinic in Gujarat, India. Mrs. Daruwala and Mr. Dudhatra are with the Sakhiya Skin Clinic in Gujarat, India. Mr. D. Sakhiya is with the B.J. Medical College, New Civil Hospital Asarwa in Gujarat, India.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Rituximab, an anticluster of differentation 20 antibody, has been shown in open series studies to be effective in treating pemphigus. In the literature, lymphoma (dose of 375mg/m2, four-week infusion) and rheumatoid arthritis (two infusions of 1,000mg each, 15 days apart) are two protocols extensively used for rituximab treatment in pemphigus.
Objective. We investigated whether a modified rheumatoid arthritis protocol, in which the patient received a single treatment course ranging from 2 to 5 infusions of 1,000mg of rituximab during an interval of four weeks is safe and effective in pemphigus management.
Methods. Patients with pemphigus were treated with a single treatment course ranging from 2 to 5 infusions of 1,000mg of rituximab during an interval of four weeks. Clinical consensus late endpoints and desmoglein 1 and desmoglein 3 indices were monitored.
Results. We enrolled 32 patients in the study: four with pemphigus foliaceus (PF) and 28 with pemphigus vulgaris (PV). The follow-up period was 98.22±20.65 weeks (range: 40–140 weeks). All 32 patients responded to therapy. Nineteen patients achieved complete remission during a median period of 46 weeks (8 on minimal therapy, 11 off therapy). Thirteen patients achieved partial remission during a median period of 46 weeks (8 on minimal therapy, 5 off therapy). Relapses were seen in five (15.63%) patients between 72 and 96 weeks (median: 96 weeks) after the start of therapy. The antidesmoglein index correlated well with clinical improvement in PV or PF.
Conclusion. Modified rheumatic arthritis protocol for rituximab was shown to be effective and safe in treating patients with pemphigus.
KEYWORDS: Pemphigus, rituximab, modified rheumatoid arthritis protocol, antidesmoglein index
Pemphigus is a group of potentially life-threatening autoimmune diseases characterized by cutaneous and/or mucosal blistering. Pemphigus can be classified into four types: pemphigus vulgaris (PV), pemphigus foliaceus (PF), paraneoplastic pemphigus, and immunoglobulin A pemphigus.1 Among these four, the two main variants are PV and PF.2 PV is associated with the presence of immunoglobulin G antibodies against desmoglein 3 (Dsg3), with or without antidesmoglein 1 (anti-Dsg1) antibodies, whereas PF is characterized by the presence of antidesmoglein 1 antibody alone.
Even though various treatment options are available, including first-line therapy with corticosteroids and adjuvant treatments with immunosuppressive, anti-inflammatory, antibiotic agents, or intravenous immunoglobulins, pemphigus remains an incurable disease, and treatment is challenging.3 Although corticosteroids are the cornerstone of pemphigus treatment, strong evidence supporting their effectiveness is lacking. Currently, rituximab is emerging as the latest treatment option with a lack of evidence.
Rituximab (MabThera; Roche Holding AG, Basel, Switzerland) is a chimeric human-mouse monoclonal antibody that binds specifically to the transmembrane antigen cluster of differentiation 20 (CD20) expressed on B-lymphocytes from the pre-B-cell stage to the preplasma-cell stage. CD20 is not expressed on hematopoietic stem cells and plasma cells.4 The binding of rituximab to CD20 leads to B-cell depletion by diverse mechanisms, such as antibody-dependent cellular cytotoxicity, complement-mediated lysis, direct disruption of signaling pathways, and triggering of apoptosis.5
United States Food and Drug Administration approved the use of rituximab in lymphoma in 1997, for rheumatoid arthritis (RA) in 2006, for chronic lymphocytic leukemia in 2010, and for Wegener’s granulomatosis in 2011. In the literature, lymphoma and RA are the two protocols most extensively used in pemphigus treatment. In the lymphoma protocol, patients received four weekly infusions of rituximab (dose of 375 mg/m2); meanwhile, the RA protocol consisted of two infusions of 1,000mg each, 15 days apart.6 Further, to treat pemphigus, various modified treatment protocols have been used and reported in the literature, with promising results.7–15
For years, rituximab has also been used as an off-label drug for distinct autoimmune diseases, such as idiopathic thrombocytopenic purpura, systemic lupus erythematosus, myasthenia gravis, Wegener’s disease, Sjögren’s syndrome, and dermatomyositis.16 From 2001 to 2006, the earliest successful treatments with rituximab in patients with blistering diseases were seen in paraneoplastic pemphigus associated with B-cell non-Hodgkin’s lymphomas, as reported by Heizmann et al,17 Schadlow et al,18 and Ahmed et al.19 Subsequently, several case reports and case series have reported a remarkable therapeutic effect of rituximab in pemphigus.20–22 In many studies, the most common dose for treatment is used as per the lymphoma protocol.23–26 Here, we report the effects of 1,000 mg of rituximab in pemphigus using a modified RA protocol in which the patient received 1 to 5 additional monthly infusions during a four-week interval.
