J Clin Aesthet Dermatol. 2019;12(11):35–44
 by Athanasios J. Stefanis, MD, MPharm; Tomas Groh, MSc, PhD; Monika Arenbergerova, MD, PhD; Petr Arenberger, MD, PhD; and Peter O. Bauer, MD, PhD
by Athanasios J. Stefanis, MD, MPharm; Tomas Groh, MSc, PhD; Monika Arenbergerova, MD, PhD; Petr Arenberger, MD, PhD; and Peter O. Bauer, MD, PhD
Drs. Stefanis, Arenbergerova, and Arenberger are with the Department of Dermatology and Venereology, Faculty Hospital Kralovske Vinohrady and Third Medical Faculty of Charles University in Prague, the Czech Republic. Drs. Groh and Bauer are with Bioinova, Ltd. in Prague, the Czech Republic.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Adipose cells organized in small clusters under the reticular dermis closely interact with hair follicular cells and regulate the hair cycle. Intradermal adipocyte progenitor cells are activated toward the end of the telogen phase to proliferate and differentiate into mature adipocytes. These cells, surrounding the hair follicles, secrete signaling molecules that control the progression of the hair cycle. Diseases associated with defects in adipocyte homeostasis, such as lipodystrophy and focal dermal hypoplasia, lead to alopecia. In this review, we discuss the potential influence of stromal vascular fraction from adipose tissue in the management of alopecia as well as its involvement in preclinical and clinical trials.
KEYWORDS: Adipose-derived stromal/stem cells, adipose tissue, alopecia, hair loss, stromal vascular fraction
Adipose tissue is a loose connective tissue composed of cells supported by an intracellular matrix as well as by vascular, lymphatic, and neural networks.1 The major cellular component of fat tissue is the adipocyte; however, a variety of other cell types are found as well, collectively labeled as stromal vascular fraction (SVF).2 SVF was first isolated by Zuk et al3 in 2001 from lipoaspirate obtained by liposuction. Specifically, SVF contains mature cells (e.g., fibroblasts, smooth muscle, endothelial, blood cells), progenitor cells (e.g., preadipocytes and endothelial, vascular, and hematopoietic progenitors), and, most importantly, stem cells (e.g., mesenchymal and hematopoietic stem cells, pericytes, and supra-adventitial cells), which are also known as adipose tissue-derived stromal cells (ASCs).4, 5
It has been found that some of the SVF cell types possess regenerative and anti-inflammatory potentials in damaged tissues due to their ability to secrete growth factors and anti-inflammatory molecules.6,7 Mesenchymal stem cells (MSCs) have also been isolated from other sources, such as bone marrow,8 the placenta,9 muscle,10 or blood.11 However, the greater the abundance of adipose tissue in the human body, the significantly higher the yield of MSCs is compared to other tissues.12 Furthermore, the simplicity of harvesting has made fat one of the most important sources for such cells.
As a result, SVF has attracted substantial attention for its potential use in regenerative medicine in various fields, including internal medicine,13–15 orthopedics,16 plastic and general surgery,17–19 and wound healing.7 More recently, the potential role of SVF in hair growth has been investigated for its anticipated contribution to alopecia management. Scalp hair is a key element in the appearance of the individual in addition to the thermoregulation and protection of the skin. Cases of alopecia can be classified by the distribution of hair loss as either diffuse, patterned, or patchy and by the absence or presence of scarring. A thorough description of the etiopathogenesis, diagnosis, and treatment options is presented elsewhere.20,21
Androgenic alopecia is the most common form of alopecia, affecting an estimated 80 percent of men and 50 percent of women by the age of 70 years.22,23 It is classified as a nonscarring patterned form of alopecia and is characterized by bitemporal recession, a gradual loss of hair in the frontoparietal region in men, and a diffuse hair thinning with preservation of frontal hairline in women.22 The underlying mechanism is believed to be a higher concentration of dihydrotestosterone (DHT) to androgen-sensitive hair follicles, causing thinning of the dermal papillae and shortening of the anagen phase.24,25 Current well-established treatment modalities focus on reducing the androgenic effects on hair follicles (5a-reductase inhibitors), stimulating hair growth (minoxidil), or transferring androgen-independent hair to the affected scalp (hair transplantation).23
Diseases associated with defects in adipocyte production, such as in lipodystrophy and focal dermal hypoplasia, leads to alopecia.26,27 Dermal adipocytes are organized in small clusters under the reticular dermis and interact with the hair follicular cells regulating the hair cycle.28 Intradermal adipocyte progenitor cells are stimulated at the end of the telogen phase, multiplying and differentiating into mature adipocytes, which then surround the hair follicles and secrete signaling molecules, such as platelet-derived growth factor (PDGF), leptin, adiponectin, and bone morphogenetic protein 2.29 The expression of these molecules induces the anagen phase of the hair cycle and promotes hair growth.29
The purpose of this review is to provide a synopsis of different SVF isolation methods and the involved components and to provide a description of possible mechanisms for SVF contribution to tissue remodeling and regeneration. It also presents a critical outline of the research concerning the application of SVF and its constituents in the treatment of hair loss where the applied SVF is expected to support the dermal adipose tissue.
