Updates in Psoriasis Management 2019

Based on Selected Presentations from Maui Derm 2019
January 26–30, 2019, Maui, Hawaii

by Jo Ann LeQuang
Ms. Lequang is Owner of LeQ Medical in Angleton, Texas, Director of Scientific Communications at NEMA Research, Inc., in Naples, Florida, and Founding Director of No Baby Blisters in Colorado Springs, Colorado.
J Clin Aesthet Dermatol 2019;12(10):S7–S19
Based on presentations by

Linda Stein Gold, MD
Dr. Stein Gold is with Henry Ford Health System in Detroit, Michigan.

Arthur Kavanaugh, MD
Dr. Kavanaugh is Professor of Medicine and Director of the Center for Innovative Therapy (CIT) at University of California, San Diego in San Diego, California.

Craig L. Leonardi, MD
Dr. Leonardi is Associate Clinical Professor of Dermatology with the St. Louis University Medical School in St. Louis, Missouri.

Bruce E. Strober, MD, PhD
Dr. Strober is Assistant Clinical Professor of Dermatology with the Department of Dermatology of Yale University in New Haven, Connecticut.
Funding: This supplement was funded by Sun Pharmaceutical Industries Ltd., Princeton, New Jersey.
Disclosures: Dr. Kavanaugh has conducted research studies for AbbVie?, Amgen?, Astra-Zeneca, BMS?, Celgene, Corrona, Galapagos/Gilead, Genentech/Roche?, ITN?, Janssen?, LCTC, LIAI, NIH, Novartis?, Pfizer, Regeneron/Sanofi?, TREG, and UCB. Ms. LeQuang has no conflicts of interest relative to the content of this supplement. Dr. Leonardi has served as a consultant, speaker, advisory board member, or investigator for Abbvie, Allergan, Amgen, Celgene, Coherus, Dermira, Eli-Lilly, Galderma, Glaxo Smith Kline, Janssen, Leo, Merck, Merck-Serono, Novartis, Pfizer, Sandoz, Sienna, UCB, and Vitae. Dr. Martin has served as a speaker, consultant, or advisory board member for Aclaris, Aqua, Celgene, DUSA/Sun Pharma, Janssen, Pfizer, Ortho/Valeant, UCB, Aclaris, Celgene, Pfizer, and UCB. Dr. Stein Gold has served as a speaker, consultant, or advisory board member for Leo Pharma, Mayne Pharma, Pfizer Inc., Taro, and Valeant. Dr. Strober has been a consultant to, speaker for, or has received honoraria from AbbVie, Almirall, Amgen, Arena, Boehringer Ingelheim, Bristol-Myers-Squibb, Celgene, CORRONA Psoriasis Registry, Dermavant, Dermira, Janssen, Leo, Eli Lilly, Kyowa Hakko Kirin, Medac, Meiji Seika Pharma, Sebela Pharmaceuticals, Menlo Therapeutics, Novartis, Pfizer, GlaxoSmithKline, UCB Pharma, Sun Pharma, Ortho Dermatologics/Valeant, Regeneron, Sanofi-Genzyme.
A Message from the Program Director and Guest Editor

George Martin, MD
Dr. Martin is Guest Editor and Program Director of MauiDerm 2019 and with Dermatology Associates in Khei, Maui, Hawaii.
Dear Colleagues:
Each year, Maui Derm strives to bring to the podium the most up-to-date and cutting edge scientific and clinical developments in the field of dermatology. Maui Derm 2019 was no exception—our outstanding faculty delivered a mind-numbing amount of clinical information in multiple areas of dermatology. One therapeutic area in particular—psoriasis—has demonstrated a rapid expansion of new therapeutic options. In this supplement to The Journal of Clinical and Aesthetic Dermatology (JCAD), we’ve captured a select group of Maui Derm 2019 presentations we feel represents the key recent clinical developments in psoriasis. Here, we provide information on newly discovered mechanisms of action, emerging and newly approved drugs, and broadening therapeutic indications for existing drugs in the field of psoriasis.
We all understand the importance of staying up to date on the latest clinical developments in dermatology so that we can provide our patients with the best care possible. As busy dermatologists, however, it isn’t always possible to attend educational events such as Maui Derm. We hope the information presented in this supplement assists clinicians in determining how best to prescribe these emerging therapies as they come to market, in order to achieve optimal treatment outcomes in patients.
We hope you can join us January 25–29, 2020, in Maui next year for what promises to be another outstanding educational event. If your schedule prevents you from joining us in 2020, however, we hope to once again provide you with highlights from some of the key presentations from our meeting faculty.
With aloha,
George Martin, MD
Introduction
Each year, the MauiDerm for Dermatologists symposium in Maui, Hawaii, offers lectures, panel discussions, and workshops that are designed to provide in-depth, cutting edge material on an extensive range of medical, cosmetic, coding/billing, and CLIA certification topics for practicing dermatologists. Among numerous other dermatological topics presented during the 15th annual 2019 MauiDerm event, several key opinion leaders presented information on the latest psoriasis treatment options, clinical trial results (including some important new head-to-head studies) in psoriasis, and novel and established therapies for the treatment and prevention of psoriasis. This article highlights insights and data presented by selected experts in the field of psoriasis based on presentations from Bruce E. Strober, MD, PhD; Arthur Kavanaugh, MD; Linda Stein Gold, MD; and Craig L. Leonardi, MD.
Intriguing Findings in Psoriasis
Based on a presentation by Bruce E. Strober, MD, PhD, Assistant Clinical Professor of Dermatology, Department of Dermatology of Yale University, New Haven, Connecticut.
Apremilast. Apremilast, a small molecule, is a phosophodiesterase-4 inhibitor (PDE4) that has been shown to be safe and effective in treating plaque psoriasis (ESTEEM study),1 scalp and nail psoriasis (ESTEEM 1 and 2),2 and psoriatic arthritis (PALACE study).3 While the association between obesity and metabolic syndrome with both psoriasis and psoriatic arthritis (PsA) is well established,4–6 it has been unclear if drug therapy might affect weight gain and A1c levels in patients with psoriasis. In a post-hoc sub-study of the two landmark psoriasis trials, ESTEEM and PALACE (n=2,242), it was found that A1c levels and weight were reduced in patients receiving apremilast with higher baseline A1c levels compared to patients taking apremilast with lower baseline A1c levels and placebo patients. In patients taking insulin and apremilast, weight loss still occurred, leading investigators to infer that PDE4s might have an effect on insulin resistance.7
Psoriasis and cardiovascular risk. Ongoing research continues to explore the relationship between psoriasis and known cardiovascular risk factors, such as arteriosclerosis. Excessive carotid intima-media thickness (IMT) is considered a marker for arteriosclerosis,8–11 and some studies have found that in patients with psoriasis or rheumatoid arthritis, treatment with anti-tumor-necrosis-factor-alpha (anti-TNF-?) resulted in a reduction of IMT.12,13 Based on that finding, a prospective study (N=53) was conducted to evaluate whether systemic or biologic agents (methotrexate or ustekinumab) might affect carotid IMT.14 The IMT of patients with psoriasis treated with these drugs tended to decrease over an eight-month follow-up, but the difference was not statistically significant; the difference was more pronounced with ustekinumab than methotrexate.14 These results are intriguing, but must be viewed with caution, because this small study was not blinded.
More evidence is suggesting that the association between psoriasis and metabolic syndrome involves risk factors for cardiovascular disease. In a 2017 study of patients with psoriasis and healthy controls, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) was used at baseline.15 Patients with moderate-to-severe psoriasis were treated with ustekinumab until a Psoriasis Area and Severity Index (PASI) of 75-percent improvement over baseline (PASI75), whereupon a 18F-FDG PET/CT scan was performed again. Decreased inflammation in the ascending aorta, descending aorta, aortic arch, and liver were used as surrogate markers in the 10 patients with psoriasis and decreased significantly.
Presented in 2018 at the American Academy of Dermatology annual meeting, Gelfand et al reported on a placebo-controlled clinical trial of aortic inflammation in 43 patients with psoriasis who were randomized 1:1 to ustekinumab or placebo. Patients were evaluated by target-to-background ratio (TBR) on 18F-FDG PET/CT at baseline and 12 weeks. At 12 weeks, 77 percent of the ustekinumab patients and 11 percent of the placebo patients had achieved PASI75. Average aortic vascular inflammation had a mean TBR value of 1.31±0.15 at baseline and decreased 6.6 percent at 12 weeks among patients receiving ustekinumab but increased 12.1 percent in the same time period in patients receiving the placebo (p=0.001).16 Following the double-blind phase (12 weeks), all patients were treated with ustekinumab and entered an open-label phase (40 weeks for patients from the ustekinumab group and 52 weeks for patients from the placebo group).
Interleukin-19. Interleukin (IL)-19 upregulates keratinocyte growth factor (KGF) transcripts, and it has been found that patients with psoriasis have statistically significantly higher IL-19 serum levels than healthy subjects.17 IL-19 serum concentrations can be correlated with increased PASI scores in patients with psoriasis. Thus, serum IL-19 might serve as a straightforward, reliable, and predictive biomarker for psoriatic disease activity that could be employed to predict early response to therapeutic regimens and even help determine interval drug dosing.
IL-36 pathway Inhibition. The role of IL-36 in keratinocyte proliferation is emerging as another important drug development target. The identified mutations in IL-36RN and their inflammation cascades have resulted in pathophysiological insights with respect to generalized pustular psoriasis.19 A new humanized anti-IL-36R antagonist has entered a Phase I study for safety and efficacy in patients with acute generalized pustular psoriasis. Preliminary results suggest that IL-36R inhibition might be a safe and effective way to treat generalized pustular psoriasis flares.18
Pediatric psoriasis. The challenges of treating pediatric psoriasis were addressed, in part, in an ongoing Phase II, open-label study that reported on the safety and pharmacokinetics of apremilast in patients between the ages of 6 and 17 years with moderate-to-severe plaque psoriasis. Pediatric patients with psoriasis that could not be adequately controlled with topical treatments were included in the study. While the study is ongoing, preliminary results appear promising.