Methods
Patients. In this retrospective, nonrandomized, single-center open case series, we treated 32 patients with PV and PF with rituximab at our private clinic between June 2015 and October 2018. The study was conducted in accordance with the 1975 Declaration of Helsinki, revised in 2000. Written informed consent was obtained from the patients prior to enrollment in the study. Diagnostic confirmation was made by clinical, histological, and immunological criteria. Eligible patients had severe, long-term disease as a result of to conventional therapy resistance or had developed major complications after steroid therapy. Conventional therapy resistance was defined as the continued development of new lesions, continued extension of old lesions, or failure of established lesions to begin to heal after three months of treatment with prednisolone doses of 5mg, 10mg, or 20 mg daily and traditional adjuvant therapies (e.g., azathioprine 50mg daily, betamethasone 2mg daily, betamethasone pulse therapy 2mg daily, deflazacort 6mg daily, cyclosporine 3 to 5mg/kg-1 daily, dexamethasone and cyclophosphamide pulse therapy).
Treatment protocol. A single course of medication is a period of continuous treatment with a single drug, sometimes with variable dosing. Based on the severity, a single course of of 1000mg of rituximab was administered 2 to 5 infusions over four weeks. As premedication, we gave ceftriaxone 1gm intravenously, hydrocortisone 100mg intravenously, paracetamol 650mg stat orally, and pheniramine maleate 2cc stat intravenously, sequentially on the day of infusion. After 30 minutes of these premedications, rituximab (1g) intravenously in 1pt of normal saline was given slowly over 6 to 8 hours.
Observation periods and endpoints. The observation periods and endpoints were defined according to the latest consensus statement of disease definition and endpoints in pemphigus.27 Time to disease control was defined as the time at which new lesions ceased to form and established lesions began to heal. The end of the consolidation phase was defined as the time at which no new lesions had developed for a minimum of two weeks and approximately 80 percent of lesions had healed. Time to late endpoint refers to the period between consolidation and the late endpoint. Late endpoints were defined as “complete remission (CR) on therapy” (CR ON), or the absence of new or established lesions while the patient is receiving minimal therapy; “CR off therapy” (CR OFF), or the absence of new or established lesions while the patient has been off all systemic therapy for at least two months; “partial remission (PR) on therapy” (PR ON), or the presence of transient new lesions that heal within one week while the patient is receiving minimal therapy, including topical steroids; or “PR off therapy” (PR OFF), or the presence of transient new lesions that heal within one week without treatment while the patient is receiving no systemic therapy. Minimal therapy was defined as up to 10mg daily of prednisolone for at least two months, while minimal adjuvant therapy indicated half of the dose required to be defined as treatment failure and relapse was the appearance of three or more new lesions that do not heal spontaneously within one week or the extension of established lesions in a patient who had previously achieved disease control.
Enzyme-linked immunosorbent assay testing for anti-desmoglein antibodies. Specific antibodies against anti-Dsg1 and anti-Dsg3 were measured in the sera of patients at the beginning of the therapy and at the end of therapy. Immunoglobulin G index values for Dsg1 and for Dsg3 were determined by the commercial enzyme-linked immunosorbent assays MESACUP desmoglein test “Dsg 1” and MESACUP desmoglein test “Dsg 3” in accordance with the manufacturer’s protocol (MBL; Nagoya, Japan).