Isolation of SVF
SVF can be extracted either enzymatically, usually with a collagenase solution or by mechanical, nonenzymatic methods that involve centrifugation and mixing steps.30 Apart from differences in the manufacturing process, the two extraction techniques can yield dissimilar concentrations of the cell populations composing the SVF—in particular, that of the ASCs.30,31 In addition, it has been postulated that the quality of the final product depends upon several other factors including the harvesting method of the adipose tissue (e.g., tumescent versus ultrasound-assisted liposuction, experience of the operator, handling and preservation of the lipoaspirate), the site of the fat extraction (e.g., abdominal versus gluteal region), and aspects of the patient history (e.g., use of cytotoxic medications, preceding radiotherapy).32–34 Moreover, other patient factors such as age and body mass index have been suggested to affect the viability and function of the ASCs similarly to in the case of MSCs isolated from other tissues.35 However, a recent review has not confirmed this argument.36 In an attempt to streamline the process of SVF isolation from lipoaspirate and eliminate the manufacturing-related variables, several automated and semiautomated devices have been developed and tested worldwide, with various outcomes.31,37
Enzyme-assisted isolation of SVF. Most common methods of SVF isolation from adipose tissue rely on digestive enzymes such as collagenases, dispase, or trypsin.4 Although each laboratory employs unique protocols, the procedure is generally composed of a series of washing steps followed by enzymatic digestion and continued with centrifugation with or without gravity-based separation and filtration.31 Usually, adipose tissue is washed with saline solutions, such as Ringer’s lactate or phosphate-buffered saline, and digestive enzymes are added to divide the sample into two parts: the upper adipocyte-rich portion and the lower aqueous portion containing the cells of interest. Subsequently, centrifugal or gravitational forces are employed to further separate the fatty and aqueous phases and to pellet the cells.38 Filtration to obtain a purer cell suspension by size selection and tissue fragment removal is also performed.31 Finally, erythrolysis is employed to reduce the high number of erythrocytes from the end-product and to limit the presence of cells of hematopoietic origin.38 The SVF can then be either stored for cryopreservation or cultured to obtain a higher number of ASCs.
Although the technique is widely used and, compared to mechanical extraction, is significantly more efficient in promoting ASC yield,31 it has several disadvantages. First of all, the use of collagenases is the subject of strict regulations by several regulatory authorities worldwide including the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), who classify SVF as a medicinal product.39 Second, the activity and purity of collagenases differ between batches even from the same manufacturers and their production is costly.31 Moreover, the process of enzymatic isolation of SVF is time-consuming30 and there are concerns about the safety and efficacy of such SVF solutions in clinical settings,40–42 although these arguments have been disputed recently.30
Nonenzymatic isolation of SVF. Considering the above limitations, there has been a great effort made worldwide to develop methodologies to replace the enzymatic step while still obtaining adequate concentrations of SVF and ASCs. In general, the strategy involves performing mechanical agitation in an attempt to break down the collagen in the extracellular matrix and release the SVF.31
The most exploited technique is centrifugation often supplemented with other methods, such as vibration and hand-shaking. In 2009, Baptista et al43 used centrifugation of lipoaspirate after an erythrocytolytic step to produce around 240,000 cells/mL of lipoaspirate, with five percent of them meeting the criteria for ASCs. In 2010, Francis et al,44 using a series of washing, red blood cells (RBC) lysis, and centrifugation steps, managed to isolate 1,000 ASCs/mL of processed lipoaspirate in 30 minutes and compared it with the 20,000 ASCs/mL produced after an eight-hour, enzymatically based isolation process. Markarian et al45 subjected lipoaspirate to three different centrifugation velocities and different processing times after an RBC lysing step and compared the obtained population of nucleated cells with that produced by way of enzymatic extraction with either collagenase or trypsin. On average, the collagenase-based method produced a significantly higher yield of SVF cells/mL of processed lipoaspirate than either the trypsin-based or nonenzymatic procedures.