Nail involvement in psoriatic disease. Nail involvement in psoriasis can be particularly resistant to treatment. Results from the PHOENIX 1 study found that ustekinumab 45mg and 90mg improved the Nail Physician’s Global Assessment (Nail PGA) significantly over baseline, with improvements showing a duration of up to one year of treatment.20 Results of the IXORA-S study have recently been presented at the European Academy of Dermatology and Venereology (EADV) in 2017 and 2018; head-to-head comparisons between ixekizumab versus ustekinumab over one year for the treatment of nail lesions in patients (n=302) with chronic plaque psoriasis reported ixekizumab provided significantly greater clearance than ustekinumab of nail lesions at 52 weeks.21 In this multicenter, double-blind study, patients receiving ixekizumab were started with a 160mg dose administered in two 80mg injections, followed by 80mg injections every two weeks for 12 weeks, and then an 80mg injection every four weeks. The ustekinumab patients received 45 or 90mg doses (based on patient weight) at baseline, at four weeks, and then every 12 weeks.21
Guselkumab versus secukinumab. Late-breaking research was presented at the Inflammatory Skin Disease Summit in 2018 comparing guselkumab to secukinumab in a head-to-head Phase III trial for patients with moderate-to-severe psoriasis (ECLIPSE study).20 In this randomized study, patients received guselkumab 100mg at baseline, Weeks 4 and 12, and then every eight weeks (n=534) or secukinumab 300mg at baseline, weekly for four weeks, and then every four weeks (n=514). Patients were treated for a total of 48 weeks. More patients in the guselkumab group achieved PASI90 at Week 48 than in the secukinumab group (84.5% vs. 70.0%, respectively, p<0.001). A secondary endpoint was the percentage of patients who achieved PASI75 or better at both Weeks 12 and 48; guselkumab was either noninferior or superior to secukinumab (84.6% vs. 80.2%) in this regard. Guselkumab was superior in the percentage of patients achieving PASI100 at 48 weeks (58.2% vs. 48.4%, p=0.001). Adverse events for both agents aligned with the established safety profiles for these agents.20
The Latest in Psoriatic Arthritis Research
Based on a presentation by Arthur Kavanaugh, MD, Professor of Medicine and Director of the Center for Innovative Therapy (CIT) at University of California, San Diego in San Diego, California
Psoriatic arthritis (PsA) is a chronic, immune-mediated, inflammatory arthropathy associated with joint and enthesis inflammation (including the axial skeleton), and is known to be comorbid with metabolic syndrome, obesity, and cardiovascular disease.23 PsA features might include enthesitis, dactylitis, nail dystrophy, uveitis, and osteitis. The prevalence of PsA is estimated to be between 0.5 and 3.15 percent in the United States,24 and a recent large-scale, population-based study of psoriasis prevalence in Canada was found to be 2.54 percent.25 Increasing prevalence might be due, in whole or part, to such factors as an aging population, extended longevity of patients with psoriasis, greater awareness (and thus diagnosis) of PsA, and more PsA treatments. The conventional treatment options for PsA include adjunctive agents, nonsteroidal anti-inflammatory drugs (NSAIDs), topical treatments, injections, physical therapy, disease-modifying antirheumatic drugs (DMARDS), biologics, jakinibs (i.e., tofacitinib), and PDE-4 inhibitors (apremilast). Experimental options include some new Jakinibs (baricitinib, filgotinib, upadacitinib, and other drugs, such as novel ?-IL-23 drugs (i.e., guselkumab, risankizumab, tildrakizumab) and anti-IL-17 drugs, such as brodalumab or bimekizumab, which inhibit IL-17RA and IL-17A/F, respectively.
About 40 percent of patients with PsA under treatment do not respond to treatment or respond partially, which underscores the need for better elucidation of the pathogenesis of PsA.23 There is a strong genetic component to PsA that is distinct from that of plaque psoriasis. Environmental factors, such as stress, trauma (particularly joint or bone trauma), and possibly streptococcal infections, might trigger PsA in genetically susceptible individuals.
Approved drugs for the treatment of PsA target the cytokines IL-12/23, IL-17A, TNF, and the Janus kinase (JAK) pathways. JAK pathways are also targets for drugs treating rheumatoid arthritis, but not cutaneous psoriasis.
Ustekinumab. Axial spondyloarthritis (axSpA) is an immune-mediated chronic inflammatory form of arthritis that typically affects the axial skeleton, but might involve peripheral joints as well. Ustekinumab was evaluated in three randomized placebo-controlled trials in patients with axSpA where patients were randomized into three arms to receive subcutaneous ustekinumab 45 or 90mg (weight-based dosing) or placebo for up to 24 weeks; at the end of this phase, patients receiving the placebo were randomized again to receive ustekinumab at 45 or 90mg weight-based doses. The primary study endpoint was the Assessment of Spondyloarthritis International Society Criteria (ASAS) of 40 percent (ASAS40). In all three of the studies, no ustekinumab dose group showed a clinically meaningful improvement versus placebo, and all three of the studies were discontinued early. Adverse events with ustekinumab aligned with those in previous studies. The population of patients with axSpA might also be treated with risankizumab, an IL-23 inhibitor. In a randomized trial, patients received 18, 90, or 180mg of risankizumab or placebo, but no dose group of risankizumab had clinically superior results than placebo.28
Ixekizumab versus adalimumab versus placebo. A Phase III, randomized, controlled (active and placebo) trial likewise presented at the 2018 ACR enrolled 341 patients with active radiographic axial spondyloarthritis who were DMARD-naïve for a 16-week study utilizing 80mg subcutaneous ixekizumab every two weeks or four weeks; or 40mg adalimumab every two weeks; or placebo.32 Significant improvements with active treatments emerged as early as the first week; compared to placebo, there were significant improvements in ASAS, BASDA150, and ASDAS responses, as well as improved spinal MRI scores. No unexpected adverse events were reported.
Bimekizumab. Bimekizumab was evaluated in a Phase IIb clinical trial of 206 patients with PsA randomized to five arms: subcutaneous bimekizumab 16mg; 160mg; 160mg after a 320mg loading dose; 320mg; or placebo every four weeks for 12 weeks. Bimekizumab inhibits IL-17A and IL-17F (Table 1). Bimekizumab offered significant improvements to patients with PsA at 12 weeks.30
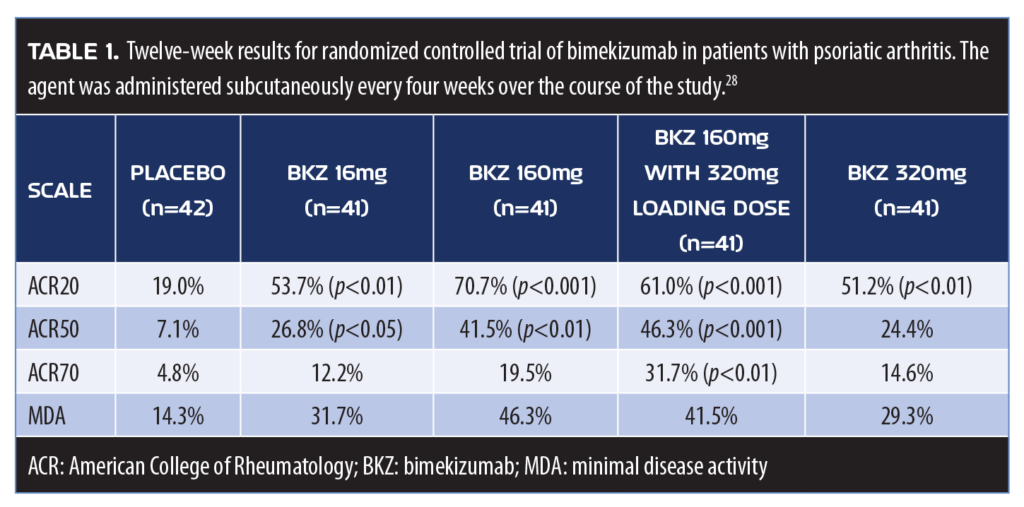
IL-17A. IL-17A is a cytokine involved in the pathogenesis of numerous immune-modulated inflammatory diseases, including PsA. IL-17 is one member of a five-subunit cytokine family; like IL-17F, IL-17A is secreted by T-helper (Th) and other cells. High levels of IL-17A have been associated with mast cells and neutrophils at the sites of skin and joint disease. IL-17A upregulates the expression of numerous inflammation-related genes in target cells, such as keratinocytes and fibroblasts, which mediate clinical features of the disease. Furthermore, IL-17A acts with other cytokines, including but not limited to TNF, resulting in additive or synergistic effects. For patients with PsA, clinical study results show no benefit over placebo from IL-17A inhibitors, although IL-17A inhibition has been effective in treating plaque psoriasis.29 Moreover, IL-17A inhibition is even less beneficial in the treatment of rheumatoid arthritis. This hierarchy of effectiveness for IL-17A inhibition (plaque psoriasis > PsA > rheumatoid arthritis) remains to be elucidated and suggests that there are likely other disease-related considerations involved.
Apremilast. In 2014, results from the PALACE-1 study were published, demonstrating the effectiveness of the PDE4 inhibitor apremilast in the treatment of PsA in terms of offering symptomatic relief and improving function.3 Last year, PALACE-1 investigators were able to release five-year data from that study, confirming the durability of the responses).
Apremilast regulates proinflammatory and anti-inflammatory cytokine pathways (TNF-?, IL-10, IL-23, and IL-17A). Sub-studies of the ESTEEEM trial investigating apremilast in skin psoriasis found that nonlinear associations and cytokine synergistic action between TNF-? and IL-17 inhibition can be predictive of an improvement in PASI score.37
Tofacitinib. Inhibition of the JAK pathways with agents such as tofacitinib represents important emerging treatment courses for PsA. In a six-month study of 395 patients with PsA who did not respond to treatment with TNF inhibition, oral tofacitinib (5 or 10mg/day) was more effective than placebo at reducing disease activity at three months, but was associated with a higher number of adverse events. At three months, ACR20 response was 50 percent with tofacitinib 5mg and 47 percent with 10mg versus 24 percent with placebo (p<0.001 for both doses).38
Combination therapy. Combination therapy is defined as the use of more than one agent with different mechanisms of action to address a single condition. Using two or more agents together might have an additive benefit, meaning the patient gets the benefits from two drugs. However, in some cases, two agents might provide a synergistic benefit in which the benefits exceed the sum of the parts.
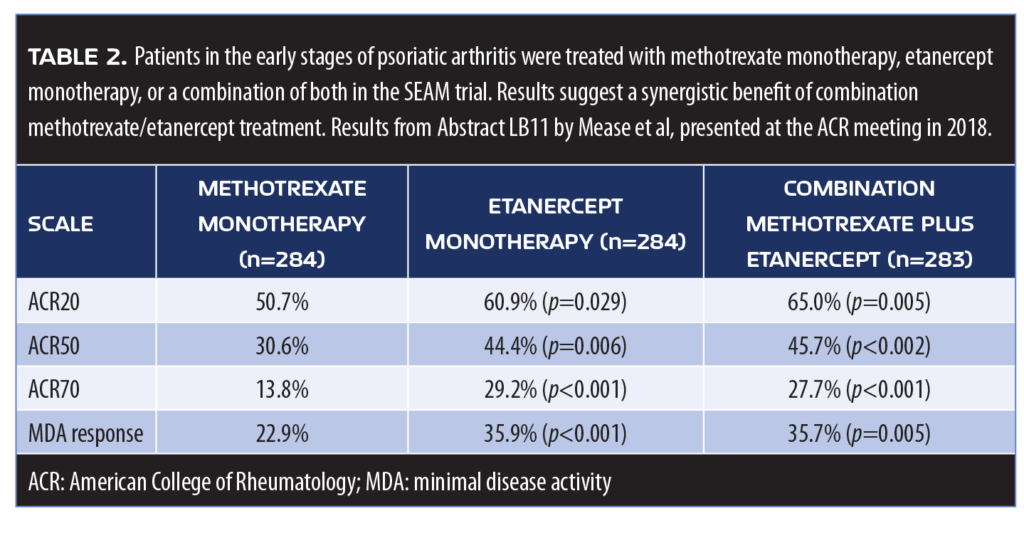
Methotrexate and etanercept. Presented at the American College of Rheumatology (ACR) meeting in 2018, the SEAM trial explored whether methotrexate and TNF inhibition (etanercept) offered synergistic treatment for PsA. With a primary endpoint of ACR 20 percent response (ACR20) and minimal disease activity (MDA), the study evaluated 851 patients who were biologic-naïve and in the early stages of PsA treatment with methotrexate monotherapy, etanercept monotherapy, or both.26,27 Patients treated with etanercept monotherapy had significantly better responses in general than patients treated solely with methotrexate, but combination patients had significantly better results in terms of the endpoints over the course of the study (median duration 0.6 years (Table 2). In terms of adverse events, nausea was more frequently reported in the two methotrexate arms of the study, but otherwise reported adverse events were similar.