Results
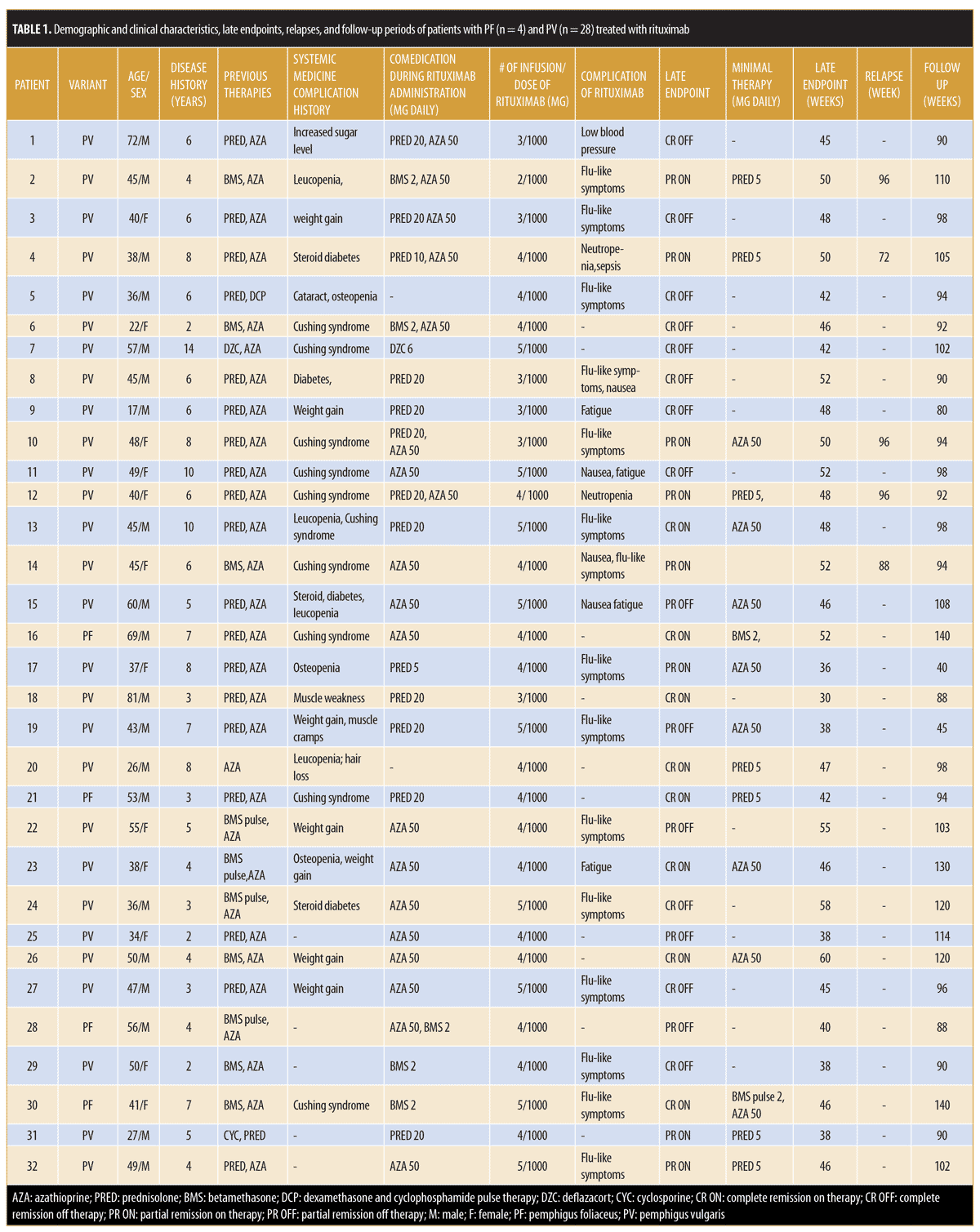
Patient characteristics. In this retrospective, open case series, 32 patients with pemphigus were enrolled, of whom four had PF and 28 had PV. The interval between the two infusions of rituximab (1,000mg) was four weeks in every patient. Patients included 20 (62.5%) men and 12 (37.5%) women with a mean age of 45.34±13.75 (range: 17–81) years and a mean disease period of 5.69±2.66 (range: 2–14) years. Prior to rituximab, study participants had received several conventional systemic agents, such as prednisolone, azathioprine, betamethasone, betamethasone pulse therapy, deflazacort, cyclosporine, and dexamethasone and cyclophosphamide pulse therapy.
