J Clin Aesthet Dermatol. 2019;12(10):35–38
 by Abraham M. Korman, MD; Kelly A. Reynolds, BA; Fadi Nabhan, MD; Bhavana Konda, MD; Manisha H. Shah, MD; and Benjamin H. Kaffenberger, MD
by Abraham M. Korman, MD; Kelly A. Reynolds, BA; Fadi Nabhan, MD; Bhavana Konda, MD; Manisha H. Shah, MD; and Benjamin H. Kaffenberger, MD
Drs. Korman and Kaffenberger are with the Division of Dermatology in the Department of Internal Medicine at The Ohio State University Comprehensive Cancer Center in Columbus, Ohio. Ms. Reynolds is with the University of Cincinnati College of Medicine in Cincinnati, Ohio. Drs. Nabhan, Konda, and Shah are with the Division of Medical Oncology in the Department of Internal Medicine at The Ohio State University Comprehensive Cancer Center in Columbus, Ohio.
FUNDING: Polypodium leucotomos extract was provided via samples from Ferndale Laboratories.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Vandetanib is a tyrosine kinase inhibitor approved by the United States Food and Drug Administration for the treatment of metastatic medullary thyroid cancer. It has been linked to a variety of dermatologic reactions, including photosensitivity. We describe the case of a 55-year-old man who developed a severe, painful, erythematous, bullous eruption in sun-exposed areas one month after the initiation of vandetanib. The eruption was initially refractory to treatment with steroids and did not resolve with strict sun avoidance, but finally cleared after a few weeks of oral supplementation with Polypodium leucotomos (P. leucotomos) extract. P. leucotomos is an extract derived from a tropical fern, with antioxidant effects that mitigate ultraviolet-induced cutaneous erythema via inflammatory interference and the promotion of other cytotoxic responses. This case illustrates the potential for P. leucotomos to be used as a safe and effective photoprotective agent for refractory phototoxic reactions. Further randomized, controlled trials are needed to better understand the mechanism of action and photoprotective properties of P. leucotomos in the treatment of tyrosine kinase-induced phototoxicity and other dermatoses.
KEYWORDS: Metastatic medullary thyroid cancer, phototoxic reaction, Polypodium leucotomos, severe cutaneous adverse reactions, tyrosine kinase inhibitors, vandetanib
In recent years, tyrosine kinase inhibitors have risen to prominence with the development of novel oncologic therapies. Vandetanib is an oral tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor 2, the epidermal growth factor receptor, and RET tyrosine kinase.1 It is approved by the United States Food and Drug Administration (FDA)for the treatment of advanced, unresectable, or metastatic medullary thyroid cancer. In clinical trials, a variety of cutaneous reactions have been identified as potential side effects of tyrosine kinase inhibitors, including acneiform rash, xerosis, eczema, and, in rare cases, phototoxicity.3 Of the few case reports of vandetanib-induced phototoxicity, the discontinuation of vandetanib resulted in improvement within one month, with postinflammatory hyperpigmentation and erythema often following the acute eruption.2–5 Here, we describe a case of a protracted vandetanib-induced phototoxic drug rash that eventually resolved with oral supplementation of Polypodium leucotomos (P. leucotomos) extract.
Case Presentation
A 55-year-old man with familial RET V804M-positive medullary thyroid cancer was treated with vandetanib 300mg daily for extensive, inoperable, metastatic disease after total thyroidectomy and radical neck dissection.
Less than one month after the initiation of vandetanib, the patient developed a severe, painful sunburn after minimal sun exposure. Vandetanib was discontinued immediately, resulting in only marginal improvement in pain and swelling over the next 24 hours. He was evaluated by the dermatology team the following day, where extensive erythema and blistering were noted in the sun-exposed areas of his upper chest, face, and neck, with sparing of skin creases and sun-protected areas (Figure 1A). Multiple painful bullae were scattered over the dorsal and palmar surfaces of his hands (Figure 1B). Aside from the recent initiation of vandetanib, he denied any other recent changes in medication use, illness, or variations in his daily regimen. Urine porphobilinogens and serum porphyrins were negative. Antinuclear antibodies were not detected.