IL-17 blockade and secukinumab. An intriguing open-label, observational study was presented at the ACR meeting in late 2018 that addressed the potential interception of PsA at the earliest possible stages using an IL-17 blockade with secukinumab.31 The subset of patients with plaque psoriasis who present with arthralgia and subclinical joint inflammation visible on magnetic resonance imaging (MRI) are considered to be at elevated risk for PsA. In this observational study of 20 patients, IL-17 inhibition with 300mg of subcutaneous secukinumab over 24 weeks resolved inflammation and led to significant improvements in skin psoriasis as well. No progression in terms of joint damage occurred. This suggests that early intervention to PsA might be feasible and of benefit.31 With these promising results, a closer look at the role of IL-17 in gut health is warranted. IL-17A and IL-17AF bind to the same receptor and share 50 percent amino acid identity, but a recent murine study suggests that IL-17F might serve as a better target for colitis treatment than IL-17A.33 The role of IL-17F in intestinal mucosal inflammation was recently described to have shaped the microbiota of the gut with antimicrobial peptides (AMPs), contributing to an inflammatory state. Since microbial dysbiosis is a sign of inflammatory bowel disease, this suggests that IL-17F inhibition might represent a new treatment strategy for colitis.34
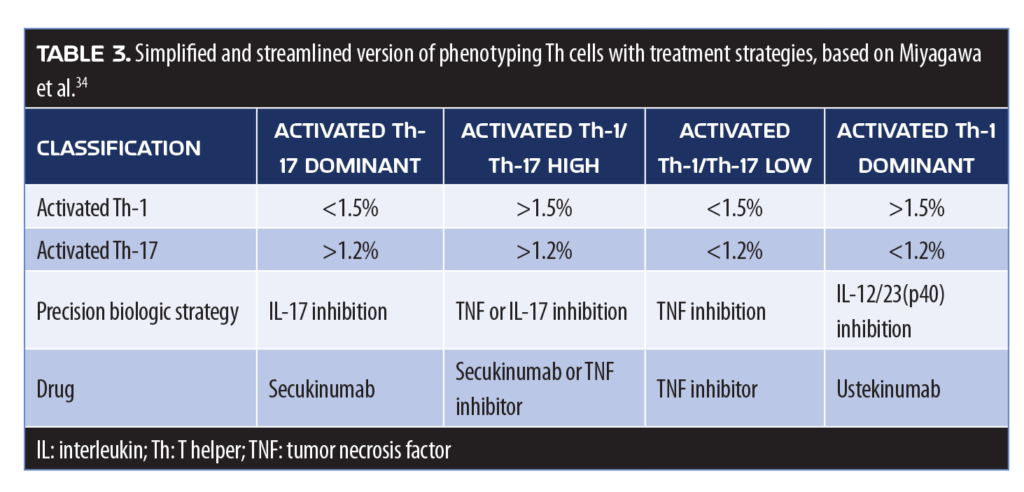
Precision medicine approach. The “Precision medicine” approaches to care might soon include the treatment of PsA, in which the patient’s T-cell immunophenotype would be used to guide the choice of biologic therapy. At the moment, there is little guidance in the literature as to which biologic therapy is most appropriate for an individual patient with PsA,35 but the precision medicine paradigm might still be of value. A proposed method for a precision-medical approach for PsA involves phenotyping peripheral lymphocytes to differentiate the stage of PsA and determine the lineage of various relevant cell lines (Th-1, Th-2, and Th-17) (Table 3).36 A flow-based therapy selection was shown to result in significantly improved ACR20 and ACR50 scores at six months compared to conventional treatment.
What’s New in Topical Therapy for Psoriasis
Based on a presentation by Linda Stein Gold, MD, Henry Ford Health System in Detroit, Michigan
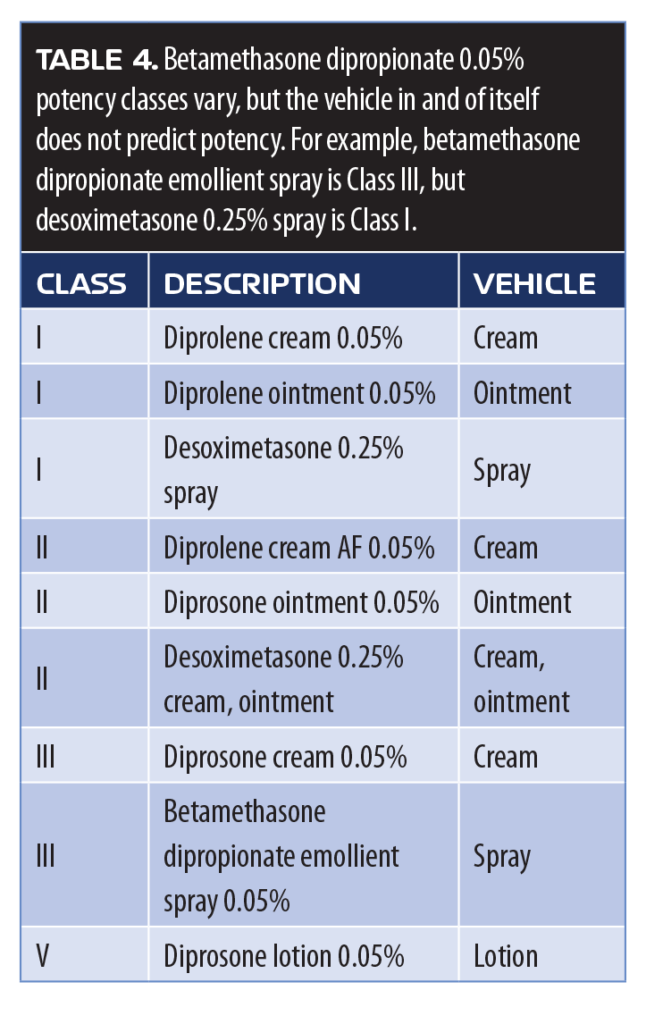
Topically administered corticosteroids, such as betamethasone dipropionate, have long been a cornerstone in psoriasis treatment, but their initial use in the 1960s focused on the active agent and neglected to give serious consideration to the vehicle. In fact, some clinicians might view claims about the vehicles used for topical products to be more marketing than actual science. However, topical products depend on adequate percutaneous penetration and effective permeation of the active ingredient for optimal results, which can be affected by the type of vehicle used. The product vehicle might also affect allergenicity, relative irritancy, and play a role in patient satisfaction, acceptance, and—in that way—might encourage patient adherence.39 The potency of topical corticosteroids is classified numerically (1-VII), with lower classes more potent than higher classes. However, potency is evaluated based on the vasoconstrictive properties of the molecule, and the same active ingredient might have different potency classes in different formulations (Table 4).
By the same token, potency does not necessarily predict efficacy. Since potency is rated by a vasoconstrictive assay (VCA)—that is, the degree of blanching in the lower levels of the skin—it serves as a good metric in terms of how much drug passes through the skin, but it does not necessarily reflect how much of the drug stays in the skin. Novel “designer” vehicles for topical psoriasis products might allow for a depot effect, which allows the steroid to penetrate the stratum corneum and then reside in a depot within the epidermis/dermis.
Betamethasone dipropionate. A study comparing a novel betamethasone dipropionate emollient spray (DFD-01) to super-potent augmented betamethasone dipropionate 0.05% lotion (diprolene) evaluated 351 adults with moderate plaque psoriasis with an affected mean body surface area (BSA) of 13 to 14 percent. The DFD-01 spray was specifically designed to allow for optimal steroid penetration into the epidermal layers. Treatment success was defined as Investigator Global Assessment (IGA) score of 0 or 1 and at least a two-grade improvement over baseline and improvement in the Total Sign Score (TSS), which sums erythema, scaling, and plaque elevation. Results found that the medium-potency DFD-01 optimized spray was similarly effective to super-potent diprolene lotion in this population, and in some patients the spray produced improvements in as early as eight days.40
Halobetasol propionate. Rapid effective treatment for plaque psoriasis was confirmed in a recent randomized, double-blind, Phase II study of 120 patients with plaque psoriasis assigned to one of three study arms: halobetasol propionate 0.01% lotion, halobetasol propionate 0.05% cream, or vehicle. Patients were treated once a day for two weeks. Treatment success was achieved in 30.0 percent of the patients treated with halobetasol propionate 0.01% lotion and 31.6 percent of those treated with halobetasol propionate 0.05% cream (not significant). Thus, the 0.02% lotion was similarly effective when compared to the higher-concentration cream product.42
Calcipotriene and betamethasone dipropionate combination approaches. Since the vehicle might play an important role in product effectiveness, developing a better vehicle could potentially improve treatment outcomes. According to mathematical modeling done in the 19th century (Frick’s Law), penetration of the agent is directly related to the amount of active drug dissolved in the product. Foam formulations incorporate propellants and can more fully dissolve the active ingredients, enhancing their penetration through the skin while minimizing systemic absorption. An aerosol foam fixed combination product of calcipotriene 0.005% and betamethasone dipropionate 0.064% was significantly more effective in treating patients with psoriasis vulgaris than calcipotriene and betamethasone dipropionate ointment. At four weeks, more patients receiving the foam treatment achieved treatment success than those receiving the ointment (p=0.005).43 This conflicts with the conventional thinking that ointments offer superior effectiveness compared to other vehicles.
A new vehicle design (PAD™ technology, mc2 therapeutics, Copenhagen, Denmark) was designed to offer 10- to 30-fold fewer surfactants compared to a traditional emulsion droplet. The inner substance is encapsulated by an aqueous film, and the oil-in-water design creates a concentration gradient that enhances penetration through the skin. The droplet design was created to enhance penetration and improve patient experience to drive greater adherence. This novel technology using a combination of calcipotriene and betamethasone in a cream formulation was compared to the same combination in a topical suspension. Adults with mild-to-moderate psoriasis vulgaris were enrolled for an eight-week trial after which there was a two-week follow-up phase. The cream product using the novel PAD™ technology exhibited a significantly higher rate of treatment success than the topical suspension.-41
Tazarotene combination approaches. Tazarotene is an established topical acetylenic retinoid used mainly in the treatment of acne vulgaris, but can be effective in the treatment of psoriasis.44 It is available in 0.1% and 0.05% cream and gel formulations. It works by inducing differentiation in keratinocytes and decreasing proliferation, and is known to have an anti-inflammatory effect.45,46 It must be used with caution due to its pregnancy category rating of X and its association with adverse events, including pruritus, burning, itching, peeling, and irritation.47 While tazarotene can be used as a monotherapy, it may also be used as part of a combination therapeutic approach with other agents. In an investigator-blinded study of 300 patients with psoriasis, tazarotene 0.1% gel combined with a moderately potent or high-potency corticosteroid topical product was significantly more effective in treating psoriasis than placebo at 12 weeks.48
A Phase II clinical study evaluated the safety and efficacy of halobetasol propionate combined with tazarotene lotion in 212 adults with moderate-to-severe plaque psoriasis. This analysis had four study arms: halobetasol propionate plus tazarotene lotion, halobetasol propionate lotion monotherapy, tazarotene lotion monotherapy, and placebo (vehicle only) over eight weeks with a four-week post-treatment follow-up. Combination therapy was significantly more effective at both eight and 12 weeks compared to monotherapy or vehicle only.49 There also appeared to be a synergistic effect of the fixed combination compared the individual ingredients used alone. In the two Phase III trials, the fixed combination was statistically superior to the vehicle at eight weeks (36%, 45% vs. 7%, and 13%). Efficacy was maintained at four weeks off treatment in 33 percent of patients.50
A new fixed-dose combination product of halobetasol propionate and tazarotene appears to provide safe, effective topical treatment for psoriasis.44 More patients reported an adverse event with the combination product than the vehicle alone (35.9% vs. 21.4%, respectively), and more patients in the combination group compared to the vehicle-only group discontinued treatment due to adverse events (6.3% vs. 3.6%, respectively).51 The drug combination of halobetasol propionate and tazarotene appears to offers synergistic treatment benefits to patients that persist up to four weeks after discontinuation of treatment.52
Tapinarof. Recent studies of tapinarof, a novel, nonsteroidal, topical molecule, show promise. Tapinarof belongs to a class of anti-inflammatory agents known as therapeutic aryl hydrocarbon receptor (AhR) modulators. The molecule binds the AhR directly, activating the body’s AhR pathways, which indirectly reduce IL-17A expression. The transcription factor AhR controls the expression of IL-21 and IL-22. Tapinarof also acts as an antioxidant by inhibiting reactive oxygen species (ROS). As such, tapinarof has a unique mechanism of action among topical agents for treating psoriasis. In a double-blind, vehicle-controlled, randomized, six-arm study, 65 percent of patients in the tapinarof 1% twice-daily group, 56 percent of tapinarof 1% once-daily group, 46 percent of tapinarof 0.5% twice-daily group, and 36 percent of tapinarof 0.5% once-daily group, compared to vehicle alone (once daily and twice daily, 5% and 11%, respectively), achieved significantly better results, defined as PGA 0 or 1 (and an improvement of ?2 grades) in 12 weeks.53 Efficacy persisted up to four weeks after treatment stopped.