Clinical efficacy of rituximab on pemphigus. All patients received a single course of rituximab, but five patients needed retreatment with rituximab due to relapse. Patients 4 and 14 experienced relapse at Weeks 72 and 88, respectively. In addition, Patients 2, 10, and 12 relapsed at Week 96. Thus, relapses were seen in five (15.63%) patients between Weeks 72 and 96 (median: 96) after the start of therapy (Figure 1).
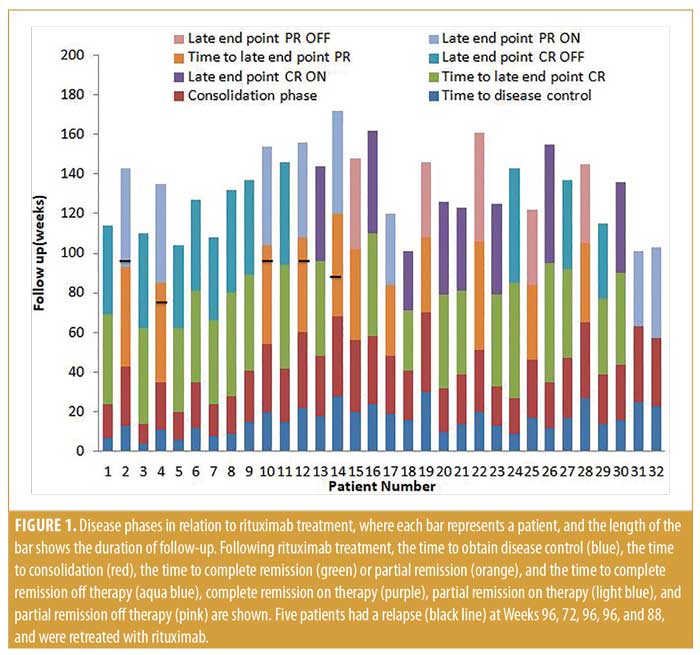
Early endpoints. The time to disease control ranged between four and 30 (median: 15.5) weeks, while the time to consolidation phase ranged between 10 and 40 (median: 27.5) weeks.
Late endpoints. At the end of the study (October 2018), after a follow-up period of 98.22±20.65 (range: 40–140) weeks, 19 of the 32 (59.37%) patients had achieved CR; eight of these were receiving minimal therapy (CR ON), and 11 did not need any medication (CR OFF). Additionally, 13 of the 32 patients demonstrated PR (40.63%), including eight on minimal therapy (PR ON) and five off medication (PR OFF) (Table 1). The mean of the late endpoints in total (CR+PR) were achieved at 46±6.54 (median: 46) weeks; for PR, the median was 46 weeks, while, for CR, it was also 46 weeks. Notably, we saw some patients still improving after the first year of treatment, achieving CR during the second year.
Adverse events of rituximab. Mild, early adverse effects were observed in 19 patients (59.38%): four had fatigue, four presented with nausea, and 14 showed flu-like symptoms. The first patient experienced low blood pressure, which was temporary and resolved by general supportive measures. Patient 12 had neutropenia, which was mild and resolved spontaneously. One serious adverse event of sepsis due to neutropenia occurred in a fourth patient at Week 26. This side effect was possibly related to comedication with azathioprine 50mg daily; after stopping this drug, the patient recovered from the neutropenia and his disease further improved. Ten (31.25%) patients had no complaints related to rituximab treatment (Table 1).
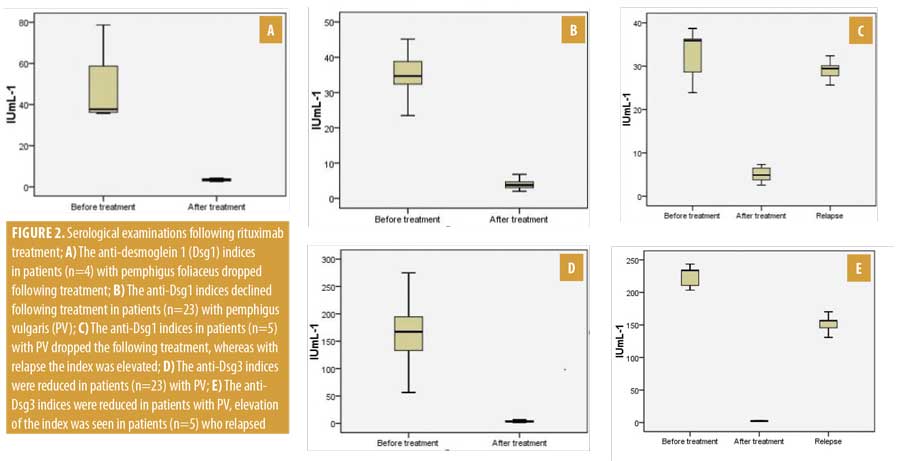
Serological evaluation of anti-Dsg1 and anti-Dsg1 autoantibodies. The anti-Dsg1 IgG index strongly decreased in all four patients with PF after rituximab and the drop in the index correlated with their clinical improvement (Figure 2a). Patients 16, 21, and 30 achieved CR ON at Weeks 52, 42, and 46, respectively. Patient 28 obtained PR OFF at Week 40. A Wilcoxon signed-rank test showed that four or five infusions of rituximab during a four-week course did not elicit a statistically significant change in anti-Dsg1 index in individuals with pretreatment anti-Dsg1 index (Z= -1.83; n=4; p=0.07). Indeed, the median anti-Dsg1 indices for pre- and posttreatment were 37.69 and 3.39 IU/mL-1, respectively. During the follow-up period, relapse of patients with PF was not observed.