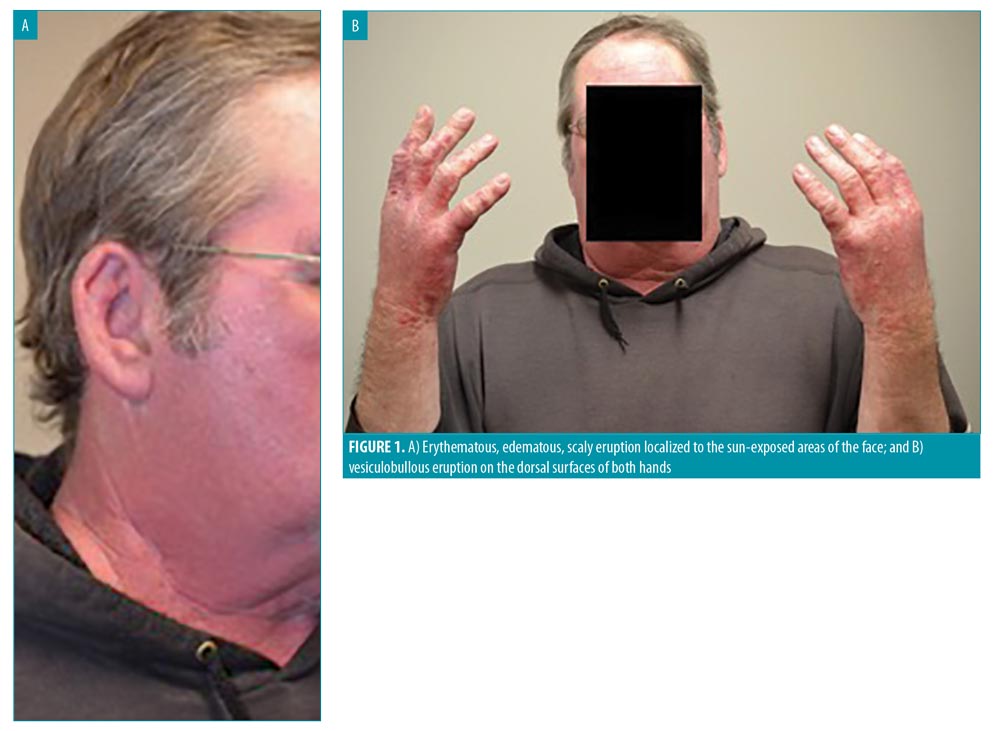
Given the temporal relationship between the photodistributed eruption and the initiation of vandetanib therapy, a presumptive diagnosis of vandetanib-induced phototoxicity was made. Vandetanib was discontinued, and the patient was prescribed betamethasone diproprionate 0.05% cream for his hands, fluticasone 0.05% for his face, and a 16-day oral prednisone taper, beginning at 40mg and tapered by 10mg every four days. He was instructed to continue sun protection factor (SPF) 70 protection at all times and practice strict sun avoidance whenever possible. An SPF 50+ hat with neck protection flap and special ultraviolet (UV) shield gloves were also recommended.
When the patient returned to the clinic six weeks later, his symptoms were only mildly improved; he continued to experience persistent, severe erythema over his face, chest, neck, and hands, despite the discontinuation of vandetanib, careful sun avoidance, and strict use of the recommended sunscreens, hats, sleeves, and gloves. He was initiated on a trial of P. leucotomos extract supplementation 240mg daily. Vandetanib use remained on hold.
When the patient returned for his repeat examination eight weeks later, his skin was completely clear. The previously erythematous and edematous sun-exposed areas of his face, neck, and chest were healed, with no postinflammatory changes or hyperpigmentation noted. The bullous eruption on his hands had fully resolved. In the interim, he had been taking the P. leucotomos supplement daily, while other factors, including limited sun exposure, remained relatively unchanged. After the resolution of the rash, the patient was offered the option to rechallenge to vandetanib while taking the P. leucotomos extract but he declined to do so.
Discussion
Vandetanib-induced phototoxicity. Vandetanib is a potent, once-daily, oral multikinase inhibitor with peak blood plasma concentrations reached in 4 to 10 hours after administration. Pharmacokinetic studies have identified that the clearance rate of vandetanib is approximately 13L/h, with an average half-life of approximately 19 days and steady-state concentration reached after approximately three months.1
While cutaneous reactions, such as acneiform rash, xerosis, and eczema, are listed as common adverse events of tyrosine kinase inhibitors, severe phototoxicity is relatively rare.3 Patel et al6 conducted a systematic review of the incidence of cutaneous side effects associated with tyrosine kinase inhibitors and concluded that Grades 3 to 4 rashes are reported in a considerable number of patients treated with imatinib, nilotinib, erlotinib, lapatinib, sorafenib, and sunitinib. In a pooled analysis of Phase I, II, and III clinical trials of sunitinib, phototoxicity occurred in less than 0.1 percent of patients.7
A few cases of severe phototoxicity with vandetanib treatment have been reported in the literature (Table 1). Chang et al2 described the case of a 60-year-old man with hepatocellular carcinoma who developed bullous lesions related to sun exposure during a trial on vandetanib. Another report by Fava et al5 described a photoallergic reaction that occured in an 80-year-old woman after two months of vandetanib therapy for metastatic nonsmall-cell lung cancer. In a review of Phase II and III studies on vandetanib, 3 of 63 patients developed severe phototoxicity, leading to the discontinuation of the drug.3