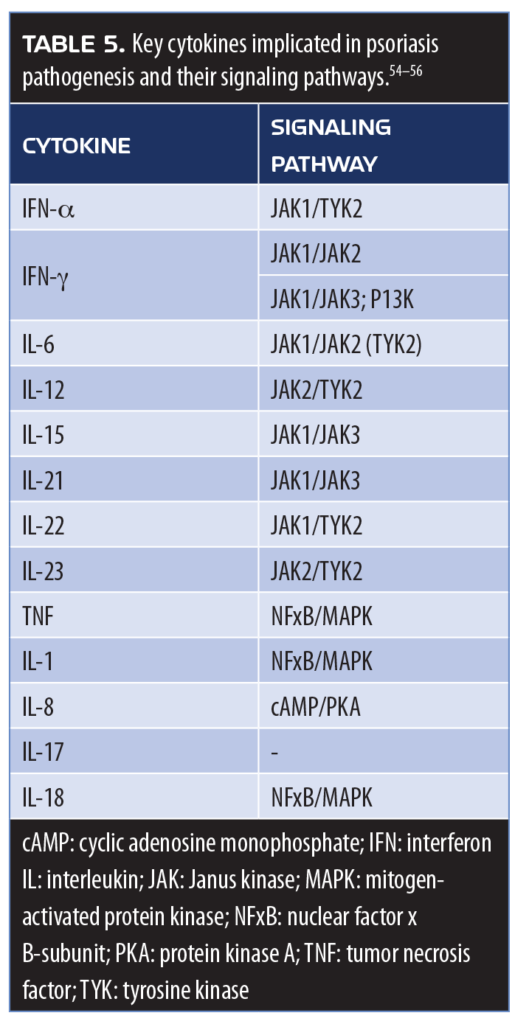
JAK1/JAK2 inhibitors. Many proinflammatory cytokines implicated in the pathogenesis of psoriasis use JAK pathways (Table 5).54–56 Topical treatments that down-regulate key inflammatory cell markers using an agent that inhibits JAK1/JAK2 are currently under investigation. INCB018424 is a novel JAK1/JAK2 inhibitor available as a 1.0% and 1.5% cream designed to block the signal transduction of multiple pro-inflammatory cytokines. Early clinical study results are encouraging. This underscores the fact that new molecules in topical therapy for psoriasis show promise and that older, established therapies, such as tazarotene, still hold a place in the psoriasis armamentarium.
New Developments in Treating Moderate-to-severe Psoriasis
Based on a presentation by Craig L. Leonardi, MD, Associate Clinical Professor of Dermatology with the St. Louis University Medical School in St. Louis, Missouri
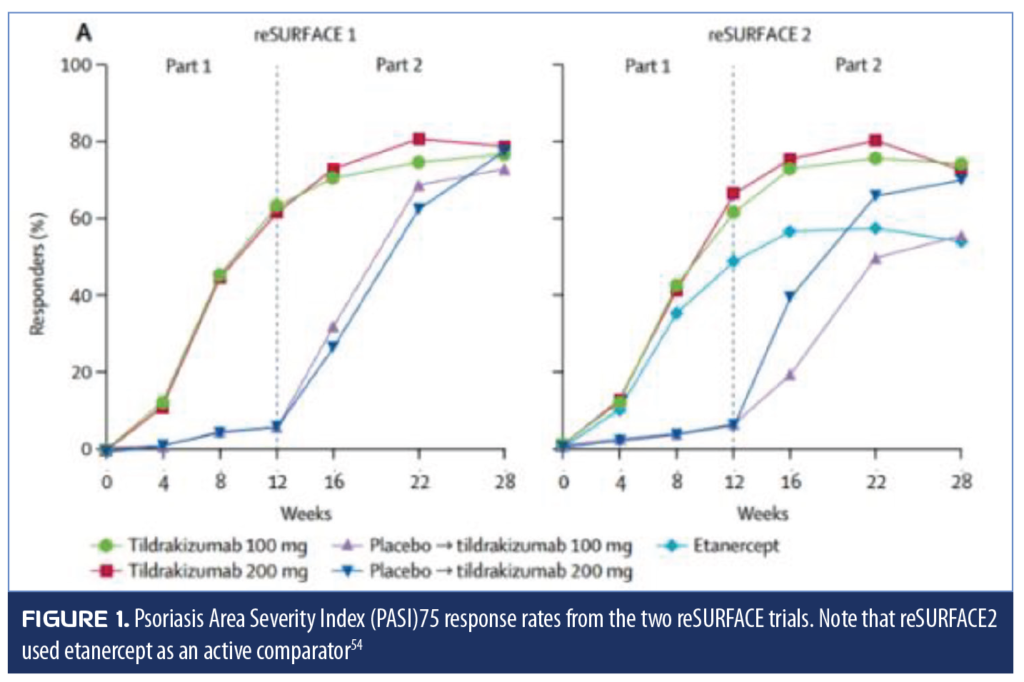
Tildrakizumab. Tildrakizumab is a high-affinity, humanized immunoglobulin G1 (igG1) monoclonal antibody that targets the IL-19 subunit of IL-23. Two large, randomized, controlled trials (reSURFACE 1 and reSURFACE 2) were conducted to assess the safety, efficacy, and tolerability of tildrakizumab in patients with moderate-to-severe plaque psoriasis.57 The multicenter trials were three-part, double-blind, parallel-group studies that randomized patients to one of the following arms: tildrakizumab 200mg, tildrakizumab 100mg, or placebo (reSURFACE 1); or tildrakizumab 200mg, tildrakizumab 100mg, etanercept 50mg, or placebo (reSURFACE 2). Tildrakizumab was administered to patients by subcutaneous injection at baseline (Week 0) and Week 4 during Part 1 of the trial and again at Week 16 during Part 2 of the trial. In the second study, the active comparator etanercept was administered twice a week in Part 1 and once a week in Part 2. Primary endpoints were the percentage of patients who achieved PASI75 and a PGA response of 0 or 1 with an improvement of at least two grades.57 By Week 12, 62 and 64 percent of the tildrakizumab 200mg and 100mg groups, respectively, had achieved PASI75, compared to six percent of patients receiving the placebo (p<0.0001). In the second trial, at 12 weeks, PASI75 was achieved by 66 and 61 percent of tildrakizumab 200mg and 100mg groups, respectively, compared to 48 percent of patients receiving etanercept and six percent of patients receiving the placebo (p<0.0001 for tildrakizumab groups vs. placebo). Tildrakizumab offered statistically significantly superior results when evaluated against the active comparator etanercept (p<0.0001 for tildrakizumab 200mg vs. etanercept and p=0.0010 for tildrakizumab 100mg vs. etanercept).57 The PGA endpoint was achieved by 59 and 55 percent of patients in the tildrakizumab 200mg and 100mg groups, respectively, and by four percent of placebo patients (p<0.0001 for both tildrakizumab arms compared to placebo) and by 48 percent of patients receiving etanercept (p=0.0031 and p=0.0663 for tildrakizumab 200 and 100mg, respectively, vs. etanercept) (Figure 1).57
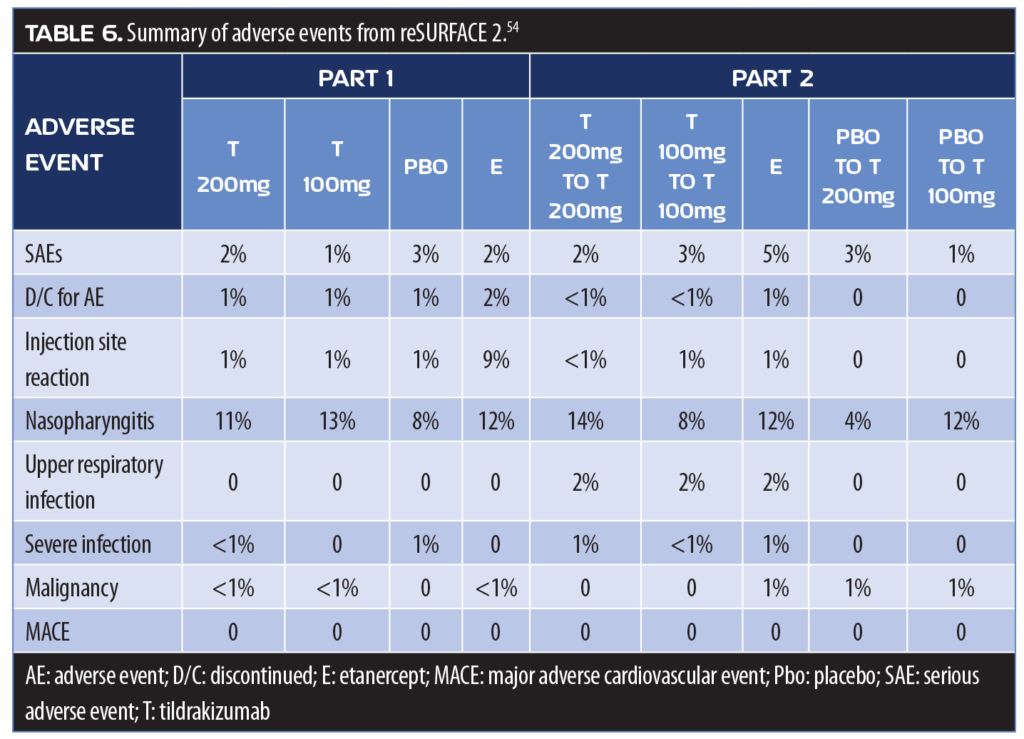
Adverse events for the reSURFACE 2 study were reported in 49 and 44 percent of patients receiving tildrakizumab 200mg and 100mg, respectively, in Part 1 and 45 and 46 percent, respectively, in Part 2. Adverse events with etanercept occurred in 54 percent of patients in Part 1 and 57 percent in Part 2.