We evaluated serological data for 23 patients with PV who experienced no relapse (Figure 2b); a Wilcoxon signed-rank test showed that rituximab infusions (range: 2–5) over four weeks demonstrated a statistically significant difference in anti-Dsg1 index among individuals with pretreatment anti-Dsg1 index (Z=-4.20; n=23; p less than or equal to 0.001). In addition, 34.67 and 3.78IU/mL-1 was the median anti-Dsg1 index for pre- and posttreatment, respectively. During a long follow-up period, we observed that five patients with PV had relapsed. Each had a positive anti-Dsg1 index before treatment, which showed a decrease after the administration of rituximab; however, during the relapse period, their anti-Dsg1 index was further increased, supporting a revised treatment course of rituximab (Figure 2c). To compare the anti-Dsg1 index difference between pretreatment, posttreatment and relapse, the Friedman test was performed and findings revealed that there was a statistically significant difference in anti-Dsg1 index among the three treatment levels (Chi2(2)=10; n=5; p=0.007). Moreover, the medians for the anti-Dsg1 index for pretreatment, posttreatment, and relapse were 35.89, 4.89, and 29.45 IU/mL–1, respectively.
Twenty-eight patients demonstrated a decrease in the anti-Dsg3 index after rituximab treatment. Patients 2, 4, 10, 12, and 14 relapsed at Weeks 96, 72, 96, 96, and 88, respectively. In each patient, the anti-Dsg3 index was elevated (Figure 2d). A comparison of the difference in anti-Dsg3 index between pre- and posttreatment was performed using a Wilcoxon signed-rank test. The analysis revealed that there was a statistically significant difference in the anti-Dsg 3(Z=-4.20; n=23; p less than or equal to 0.001) index between pre- and posttreatment. Apart from these, 167.34 and 3.46IU/mL-1 were the median anti-Dsg1 indices for pre- and posttreatment, respectively. In five patients who relapsed, the Dsg3 index was consistently high during the entire follow-up period (Figure 2e). The result of the Friedman test suggested that there was a statistically significant difference in anti-Dsg3 index among pretreatment, posttreatment, and relapse, Chi2(2)=10; n=5; p=0.007. In addition, the median for the anti-Dsg1 index for pretreatment, posttreatment, and relapse was found to be 233.67, 2.38, and 156.45 IU/mL-1, respectively.
Discussion
In recent years, pemphigus has become the most widely studied condition in relation to the effects of rituximab therapy. Consideration of various factors associated with pemphigus, including the correlation between disease activity and autoantibody titer, the availability of commercial enzyme-linked immunosorbent assays for autoantibody quantification, consensus disease definitions, and disease activity instruments to standardize reporting in clinical trials make pemphigus an ideal disease for study. All of these elements motivated our study.27
To the best of our knowledge, our series is the first comprehensive collection of patients with pemphigus treated with rituximab.14,29 Here, we used a modified RA protocol involving varying infusions of 1,000mg that has not been previously reported in the literature. Our findings suggested that all of our patients responded with clinical improvement and, moreover, 59.37 percent of the patients achieved CR. This is in favor of focusing a clinician’s attention towards rituximab therapy in pemphigus.