In another study, Shah et al46 managed to isolate viable ASCs significantly faster than when using a standard collagenase-based method with a series of washing steps with phosphate-buffered saline, manual shaking, and centrifugation (taking one hour versus three hours), albeit achieving a considerably reduced quantity (19-fold).
The combination of centrifugation and shaking by vibration as a means of mechanical isolation was tested by Raposio et al,47 who managed to extract around 125,000 SVF cells/mL of processed lipoaspirate, with five percent of them being ASCs. Moreover, Condé-Green et al30 completed vortexing and centrifugation of lipoaspirate and compared the outcome with either centrifugation alone or collagenase digestion. They reported that the SVF and the ASC yields from enzymatic extraction were 10 times higher than those after centrifugation alone and as achieved by the vortexing–centrifugation combination.
On the other hand, we were able to obtain SVF containing on average 18 percent of multipotent stromal cells by a specific series of centrifugation and washing steps (unpublished observation; T. Groh, P. O. Bauer, Bioinova Ltd., 2016).
Overall, although most of the mechanical techniques have not been proved to be as efficient as their enzymatic counterparts, so far, it appears that they are time-saving and less expensive procedures without the use of xenobiotic enzymes, avoiding the regulatory scrutiny.30,31,40
Automated and semiautomated SVF isolation systems. As can be concluded from above, the use of manual isolation, either enzymatic or mechanical, is characterized by enormous heterogeneity that hinders the drawing of safe conclusions and comparisons in the quality and quantity of extracted products. Further, although all methods must comply with the rules of good manufacturing practices, there is always a risk of contamination. Therefore, in recent years, several automated and semiautomated closed systems, both enzymatic and nonenzymatic in nature, have been developed and used worldwide to standardize the procedure, ensure sterility,30 and improve the yield.31 Although these systems might require less training to handle than the manual methods and have been designed for clinical use, they are expensive, with high operating costs.37,40 There is also a great disparity in the quantity and quality of isolated cell populations amongst various systems.37 Research on the development and optimization of SVF isolation devices is ongoing and several examples have been reviewed and presented in the literature.31,37
Mechanism of action. As mentioned earlier, SVF is a heterogeneous mixture of cells that can be largely divided into mature, progenitor, and stem cells.5 Mature cells such as fibroblasts, M2 macrophages, and endothelial cells are intermingled with preadipocytes and endothelial, vascular, and hematopoietic progenitors in addition to ASCs, hematopoietic stem cells, and pericytes. Surface antigens known as clusters of differentiation (CD) enable the characterization and identification of each cellular type. Based on CDs, there has been an international attempt4 to set minimum criteria for the composition and identification of SVF components, although research in the field is still ongoing.
The main cell types that make up the SVF are presented henceforth, followed by a discussion of their postulated roles in tissue rejuvenation.
ASCs
ASCs are multipotent stem cells of mesenchymal origin with the ability to differentiate at least into adipogenic, osteogenic, and chondrogenic lineages.3,4 When freshly isolated from SVF, they are identified by the presence of CD34, CD73, CD13, CD105, CD90, and CD29 and the absence of CD31, CD45, and CD144; however, the CD34 marker disappears after eight to 12 divisions and, therefore, is absent in cultured ASCs.4,48,49 Similarly to MSCs from other tissues, ASCs are able to secrete a number of angiogenic and immunoregulatory growth factors such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-beta),6 insulin-like growth factor 1 (IGF-1),50 and the anti-inflammatory prostaglandin phosphodiesterase 2.51 They were also found to enhance antioxidant enzyme activity.52,53 According to Bourin et al,4 they are present at a concentration of at least 2 to 10 percent in SVF regardless of the method of isolation.