The rate of serious adverse events was low in all groups and were similar. In both parts, one percent or fewer of patients receiving tildrakizumab or placebo discontinued the study due to adverse events; two percent of patients receiving etanercept discontinued due to adverse events in Part 1, and one percent terminated their participation due to adverse events in Part 2 (Table 6).57
Risankizumab. Like tildrakizumab, risankizumab is a humanized IgG1 monoclonal antibody that inhibits IL-23 by binding to the IL-19 subunit. In head-to-head, placebo-controlled clinical trials comparing risankizumab to ustekinumab (an IL-12 and IL-23 inhibitor), risankizumab demonstrated superior efficacy both to ustekinumab and placebo in treating moderate-to-severe plaque psoriasis.58 These two studies, UltiMMa-1 (n=506) and UltiMMa-2 (n=577), had a relatively high proportion of patients (34% to 41%) with prior exposure to biologics, which suggests a population of more treatment-resistant patients. The co-primary endpoints were PASI90 and sPGA “clear” or “almost clear.” At 16 weeks, 73.2 percent of patients risankizumab had achieved PASI90 compared to 42 percent of patients receiving ustekinumab and 2.0 percent of patients receiving placebo; 83.5 percent were “clear” or “almost clear” on sPGA compared to 63 percent of ustekinumab and 7.0 percent of patients receiving placebo.58 At 16 weeks, 47.2 percent of patients receiving risankizumab achieved PASI100. Among treatment-emergent adverse events, there were no cases of serious infection, active or latent tuberculosis, serious hypersensitivity, or MACE with risankizumab compared to one case each of serious infection, latent tuberculosis, and adjudicated MACE in the placebo arm; there were three cases of malignancies (n=3) with risankizumab and none in the placebo group.
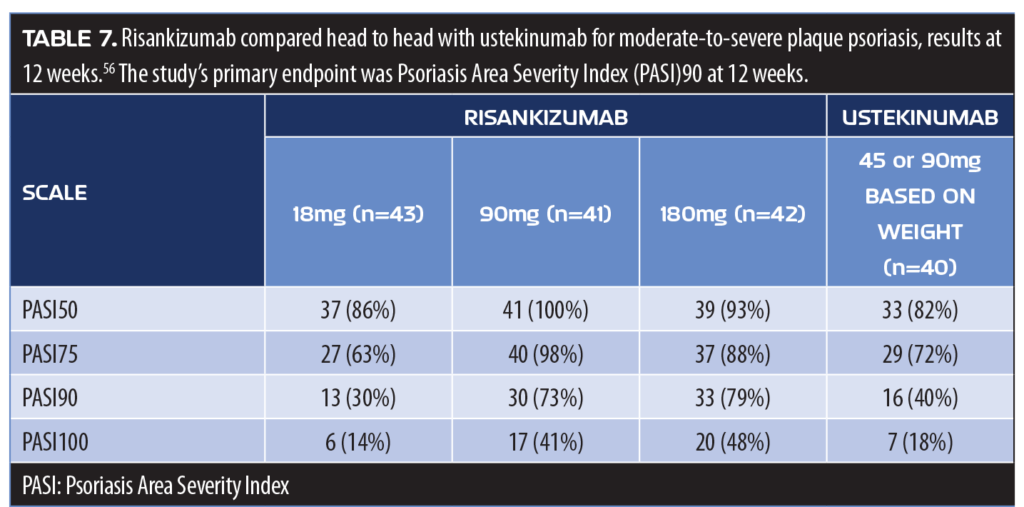
In a head-to-head clinical study, 166 adults with moderate-to-severe plaque psoriasis were randomized to receive either subcutaneous risankizumab (one 18mg dose at Week 0; or 90 or 180mg doses at Weeks 0, 4, and 16) or ustekinumab 45 or 90mg based on body weight at Weeks 0, 4, and 16.59 At 12 weeks, 77 percent of patients receiving risankizumab in the 90 and 180mg groups (pooled) and 40 percent of patients receiving ustekinumab achieved PASI90 (p<0.001) (Table 7). Patients receiving risankizumab (90 and 180mg results pooled) were also more likely to achieve PASI100 compared to patients receiving ustekinumab (45% vs. 18%, respectively). Serious adverse events were reported in the risankizumab group (12% of 18mg, 0% of 90mg, and 15% of 180mg groups) and in eight percent of patients receiving ustekinumab.59
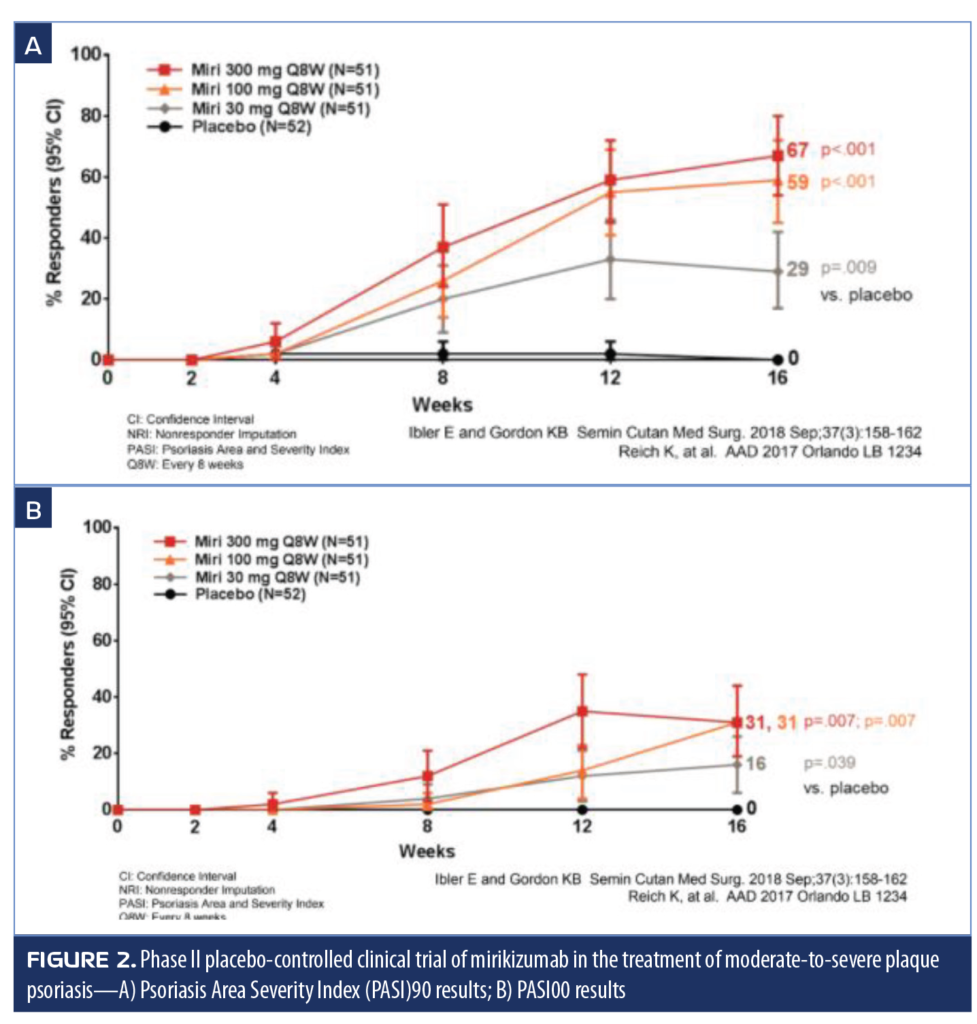
Mirikizumab. Mirikizumab is a humanized monoclonal antibody that binds to the IL-19 subunit of IL-23 and has recently completed Phase II clinical dose-ranging studies for the treatment of moderate-to-severe plaque psoriasis. In this study, 205 adult patients were randomized to 1 of 4 arms: mirikizumab 300mg every eight weeks (Q8W); 100mg Q8W; 30mg Q8W; or placebo.60 At 16 weeks, 67 percent of patients in the 300mg group achieved PASI90 (p<0.001 vs. placebo), and 31 percent achieved PASI100 (p=0.007 over placebo, placebo rate 0) (Figure 2). Treatment-emergent adverse events occurred in 48.1 percent of patients receiving the placebo and 47.1 to 51.0 percent of patients receiving mirikizumab.
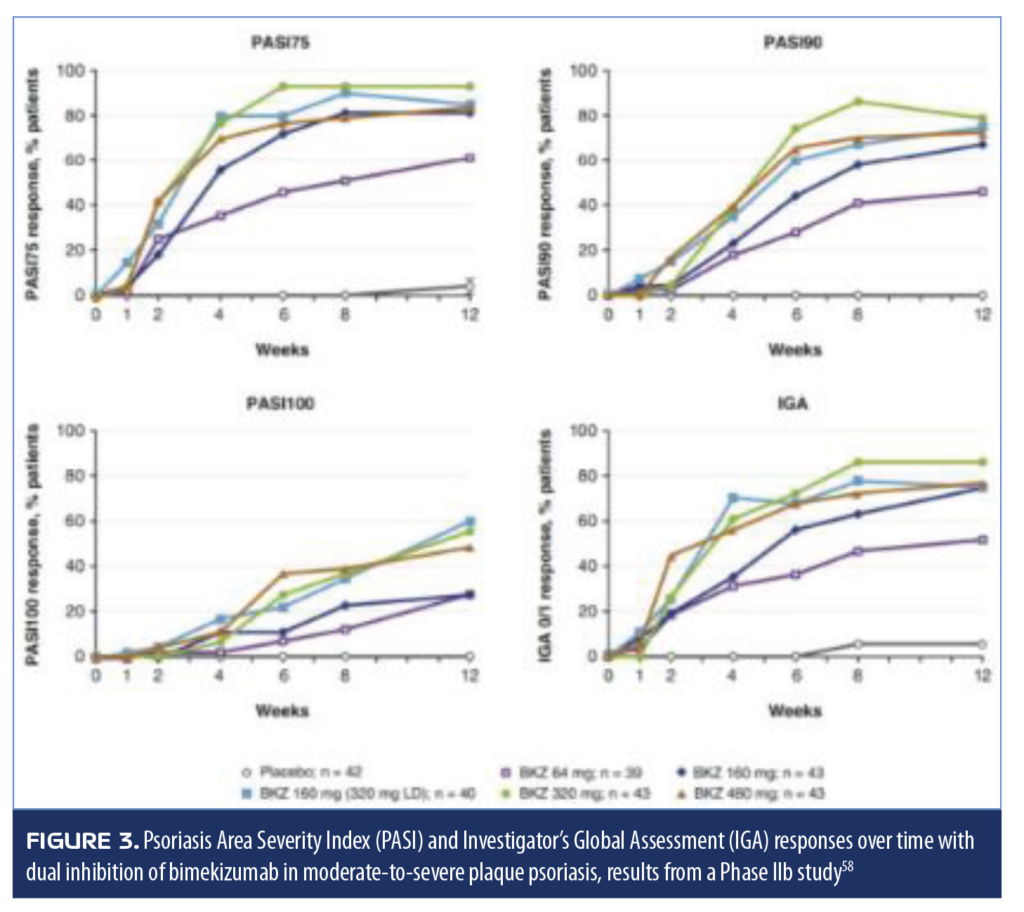
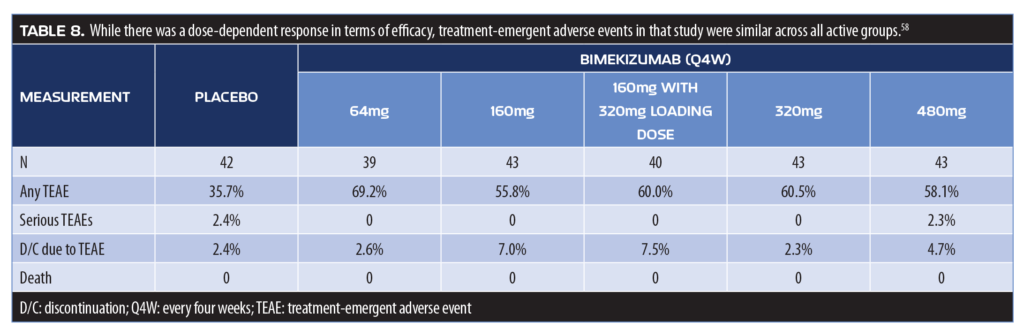
Bimekizumab. Bimekizumab is a monoclonal antibody that selectively inhibits IL-17A and IL-17F and has recently completed a randomized, double-blind, Phase IIb clinical study. It was developed based on the concept that the interplay between IL-17A and IL-17F might contribute to inflammation, and thus the inhibition of both of these cytokines would provide a more complete response to psoriasis than other biologics. Adult patients with moderate-to-severe plaque psoriasis (N=250) were randomized to 1 of 5 arms to receive placebo or subcutaneous injections of bimekizumab of 64mg; 160mg; 160mg plus a 320mg loading dose; 320mg; and 480mg every four weeks.61 The primary endpoint of this 12-week study was achievement of PASI90. The study found a dose-dependent response in meeting the primary endpoint, and all bimekizumab groups had significantly more patients achieve PASI90 than placebo (no placebo patient had PASI90, p<0.0001, all doses). No patient receiving placebo achieved PASI100, but 27.9 to 60.0 percent of patients receiving bimekizumab did (p?0.0002, all doses) (Figure 3). Treatment-emergent adverse events were reported by 61 percent of patients receiving bimekizumab and 36 percent of patients receiving placebo
(Table 8).61
Tyrosine kinase 2 (Tyk2) enzyme. One of the key targets in drug development for the treatment of a variety of autoimmune diseases, such as psoriasis, rheumatoid arthritis, multiple sclerosis, Crohn’s disease, and others, has been the tyrosine kinase 2 (Tyk2) enzyme, which serves as an important signal-transduction kinase for certain pro-inflammatory cytokine receptors associated with IL-23, IL-12, and interferon-? and –?.62 Certain types of cancer seem to be driven by hyperactivation of Tyk2 and, conversely, respond to Tyk2 inhibition.63 It should be noted that Tyk2 signaling pathways are distinct from JAK pathways.