However, though rituximab is extensively used, its optimal dosing regimen in autoimmune blistering diseases is still debatable. As mentioned earlier, apart from two protocols (lymphoma and RA), no definite treatment strategies have been reported in the literature and several authors report treating the disease according to their own modified protocols.7–15 As there is a lack of uniformity in methodology, making any conclusion is quite difficult.
Schmidt et al8 and Kim et al14 compared the clinical efficacy between two infusions and three or more infusions of rituximab therapy for lymphoma protocol and from their research; they established that the clinical efficacy of three or more infusions of rituximab is more effective than two infusions for the treatment of pemphigus. Here, we used two or more infusions to treat pemphigus, which was demonstrated to be effective and safe. We recommend that further studies be conducted to compare clinical efficacy of two infusions versus three or more infusions of rituximab therapy for modified RA protocol.8,14
To date, four case series have been reported in the literature using the RA protocol.29–32 Labib et al33 combined these data and conducted an analysis of 75 patients. On the basis these findings, CR was reported in 59 (78.67%) patients, of whom 44 (58.67%) were off therapy; 11 (14.67%) were on therapy; and, in four (5.33%) patients, the therapy was unclear. The mean duration of follow-up was 18.66 (range: 8.35–29) months. The use of a modified RA protocol has not been previously reported in the literature. In our study, a modified RA protocol was used and the findings were reported after a follow-up period of 98.22±20.65 (range: 40–140) weeks concluded that 19 of the 32 (59.37%) patients had achieved CR: eight received minimal therapy (CR ON), and 11 did not need any medication (CR OFF).
The late endpoints achieved at a median of 45 weeks for both PR and CR are longer than those reported for patients on the lymphoma regimen (13 weeks and 26 weeks)21,22 or those found by Kim et al.14 Despite the longer periods taken to reach CR or PR in our study, the relapse rate seemed to be less. According to the data collection and analysis from Labib et al, relapse occurred in 28 (37.33%) patients, whereas, in our study, relapse occurred in five (15.63%) patients. The author concluded that, after the use of a modified RA protocol, the relapse rate was thought to be less relative to that of the authentic RA protocol.
Except for one case of sepsis due to neutropenia, in our study, patients (n=19; 59.38%) had mild adverse events. Moreover, low blood pressure and neutropenia were also observed. Further serious infections were not reported in our series; however, some cases using the RA protocol have been published.29–32
Our serological investigations demonstrated that the clinical response to rituximab in patients with PF was closely related to the evolution of anti-Dsg1 antibodies as reported previously.21 The anti-Dsg3 level correlated with the mucous membrane disease activity in PV. In the relapsed patient with PV, anti-Dsg1 antibodies and the anti-Dsg3 level was further increased, suggesting the need for retreatment with rituximab, whereas no relapse was seen among patients with PF.
One major drawback of the study is that it is a retrospective, nonrandomized, single-center open case series with no placebo to compare. Furthermore, specific antibodies against Dsg1 and Dsg3 were measured in the sera of patients at the beginning and end of the therapy; however, for better clarification, autoantibody titer measurements should be collected at every scheduled visit. Apart from these, the level of CD20+ B-cells should also be measured, as this was restricted in our study due to it being retrospective. Our recommendation is that, for genuine justification, further randomized controlled research should be planned.
Conclusion
A modified RA protocol for rituximab was shown to be effective and safe in treating patients with pemphigus. Clinicians should focus on adopting rituximab modified RA protocols in patients with pemphigus; however, further research should aim to delineate a more precise judgment.
References
- Wolff K, Goldsmith L, Katz S, et al. Flitzpatric’s dermatology in General Medicine. 7th edition. New York, NY: McGraw-Hill; 2008.
- Kanwar AJ, Ajith AC, Narang T. Pemphigus in North India. J Cutan Med Surg. 2006;10(1):21–25.
- Martin LK, Agero AL, Werth V, et al. Interventions for pemphigus vulgaris and pemphigus foliaceus. Cochrane Database Syst Rev. 2009;(1):CD006263.
- Hertl M, Eming R, Borradori L. Rituximab (anti-CD20 monoclonal antibody) ultimate or first choice in pemphigus?. Dermatology. 2007;214(4):275-277.
- Carr DR, Heffernan MP. Off-label uses of rituximab in dermatology. Dermatol Ther. 2007;20(4): 277–287.
- Food and Drug Administration. Rituxan label. Revised March 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103705s5373lbl.pdf. February 12, 2020.