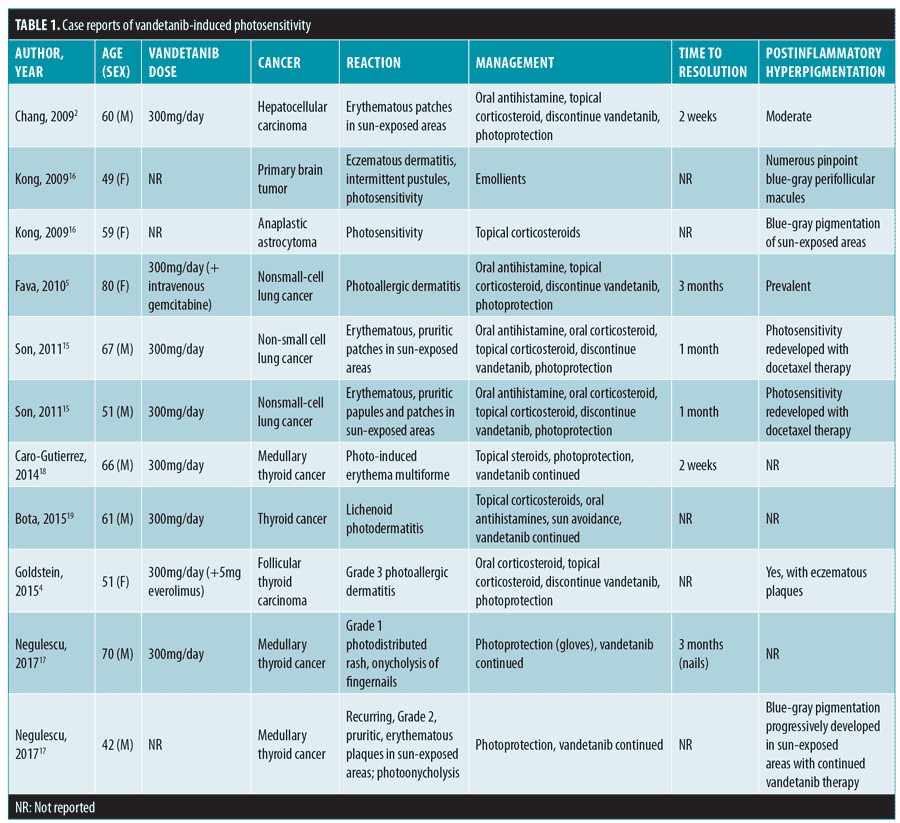
Although dermatoses commonly occur during treatment with tyrosine kinase inhibitors, there are insufficient data to establish treatment guidelines for these reactions.6 In the reported cases of tyrosine kinase-induced phototoxicity, common management strategies include topical corticosteroids, oral corticosteroids, systemic antihistamines, and aggressive photo-protection.2–5 In many cases, resolution of the eruption usually occurred at less than one month after the discontinuation of vandetanib, often with prevalent residual postinflammatory hyperpigmentation following clearance of the rash.2–5
In general, phototoxic reactions occur as a result of the damaging effects of UV light-activated cytotoxic compounds. Phototoxins are typically low molecular weight molecules
(200–500Da) with planar, polycyclic structures, which have a low threshold for excitation by visible-light photons.8 Exposure of these toxins to light results in the production of free radicals, which can cause destruction of neighboring tissues.8 Given that vandetanib is a small molecule with an aromatic structure, it has been theorized to have direct phototoxic properties.2–4
Salvador et al9 demonstrated the phototoxic potential of vandetanib in vitro in human keratinocytes. The authors observed a vandetanib-induced, dose-dependent decrease in keratinocyte viability with UVA irradiation, and postulated that the phototoxic potential of vandetanib is due to the formation of a highly reactive aryl radical, which has the potential to cause massive DNA photodamage, apoptosis, and the oxidation of lipids and proteins.
P. leucotomos. P. leucotomos is a fern species native to South America. Extract of P. leucotomos has been used to treat of a variety of skin conditions, including polymorphic light eruption and melasma.10 P. leucotomos has been shown to have photoprotective properties at cellular and molecular levels.11 The phenolic components of the extract, including caffeic acid and ferulic acid, act as potent inhibitors of free radicals.11,12 P. leucotomos has also been shown to inhibit the phototransformation of transurocanic acid in the stratum corneum, which has UV-absorbing properties.13 On a cellular level, P. leucotomos has been shown to improve cell membrane integrity and elastin expression.11
In a study by Middlekamp-Hup et al14 P. leucotomos demonstrated photoprotective effects in patients undergoing psoralen and UVA (PUVA) therapy. Clinically, patients treated with P. leucotomos developed less erythema and had lower PUVA phototoxicity scores compared to controls. The histological examination of skin biopsies of patients treated with P. leucotomos demonstrated significantly fewer sunburn cells in the epidermis.14
Although P. leucotomos has not been specifically studied in the management of phototoxic reactions, our patient’s dramatic improvement after P. leucotomos supplementation suggests that P. leucotomos might be a feasible option for preventing and managing photosensitivity. The photoprotective properties of P. leucotomos might help counteract damage caused by phototoxins— at the cellular level, by scavenging reactive oxygen species, and the molecular level, by strengthening cell membranes—although more studies are needed to further elucidate this mechanism.