Endothelial Progenitor Cells (EPCs)
EPCs are essential for angiogenesis in the embryonic stage of development but can also assist in the repair of vascular endothelium in adults in response to injury.54 In SVF, they have been identified by the markers CD34+, CD31+, CD133+, CD146+, and CD45-4,55 as well as CD144+, CD73+, and CD105+,56 whereas their descendants, endothelial cells (ECs), can be recognized by the presence of CD31 and factor VIII and the absence of CD34.4 EPCs secrete various factors such as VEGF, PDGF-BB, and bFGF.57 In combination, EPC and EC account for 7 to 30 percent of cells in the SVF.39,48
Pericytes
Recognized as CD146+, CD90+, CD73+, CD44+, CD29+, CD13+, CD34–, CD45–, and CD56–, pericytes55,58 are perivascular, contractile vessels that are recruited in response to PDGF-B secretion by the ECs to envelop the newly formed vessels.59 They monitor blood flow59; participate in vascular remodeling,60 blood–brain barrier formation and function,61,62 hemostasis,63 and vitamin A metabolism64; and possess anti-inflammatory65 and even phagocytic properties.66 These cells can differentiate into adipogenic, chondrogenic, and osteogenic lineages.67,68 According to Zimmerlin et al,55 they are extremely potent, although constituting only 0.44 to 2.38 percent of cells in SVF.
Monocytes/Macrophages
Around 10 percent of SVF cells are positive for CD14, a monocyte marker.69 Apart from immunoregulation, they secrete various cytokines—specifically, VEGF and bFGF—that contribute to vascular remodeling.70,71 Furthermore, the more abundant macrophages (e.g., CD45+, CD14+, CD206+)38 account for around 20 percent of the SVF population, with 70% of them being of the M2 subtype (e.g., CD301+).72 M2 macrophages are known to possess anti-inflammatory and proangiogenic functions by expressing and secreting several molecules such as the CD163 receptor with anti-inflammatory and homeostatic properties73; matrix metalloproteinase 9 (MMP-9),74 which is implicated in stroma remodeling and angiogenesis; TGF-beta, which promotes reepithelization in tissue injury75; and anti-inflammatory interleukin (IL) 1076 and IL-1 receptor antagonist (IL-1RA).77 They can also act as chaperones, facilitating vascular anastomosis,78 and a CD34+ subpopulation has been shown to possess pluripotency, adherence to plastic surfaces, and stem cell–like morphology.79
Regulatory T-cells (Tregs)
In total, lymphocytes compose 10 to 15 percent of SVF, with Tregs55 accounting for 5 to 70 percent of the CD4+T-cell population.39 They express receptors for CD4, CD25, and forkhead box P349 and play a pivotal role in immunoregulation by expressing high levels of inhibitory cytokines such as TGF-beta,80 IL-10,81 IL-35,82 and granzyme B,83 leading to the activation of the apoptotic cascade in target cells. As a result, they can prevent the overactivation of effector T-cells and induce the activation of M2 macrophages,84 thus preventing autoimmunity and maintaining homeostasis.85
In contrast with previous hypotheses, it has recently been postulated that the regenerative potential of SVF is mainly exerted by the synergistic action of the individual components and their interaction with surrounding tissues via extensive paracrine and juxtacrine signaling.48,86 When SVF is injected into the site of interest, the ASCs, in response to injury or inflammation, direct themselves around injured vessels87 and secrete cytokines and chemokines, enhancing the differentiation, proliferation, and recruitment of various cell types such as progenitor cells, fibroblasts, and macrophages.88 For example, VEGF secreted by ASCs attracts and promotes the differentiation of EPCs to ECs, which, in turn, releases PDGF-BB, enabling further proliferation and migration of ASCs and attracting others cell types such as pericytes for angiogenesis. Following VEGF, bFGF is released, leading to fibroblast proliferation and collagen production. Another factor produced by the ASCs, HGF, has been shown to play a crucial role in the migration, differentiation, and survival of the EPCs.89 Similarly, IGF-1 leads to the differentiation of ASCs into adipocytes.90 Also, the presence of Tregs and M2 macrophages in the SVF, together with stem and progenitor cells, can prevent excessive inflammation in the site and stimulate angiogenesis by secreting anti-inflammatory and immunoregulatory factors such as IL-10, IL-1RA, granzyme B, and TGF-beta. Further, fibroblasts in the SVF produce collagen and other extracellular matrix proteins, which interact with integrins to form a cytoskeleton, which serves as a structural support for new-vessel formation.91 Finally, monocytes and macrophages, apart from releasing growth factors such as VEGF and bFGF, secrete metalloproteinases such as MMP-9 that are involved in the degradation and remodeling of the extracellular matrix, releasing peptides that attract more EPCs to the area.92 The trophic and immunoregulating action of the SVF has led to an extensive research of its effects in a wide range of clinical conditions and ex-vivo applications such as in myocardial infarction,13 heart failure,14 wound healing,93,94 diabetic retinopathy,15 osteoarthritis,16 and systemic sclerosis.95
Studies of SVF and its products in hair growth and alopecia
In-vitro and ex-vivo studies. Won et al,96 in a series of experiments, demonstrated the paracrine effects of ASCs on hair growth. The ASC-conditioned medium (ASC-CM) was applied at different concentrations into the human dermal papilla cell (hDPC) and immortalized keratinocyte (HaCaT) cultures. Following a 48-hour incubation period, the proliferation rates of hDPC and HaCaT cells were increased by 100 to 140 percent and 10 to 30 percent, respectively. Moreover, upregulation of the signaling molecules pAkt and pErk, cyclin D1, and CDK2 enhancing the cell cycle were observed in hDPCs.