- Belgi AS, Azeez M, Hoyle C, et al. Response of pemphigus vulgaris to anti-CD20 antibody therapy (rituximab) may be delayed. Clin Exp Dermatol. 2006;31(1):143.
- Schmidt E, Seitz CS, Benoit S, et al. Rituximab in autoimmune bullous diseases: mixed responses and adverse effects. Br J Dermatol. 2007;156(2):352–356.
- Barrera MV, Mendiola MV, Bosch RJ, et al. Prolonged treatment with rituximab in patients with refractory pemphigus vulgaris. J Dermatol Treat. 2007;18(5):312–314.
- Faurschou A, Gniadecki R. Two courses of rituximab (anti-CD20 monoclonal antibody) for recalcitrant pemphigus vulgaris. Int J Dermatol. 2008;47(3):292–294.
- Craythorne EE, Mufti G, DuVivier AW. Rituximab used as a first-line single agent in the treatment of pemphigus vulgaris. J Am Acad Dermatol. 2011;65(5):1064–1065.
- Horváth B, Huizinga J, Pas HH, et al. Low-dose rituximab is effective in pemphigus. Br J Dermatol. 2012;166(2):405–412.
- Craythorne E, Du Viver A, Mufti GJ, et al. Rituximab for the treatment of corticosteroid—refractory pemphigus vulgaris with oral and skin manifestations. J Oral Pathol Med. 2011;40(8):616–620 .
- Kim JH, Kim YH, Kim MR, et al. Clinical efficacy of different doses of rituximab in the treatment of pemphigus: a retrospective study of 27 patients. Br J Dermatol. 2011;165(3):646–651.
- Kasperkiewicz M, Shimanovich I, Ludwig RJ, et al. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. 2011;65(3):552–558.
- Arin MJ, Hunzelmann N. Anti-B-cell-directed immunotherapy (rituximab) in the treatment of refractory pemphigus—an update. Eur J Dermatol. 2005;15(4):224–230.
- Lombardi T, Samson J, Orradori L, et al. Anti-CD20 monoclonal antibody (rituximab) for refractory erosive stomatitis secondary to CD20(+) follicular lymphoma-associated paraneoplastic pemphigus. Arch Dermatol. 2001;137(3):269–272.
- Heizmann M, Itin P, Wernli M, et al. Successful treatment of paraneoplastic pemphigus in follicular NHL with rituximab: report of a case and review of treatment for paraneoplastic pemphigus in NHL and CLL. Am J Hematol. 2001;66(2): 142–144.
- Schadlow MB, Anhalt GJ, Sinha AA. Using rituximab (anti-CD20 antibody) in a patient with paraneoplastic pemphigus. J Drugs Dermatol. 2003;2(5):564–567.
- Ahmed AR, Spigelman Z, Cavacini LA, et al. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355(17):1772–1779.
- Joly P, Mouquet H, Roujeau JC, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357(6):545–552.
- Cianchini G, Corona R, Frezzolini A, et al. Treatment of severe pemphigus with rituximab. Arch Dermatol. 2007;143(8):1033–1038.
- Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84(8):2457–2466.
- Maloney DG, Grillo-Lopez AJ, White CA et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90(6):2188–2195.
- Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2(5):932–937.
- Looney RJ, Anolik JH, Campbell D, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I /II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50(8):2580–2589.
- Murrell DF, Dick S, Ahmed AR, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. 2008;58(6):1043–1046.
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27(1):e17–e23.
- Kasperkiewicz M, Shimanovich I, Meier M, et al. Treatment of severe pemphigus with a combination of immunoadsorption, rituximab, pulsed dexamethasone and azathioprine/mycophenolate mofetil: a pilot study of 23 patients. Br J Dermatol. 2012;166(1):154–160.
- Matsukura S, Knowles SR, Walsh S, et al. Effect of a single-cycle alternative dosing regimen for rituximab for recalcitrant pemphigus. Arch Dermatol. 2012;148(6):734–739.
- Cianchini G, Lupi F, Masini C, et al. Therapy with rituximab for autoimmune pemphigus: results from a single-center observational study on 42 cases with long-term follow-up. J Am Acad Dermatol. 2012;67(4):617–622.
- Zakka LR, Shetty SS, Ahmed AR. Rituximab in the treatment of pemphigus vulgaris. Dermatol Ther (Heidelb). 2012;2(1):17.