Limitations. Because our patient declined to undergo a rechallenge with vandetanib, we were unable to rule out the prolonged discontinuation of the drug as the primary contributer to the clearance of the rash.
Conclusion
Clinicians should be aware of the risk potential of vandetanib and other multikinase inhibitors for severe phototoxicity. Given its multiple proposed photoprotective mechanisms of action, P. leucotomos might constitute a promising adjunctive treatment for severe photosensitivity, especially in patients whose conditions are refractory to conventional management with sun avoidance and protection. Further studies are necessary to better understand the role of P. leucotomos in managing severe phototoxic reactions.
NOTE: Signed photoconsent was obtained from patient for use of photos presented in this article.
References
- Clinical Pharmacology Review: Vandetanib. US Food and Drug Administration, Center for Drug Evaluation and Research. 2010.
- Chang CH, Chang JW, Hui CY, Yang CH. Severe photosensitivity reaction to vandetanib. J Clin Oncol. 2009;27(27):e114–e115.
- Giacchero D, Ramacciotti C, Arnault JP, et al. A new spectrum of skin toxic effects associated with the multikinase inhibitor vandetanib. Arch Dermatol. 2012;148(12):1418–1420.
- Goldstein J, Patel AB, Curry JL, et al. Photoallergic reaction in a patient receiving vandetanib for metastatic follicular thyroid carcinoma: a case report. BMC Dermatol. 2015;15(2):1–5.
- Fava P, Quaglino P, Fierro MT, et al. A rare vandetanib-induced photo-allergic drug eruption. Dermatol Ther. 2010;23(5):554–555.
- Huang X, Patel S, Ahmed N, et al. Severe toxicity of skin rash, fever and diarrhea associated with imatinib: case report and review of skin toxicities associated with tyrosine kinase inhibitors. Drug Des Devel Ther. 2009;2:215–219.
- Rosenbaum SE, Wu S, Newman MA, et al. Dermatological reactions to the multitargeted tyrosine kinase inhibitor sunitinib. Support Care Cancer. 2008;16(6):557–566.
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention, and management. Drug Safety. 2002;25(5):345–372.
- Salvador A, Vedaldi D, Brun P, Dall’Acqua S. Vandetanib-induced phototoxicity in human keratinocytes NCTC-2544. Toxicol In Vitro. 2014;28(5):803–811.
- Nestor MS, Berman B, Swenson N. Safety and efficacy of oral Polypodium leucotomos extract in healthy adult subjects. J Clin Aesthetic Derm. 2015;8(2):19–23.
- Bhatia N. Polypodium leucotomos: a potential new photoprotective agent. Am J Clin Dermatol. 2015;16(2):73–79.
- Choudhry SZ, Bhatia N, Ceilley R, et al. Role of oral Polypodium leucotomos extract in dermatologic diseases: a review of the literature. J Drugs Dermatol. 2014;13(2):148–153.
- Capote R, Alonso-Lebrero JL, Garcia F, et al. Polypodium leucotomos extract inhibits trans-urocanic acid photoisomerization and photodecomposition. J Photochem Photobiol B. 2006;82(3):173–179.
- Middelkamp-Hup MA, Pathak MA, Parrado C, et al. Oral Polypodium leucotomos extract (PLE) decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004;51(6):910–918.
- Son Y, Roh J, Cho E, Lee J. Photosensitivity reactions to vandetanib: redevelopment after sequential treatment with docetaxel. Ann Dermatol. 2011;23(Suppl 3):S314–S318.
- Kong HH, Fine HA, Stern JB, Turner MLC. Cutaneous pigmentation after photosensitivity induced by vandetanib therapy. Arch Dermatol. 2009;145(8):923–925.
- Negulescu M, Zerdoud S, Boulinguez S, et al. Development of photoonycholysis with vandetanib therapy. Skin Appendage Disord. 2016;2(3-4):146–151.
- Caro-Gutiérrez D, Floristán Muruzábal M, Gómez de la Fuente E, et al. Photo-induced erythema multiforme associated with vandetanib administration. J Am Acad Dermatol. 2014;71(4):e142–e144.
- Bota J, Harvey V, Ferguson C, Hood A. A rare case of late-onset lichenoid photodermatitis after vandetanib therapy. JAAD Case Rep. 2015;1(3):141–143.