BMS-986165 is a novel oral Tyk2 inhibitor that binds to the pseudokinase domain of the enzyme and has recently been evaluated in a Phase II, double-blind trial in patients with moderate-to-severe plaque psoriasis who had not responded to other agents targeting cytokine signaling mechanisms.64 Patients (N=267) were randomized to one of six groups: 3mg of BMS-986165 every other day, 3mg daily, 3mg twice a day, 6mg twice a day, 12mg daily, or placebo. At 12 weeks, 75 percent of the 12mg group, 67 percent of the 6mg twice daily group, 69 percent of the 3mg twice daily group, and 39 percent of the 3mg daily group achieved PASI75 versus seven percent of patients receiving placebo (p<0.001 for each group compared to placebo); nine percent of patients in the 3mg every other day group achieved PASI75, which was not statistically significantly superior to placebo. Adverse events were reported in 51 percent of placebo and 55 to 80 percent of patients in the active treatment group. Adverse events that caused patients to discontinue study participation occurred in four percent of placebo and 2 to 7 percent of patients in the active treatment group. Five serious adverse events were reported (n=4), two of which occurred in patients receiving placebo and one each in groups who were administered 3mg every other day (gastroenteritis due to rotavirus), 3mg daily (accidental eye injury), and 3mg twice a day (dizziness due to vestibular dysfunction in a patient with a history of this condition). In one patient, a malignant melanoma in situ (Stage 0) or typical nevus was diagnosed on skin biopsy after the patient received a first dose of 3mg; that patient was in the 3mg daily group.64
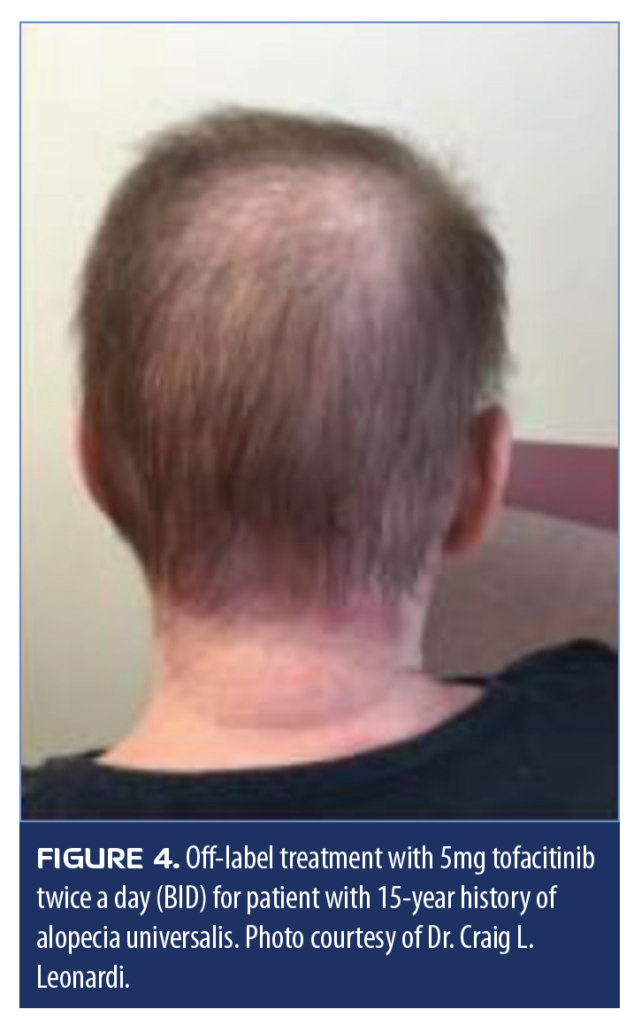
Tofacitinib. Tofacitinib is a JAK inhibitor that is being used off-label to treat alopecia areata,65 nail dystrophy associated with alopecia universalis,66 severe atopic dermatitis,67 and vitiligo.68 A 42-year-old female Caucasian patient with severe atopic dermatitis and a 15-year history of alopecia universalis, managed on a regimen of 30mg prednisone a day, experiencing complete adrenal axis suppression for 25 years, was effectively treated with 5mg tofacitinib twice a day (Figure 4). Zoster must be considered in such patients as a potential adverse event.
Head-to-head trials. Of special interest are recent head-to-head trials that can provide guidance to clinicians seeking optimal therapy for patients with plaque psoriasis (Table 9). In some cases, head-to-head study results might appear in package inserts, as is the case with TALTZ®, the labeling of which offers study results from an active comparator trial with etanercept.
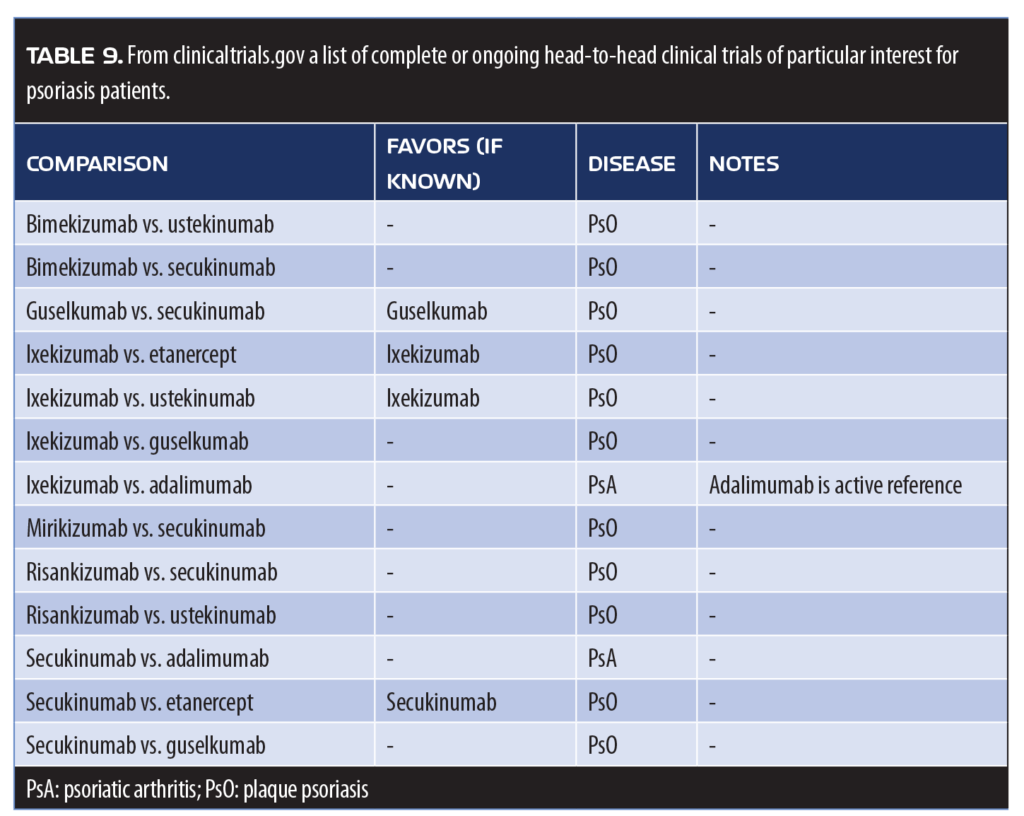
Of particular interest are the results of the IXORA trial (ixekizumab vs. ustekinumab) and the Eclipse study, in which guselkumab was compared head-to-head with secukinumab for 48 weeks. The IXORA trial randomized all patients to receive either ixekizumab (at a starting dose of 160mg, then 80mg every two weeks for 12 weeks, then 80mg every four weeks for the duration of the study) or ustekinumab (weight-based doses of 45 or 90mg). The primary endpoint was PASI90 at 12 weeks, which was achieved by 72.8 percent of patients receiving ixekizumab compared to 42.2 percent of patients receiving ustekinumab (p<0.001). Response rates for PASI75, PASI100, and sPGA (0 or 1) were significantly higher for ixekizumab compared to ustekinumab (p<0.05 for all). Adverse event rates were similar between study arms, and no deaths occurred in the study. The superior effectiveness of ixekizumab was maintained through Week 24.69 Results of the study extension to 52 weeks are shown in Figure 5.