In addition, culturing human hair follicles with of 12.5 percent ASC-CM or 1 mM of minoxidil in William’s E medium (positive control) for six days resulted in hair elongation outcomes of 44 and 40 percent, respectively, which are both significantly higher when compared to the results achieved with the negative control (i.e., one-half volume of alpha-MEM mixed with Williams E media yielded a 30% increase in length).96 In this study, however, it was not specified as to whether the concentration of minoxidil was chosen based on the absorption of the standard doses used in humans (5% or 2% once or twice per day).
The trophic action of ASC on dermal papilla cells (DPCs) and hair follicles was also demonstrated by increased alkaline phosphatase activity in DPCs after seven days of culturing of these cells with the conditioned media of rat-derived ASCs.97
In a more recent ex-vivo experiment, Won et al98 reaffirmed the positive role of ASCs in hair growth. They treated cultured human hair follicles with different concentrations of ASC-CM followed by the hair growth-suppressing factors DHT or H2O2. When compared to the ASC-CM–free control, the inhibition of growth was significantly reduced in a manner dependent on ASC-CM or DHT/H2O2 concentration.
Preclinical studies. In 2010, Won et al96 showed that three subcutaneous injections (once every three days) of the ASC suspension into the shaved dorsum of male C3H/HeN mice induced the hair follicles to move to the telogen phase and significantly increased the hair growth as compared with in the sham-injected control group after 12 weeks. Similar results, according to the authors, were obtained with a topical application (performed after 0.2-mm mesoroller application to increase the skin absorption) of the solution with ASC-CM (three single applications every three days) on the shaved back of an unspecified number of C3H/HeN mice and comparing the hair growth with a control group exposed only to the William’s E culture medium for 12 weeks.
In another 12-week in-vivo experiment, Park et al99 subcutaneously injected three subsequent doses of ASC-CM into 15 shaved C3H/HeN mice with all hair follicles in the telogen stage under normoxic or hypoxic conditions. The hair growth was compared to a control group receiving only injections with the minimum essential medium. After 12 weeks, 4 of 5 mice injected with hypoxic ASC-CM demonstrated hair growth in comparison with 2 of 5 mice with normoxic ASC-CM and 1 of 5 in the control group. Also, the hair growth in the first group with hypoxic ASC-CM was more amplified and rapid. After an analysis of the ASC-CM, the authors concluded that there was a significant increase in the density of most growth factors in the hypoxic ASC-CM.
Similarly, Jeong et al100 investigated the effects of preconditioning of ASCs with low-dose (<20mJ/cm2) ultraviolet (UV)B radiation. UVB-irradiated ASCs or their respective ASC-CM were injected to the dorsum of shaved C3H/HeN mice with hair in the telogen stage and their dorsal hair weight was compared after 18 days to the outcomes of the control groups that received nonirradiated ASCs or ASC-CM. Overall, hair appeared in all mice but the dorsal hair of the irradiated groups was three times heavier than that of the control groups, demonstrating hair transition to the anagen phase. By in-vitro/ex-vivo experiments, the authors found that the UVB generates reactive oxygen species and upregulates Nox4 in ASCs, leading to increased production of the growth factors.