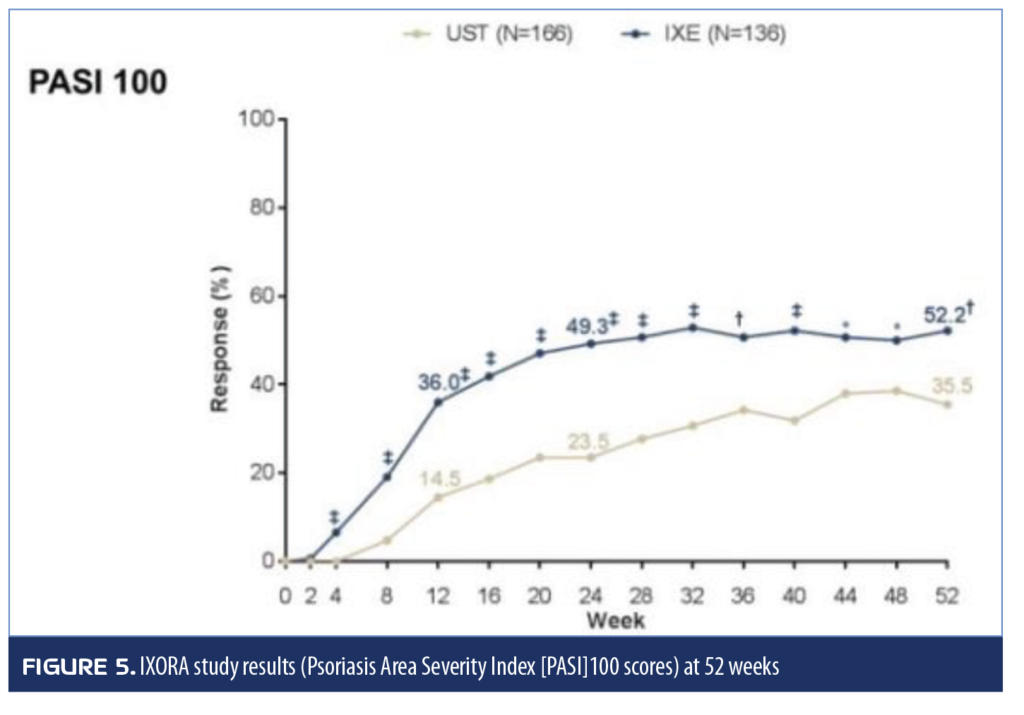
Guselkumab was compared to secukinumab in the Eclipse head-to-head trial, the results of which are not yet published in full article format. A total of 1,048 patients with plaque psoriasis were enrolled and randomized to receive guselkumab 100mg at Weeks 0, 4, and 12 then one dose every eight weeks while secukinumab patients received 300mg at Weeks 0, 1, 2, 3, and 4, and then one dose every four weeks. The primary endpoint was PASI90 rates at 48 weeks, which was achieved by 84.5 percent of patients receiving guselkumab and 70.0 percent of patients receiving secukinumab (p<0.001).70 A summary of secondary endpoints from the Eclipse study appears in Table 10.
Treating troublesome areas. Recent study results might help guide psoriasis treatment for particularly difficult-to-treat areas of the body, such as palms of the hand, soles of the feet, nails, and genitals. A randomized, controlled trial (GESTURE) found secukinumab to be effective in the treatment of palmoplantar psoriasis (n=205). At 16 weeks, 33.1 percent (300mg) and 22.1 percent (150mg) of patients receiving secukinumab achieved clear or almost clear palms and soles compared to 1.5 percent of patients receiving placebo (p<0.001). The safety of secukinumab in this study was similar to results from other secukinumab studies.71
A double-blind placebo-controlled study comparing etanercept (50mg twice weekly) to ixekizumab (80mg injection every 2 or 4 weeks with a 160mg starting dose) found significant improvements in fingernail psoriasis at 12 weeks with ixekizumab and results improved in 60 weeks with maintenance ixekizumab injections every four weeks. At 60 weeks, more than half of the patients (both groups) achieved complete resolution of nail psoriasis.72
There are few controlled studies of genital psoriasis treatment, but a recent randomized double-blind study compared ixekizumab to placebo in 149 patients with moderate-to-severe genital psoriasis with a BSA involvement of one percent or more. At 12 weeks, ixekizumab was superior to placebo for achievement of static physician’s global assessment of genitalia (sPGA-G) of 0 or 1, with 73 percent of ixekizumab patients achieving the endpoint compared to eight percent of patients receiving placebo (p<0.001). Genital itch as also reduced in 60 percent of patients receiving ixekizumab versus eight percent of patients receiving placebo (p<0.001). One serious adverse event was reported (placebo patient) and no deaths or candidiasis occurred.73
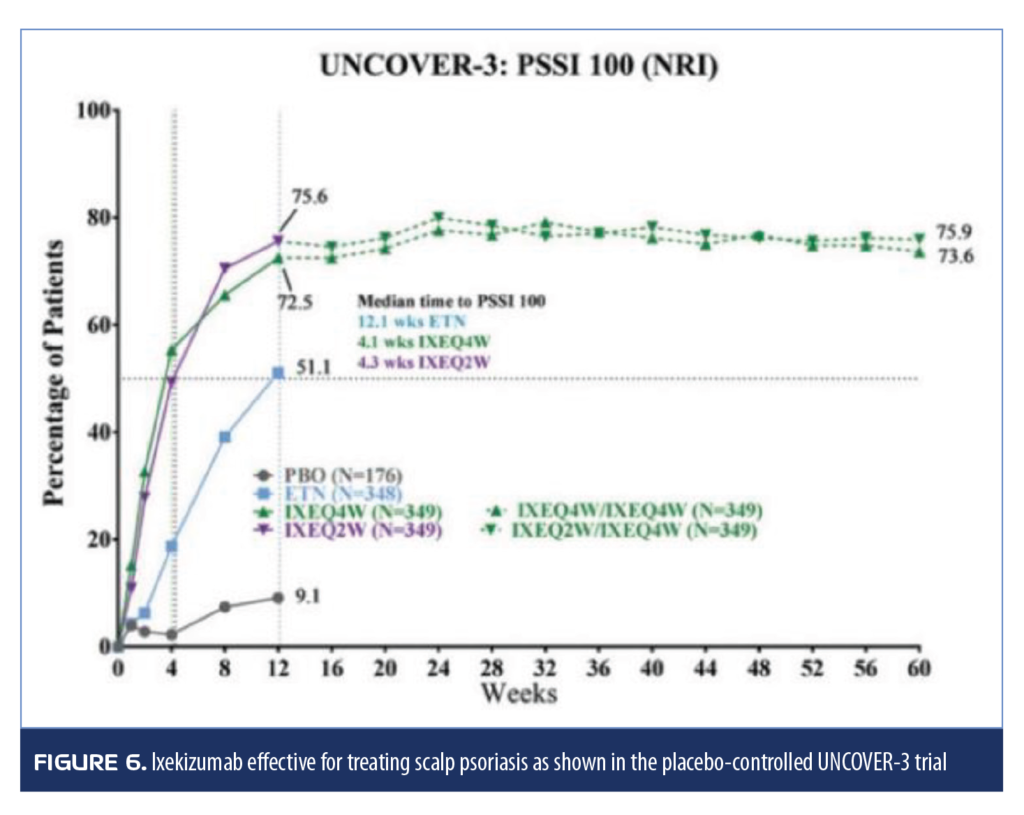
Three clinical trials (UNCOVER 1, 2, and 3) with 1,296; 1,224; and 1,346 adult patients with moderate-to-severe psoriasis, respectively, analyzed the effectiveness of ixekizumab (80mg injection every 2 or 4 weeks following an initial dose of 160mg) compared to placebo at 12 weeks. In two of the trials (UNCOVER 2 and 3), patients could be randomized to a 50mg biweekly dose of etanercept. After 12 weeks, UNCOVER-3 patients could enter the long-term extension phase and receive ixekizumab every four weeks. The long-term extension phase of the other two studies was a blinded maintenance phase in which patients were randomized to ixekizumab 80mg every four weeks or every 12 weeks for 60 weeks. In all studies, ixekizumab was more effective than placebo in treating scalp psoriasis, with most patients achieving complete or almost complete resolution of scalp psoriasis at 12 weeks and maintaining this response for 60 weeks (Figure 6).74
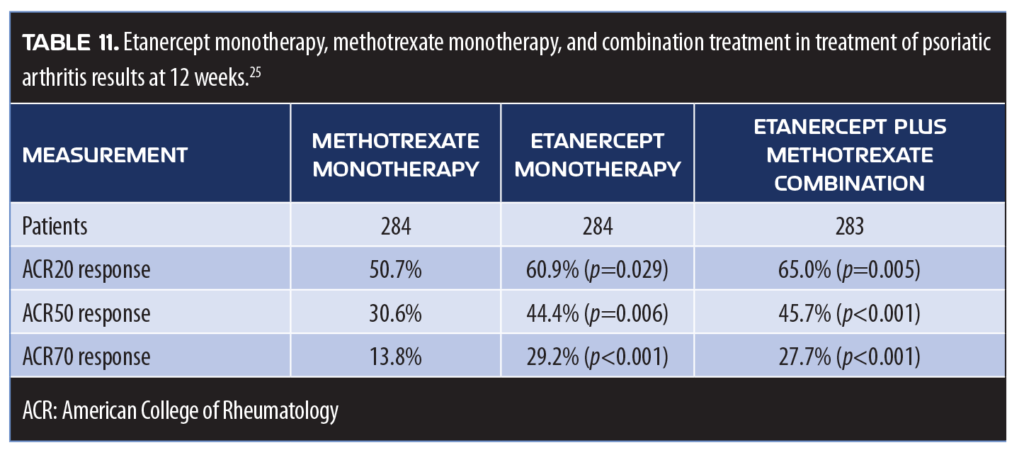
Psoriatic arthritis. An important head-to-head study comparing etanercept to methotrexate in the treatment of psoriatic arthritis (PsA) was recently published in Arthritis Rheumatology. This placebo-controlled study used etanercept and methotrexate as active monotherapeutic options, as well as in combination to treat 851 patients with PsA. The ACR20 and MDA response at 24 weeks were the main study endpoints and etanercept monotherapy and etanercept/methotrexate therapy resulted in superior ACR and MDA responses, as well as improvements in radiographic progression. Combination therapy was similarly effective to etanercept monotherapy, that is the addition of methotrexate offered no significant or synergistic benefit to etanercept monotherapy (Table 11).27
Conclusion
The old stepwise treatment paradigm for psoriasis has become outmoded. In the old model, psoriasis was treated first with over-the-counter (OTC) topicals, then prescription topicals, then phototherapy, in that order, and it was only when phototherapy failed that patients advanced to systemic treatments. Evidence backs the new treatment paradigm, which trials topical therapy first and then, if that fails, advances to any of three major treatment plans: biologics, traditional systemics, or phototherapy. This more individualized approach is safe and effective but requires clinicians to know a wide range of available treatments to tailor therapy for each individual patient.
References
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a Phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49.
- Rich P, Gooderham M, Bachelez H, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 Phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74(1):134–142.
- Kavanaugh A, Mease P, Gomez-Reino J, et al. Treatment of psoriatic arthritis in a Phase III randomised, placebo-controlled trial with apremilast, an oral phosophodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):
1020–1026. - Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey,
2003–2006. Arch Dermatol. 2011;147(4):419–424. - Haroon M, Gallagher P, Heffernan E, FitzGerald O. High prevalence of metabolic syndrome and of insulin resistance in psoriatic arthritis is associated with the severity of underlying disease. J Rheumatol. 2014;41(7):1357–1365.
- Bhole VM, Choi HK, Burns LC, et al. Differences in body mass index among individuals with PsA, psoriasis, RA and the general population. Rheumatology (Oxford). 2012;51(3):552–556.
- Puig L, Korman N, Greggio C, et al. Hemoglobin A1c and weight changes with apremilast in patients with psoriasis and psoriatic arthritis: pooled laboratory analysis of the Phase III ESTEEM and PALACE trials. Poster presented at SDPA 16th Annual Fall Conference; Nov 1–4, 2018; Orlando, FL.
- Fang N, Jiang M, Fan Y. Association between psoriasis and subclinical atherosclerosis: a meta-analysis. Medicine. 2016;95(20):e3576.
- Robati RM, Partovi-Kia M, Haghighatkhah HR, et al. Increased serum leptin and resistin levels and increased carotid intima-media wall thickness in patients with psoriasis: is psoriasis associated with atherosclerosis? J Am Acad Dermatol. 2014;71(4):642–648.
- Ibanez-Bosch R, Restrepo-Velez J, Medina-Malone M, et al. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients: a study based on carotid ultrasound. Rheumatol Int. 2017;37(1):107–112.
- Cicero-Alvarez A, Leon-Hernandez SR, Gutierrez-Enriquez K, Zapata-Rivera S. [Prognostic factors for post surgical complications in bone infection and pseudarthrosis]. Acta Ortopedica Mexicana. 2016;30(5):236–240.
- Jokai H, Szakonyi J, Kontar O, et al. Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol. 2013;69(4):523–529.
- Acher C, Acher CW, Marks E, Wynn M. Intraoperative neuroprotective interventions prevent spinal cord ischemia and injury in thoracic endovascular aortic repair. J Vasc Surg. 2016;63(6):1458–1465.
- Martinez-Lopez A, Blasco-Morente G, Perez-Lopez I, et al. Studying the effect of systemic and biological drugs on intima-media thickness in patients suffering from moderate and severe psoriasis. J Eur Acad Dermatol Venereol. 2018;32(9):1492–1498.
- Lee WK and Kim BS. Effects of ustekinumab on systemic/vascular inflammation in Korean patients with moderate to severe psoriasis assessed by FDG PET/CT. Poster presented at American Academy of Dermatology 75th Annual Meeting: March 3–7, 2017; Orlando, FL. Abstract p5608.
- Dermatology Advisor site. Psoriasis treatment ustekinumab reduces vascular inflammation. 27 Feb 2018. https://www.dermatologyadvisor.com/home/meetings/aad-2018/psoriasis-treatment-ustekinumab-reduces-vascular-inflammation/ Accessed 19 Aug 2019.
- Li HH, Lin YC, Chen PJ, et al. Interleukin-19 upregulates keratinocyte growth factor and is associated with psoriasis. Br J Dermatol. 2005;153(3):591–595.
- Bachelez H, Choon S, Marrakchi S, et al. Efficacy and safety of BI 655130, an anti-interleukin-36 receptor antibody, in patients with acute generalized pustular psoriasis. Poster presented at EADV Congress; September 12–16, 2018; Paris, France.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178(3):614–618.
- Rich P, Bourcier M, Sofen H, et al. Ustekinumab improves nail disease in patients with moderate-to-severe psoriasis: results from PHOENIX 1. Br J Dermatol. 2014;170(2):398–407.
- Palmer W. EADV report shows that ixekizumab effectively treats nail psoriasis. 14 Sep 2018. Dermatology Times site. https://www.dermatologytimes.com/eadv/eadv-report-shows-ixekizumab-effectively-treats-nail-psoriasis. Accessed 19 Aug 2019.
- Langley R, Blauvelt A, Armstrong A, et al. Guselkumab demonstrates superior long-term responses to secukinumab at Week 48 in the treatment of moderate to severe psoriasis: Results from the ECLIPSE trial. Poster presented at 3rd Annual Inflammatory Skin Disease Summit; December 12–15, 2018: Vienna, Austria.
- Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–2284.
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Eder L, Widdifield J, Rosen CF, et al. Trends in the prevalence and incidence of psoriasis and psoriatic arthritis in Ontario, Canada: a population-based study. Arthritis Care Res (Hoboken). 2019;71(8):1084–1091.
- Merola JF, Ogdie A. SEAM-PsA: Seems like methotrexate works in psoriatic arthritis? Arthritis Rheumatol. 2019;71(7):1027–1029
- Mease PJ, Gladman DD, Collier DH, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled Phase III trial. Arthritis Rheumatol. 2019;71(7):1112-1124
- Baeten D, Ostergaard M, Wei JC, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding Phase II study. Ann Rheum Dis. 2018;77(9):1295–1302.
- Kirkham BW, Kavanaugh A, Reich K. Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology. 2014;141(2):133–142.
- Ritchlin C, Kavanaugh A, Merola J, et al. Dual neutralization of IL-17A and IL-17F with bimekizumab in patients with active PsA: Results from a 48-week Phase IIb, randomized, double-blind, placebo-controlled, dose-ranging study. Poster presented at American College of Rheumatology Meeting 2018; October 19–24, 2018: Chicago, IL.
- Kampylafka E, Oliveira I, Linz C, et al. Disease interception in psoriasis patients with subclinical joint inflammation by interleukin 17 inhibition with secukinumab—data from a prospective open label study. Poster presented at American College of Rheumatology Meeting 2018; October 19–24, 2018: Chicago, IL
- van der Heijde D, JC W, Dougados M, et al. Ixekizumab significantly improves signs, symptoms, and spinal inflammation of active ankylosing spondylitis/radiographic axial spondyloarthritis: 16-week results of a Phase III randomized, active and placebo-controlled trial. Poster presented at American College of Rheumatology Meeting 2018; October 19–24, 2018: Chicago, IL
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol. 2018;19(7):755–765.
- Hall A, Towne K, Plevy S. Get the IL-17F outta here! Nat Immunol. 2018;19:648–650.
- Maneiro J, Souto A, Salgado E, et al. Predictors of response to TNF antagonists in patients with anklosing spondylitis and psoriatic arthritis: systematic review and meta-analysis. RMD Open. 2015;1:e000017.
- Miyagawa I, Nakayamada S, Tanaka Y. Optimal biologic selection for treatment of psoriatic arthritis: the approach to precision medicine. Curr Rheumatol Rep. 2019;21(5):21.
- Garcet S, Nograles K, Correa da Rosa J, et al. Synergistic cytokine effects as apremilast response predictors in patients with psoriasis. J Allergy Clin Immunol. 2018;142(3):
1010–1013.e6. - Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):
1525–1536. - Del Rosso JQ, Kircik LH, Zeichner J, Stein Gold L. The clinical relevance and therapeutic benefit of established active ingredients incorporated into advanced foam vehicles: vehicle characteristics can influence and improve patient outcomes. J Drugs Dermatol. 2019;18(2s):s100–s107.
- Fowler JF, Jr., Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15(2):154–162.
- MC2 Therapeutics Announces Positive Top-line Results from Phase 3 Clinical Trial Comparing MC2-01 Cream to Taclonex® in Adults with Psoriasis. Business Wire site. 21 Aug 2019. https://www.businesswire.com/news/home/20180820005642/en/. Accessed 19 Aug 2019.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A Phase II, multicenter, double-blind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis. J Dermatolog Treat. 2018:1–7.
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris—a randomized Phase II study. J Dermatolog Treat. 2016;27(2): 120–127.
- Tanghetti E, Lebwohl M, Stein Gold L. Tazarotene revisited: safety and efficacy in plaque psoriasis and its emerging role in treatment strategy. J Drugs Dermatol. 2018;17(12):1280–1287.
- Weinstein GD. Tazarotene gel: efficacy and safety in plaque psoriasis. J Am Acad Dermatol. 1997;37(2 Pt 3):S33–38.
- Duvic M, Nagpal S, Asano AT, Chandraratna RA. Molecular mechanisms of tazarotene action in psoriasis. J Am Acad Dermatol. 1997;37(2 Pt 3):S18–24.
- Guenther LC. Optimizing treatment with topical tazarotene. Am J Clin Dermatol. 2003;4(3):197–202.
- Lebwohl MG, Breneman DL, Goffe BS, et al. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;39(4 Pt 1):590–596.
- Stein Gold L, Bagel J, Lebwohl M, et al. Halobetasol and tazarotene: further defining the role of a unique fixed combination topical lotion in moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2018;17(12):1290–1296.
- Stein Gold L, Kircik LH, Pariser D, et al. Rapid onset of action in patients with moderate-to-severe plaque psoriasis with halobetasol 0.01%/tazarotene 0.045% fixed combination. J Drugs Dermatol. 2018;17(8):863–868.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two Phase III studies. J Drugs Dermatol. 2018;17(8):
855–861. - Pariser DM, Green LJ, Stein Gold L, et al. Halobetasol 0.01%/tazarotene 0.045% lotion in the treatment of moderate-to-severe plaque psoriasis: maintenance of therapeutic effect after cessation of therapy. J Drugs Dermatol. 2018;17(7):723–726.
- Robbins K, Bissonnette R, Maeda-Chubachi T, et al. Phase II, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. J Am Acad Dermatol. 2019;80(3):714–721.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
- O’Sullivan LA, Liongue C, Lewis RS, et al. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Mol Immunol. 2007;44(10):2497–2506.
- Wojas-Pelc A, Ciszek M, Kurnyta M, Marcinkiewicz J. Cytokine network in psoriasis. Crosstalk between keratinocytes and cells. Cent Eur J Immunonology. 2006;31(3-4):111–116.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, Phase III trials. Lancet. 2017;390(10091):276–288.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled Phase III trials. Lancet. 2018;392(10148):650–661.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–1560.
- Ibler E, Gordon KB. IL-23 inhibitors for moderate-to-severe psoriasis. Semin Cutan Med Surg. 2018;37(3):158–162.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled Phase IIb trial. J Am Acad Dermatol. 2018;79(2):277–286.e210.
- Miao W, Masse C, Greenwood J, et al. Potent and selective Tyk2 inhibitor highly efficacious in rodent models of inflammatory bowel disease and psoriasis. Arthritis Rheumatol. 2016;68:Abstract 1911.
- Miao R, Wu Y, Zhang H, et al. Utility of the dual-specificity protein kinase TTK as a therapeutic target for intrahepatic spread of liver cancer. Sci Rep. 2016;6:33121.
- Papp K, Gordon K, Thaci D, et al. Phase II trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–1321.
- Falto-Aizpurua L, Choudhary S, Tosti A. Emerging treatments in alopecia. Expert Opin Emerg Drugs. 2014;19(4):545–556.
- Dhayalan A, King BA. Tofacitinib citrate for the treatment of nail dystrophy associated with alopecia universalis. JAMA Dermatol. 2016;152(4):492–493.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73(3):395–399.
- Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151(10):1110–1112.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a Phase III study. Br J Dermatol. 2017;177(4):1014–1023.
- Han D. Guselkumab compared with secukinumab in head-to-head plaque psoriasis trial. Monthly Prescribing Reference. https://www.empr.com/home/news/guselkumab-compared-with-secukinumab-in-head-to-head-plaque-psoriasis-trial/. Published December 12, 2018. Accessed April 26, 2019.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: Results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70–80.
- van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31(3):477–482.
- Ryan C, Menter A, Guenther L, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled Phase IIIb study of patients with moderate-to-severe genital psoriasis. Br J Dermatol. 2018;179(4):844–852.
- Reich K, Leonardi C, Lebwohl M, et al. Sustained response with ixekizumab treatment of moderate-to-severe psoriasis with scalp involvement: results from three Phase III trials (UNCOVER-1, UNCOVER-2, UNCOVER-3). J Dermatolog Treat. 2017;28(4):282–287.