To preserve characteristics of dermal papillae and to demonstrate the ASCs’ effects on hair growth, Huang et al,97 constructed various chitosan-coated spheres containing ASCs and DPCs and injected them into nude mice. The formation of hair-like structures after four weeks was significantly higher after application of ASC-shell/DPC-core spheres as compared with other combinations or the non-ASC-containing spheres.
In a different study, Lee et al101 isolated ASCs from dogs, differentiated them into dermal papilla-like structures, and injected them into nude mice to assess their trichogenic potential. During the 30-day monitoring period, the authors noticed significant increases occurred in new hair follicle and sebaceous gland formation, especially during the first 20 days, while improved vascularization in the test group was also noted due to the production of VEGF from the dermal papilla cells, supporting the paracrine influence of ASCs on hair regeneration.
Clinical studies. Cohen et al5 stated in their review in 2010 that fat enriched with SVF was injected into the scalp of a woman with alopecia, resulting in a “marked improvement in hair growth” after nine months of follow-up.
In 2012, Fukuoka et al102 injected ASC-CM fortified with proteins and vitamins into the scalps of 25 male and female patients with androgenic alopecia during a four-month period and observed the effects of treatment in terms of hair count and density by trichogram and subjectively by visual analog scale (VAS) score. The authors reported a statistically significant increase (p=0.01) in patient satisfaction as observed by an average VAS score increase from 2.52 to 3.72 after one year. The drawback of the study was that five male patients were administered finasteride before or during the treatment. Finasteride, a Type II 5alpha-reductase inhibitor, has been shown to prevent male-pattern hair loss through effects on androgen metabolism.103 Therefore, its administration may have affected the evaluation of the ASC-CM–mediated clinical outcomes. In addition, the sample size was small, there were significant observer and response biases, and there was no placebo group included. Moreover, objective data such as anagen/telogen ratio and hair count and thickness were not included.
Fukuoka and Suga104 performed a six-month prospective study on 32 patients with androgenic alopecia of male and female types with monthly scalp injections with a commercially available ASC-CM (AAPE®; Prostemics, Seoul, Korea). In 10 of these patients, injections were only applied to one side of the scalp. After examination with trichogram at the start and at the end of the six-month period, the authors reported a statistically significant (p<0.05) mean increase in hair count by 29 hairs/cm2 for male and 15.6 hairs/cm2 for female patients. In this half-side comparison study, the increase in hair count between the treated and untreated scalp areas was also reported to be significant. The study was limited by its small sample size, selection and observer biases, and the lack of a control group and objective measurements.
In another study, Perez et al105 injected SVF-enriched autologous fat in five patients aged 18 to 55 years with early male- and female-pattern hair loss. The liposuction and the administration of the SVF solution were performed on the same day and patients were followed up with for up to 24 weeks postoperatively. During visits at regular intervals, progress was documented by macrophotography and global photography, whereas the hair count, anagen/telogen percentages, and hair thickness were measured by computer software. On average, increases of 23 and 24.2 percent in hair count and thickness were reported with a rise of 93 percent and a drop of 35 percent in the amount of anagen and telogen hairs, respectively. However, data from anagen/telogen percentages were statistically insignificant (p=0.15). Limitations of this study included its small sample size, lack of placebo group, and observer and response biases.
In a retrospective case series, Shin et al106 evaluated the clinical efficacy of ASC-CM for the treatment of 27 women with female-pattern hair loss. Using trichoscopy, the authors measured the hair density and thickness from the mid-line scalp before and after applying the AAPE® ASC-CM. The treatment protocol consisted of repeated applications of the ASC-CM once per week for 12 weeks. Prior to these applications, the scalp area was cleansed using a microneedle roller. At the end of the period, mean increases of 16.4 percent in hair density and 11.3 percent in hair thickness were observed and considered as clinically significant. The treatment effects were sustained during a six-month follow-up period and no adverse events were reported. However, the lack of a control group and the small sample size could limit the interpretation of these findings.
Recently, in the preliminary report from a prospective single-blinded clinical trial (STYLE trial, Table 1), Aronowitz et al.107 demonstrated a significant increase (p=0.01) in hair count by 14 percent at six months after the administration of SVF-enriched autologous fat into the scalp of eight males and one female with hair loss. In addition, the anagen-phase hair count was increased by 34 percent.
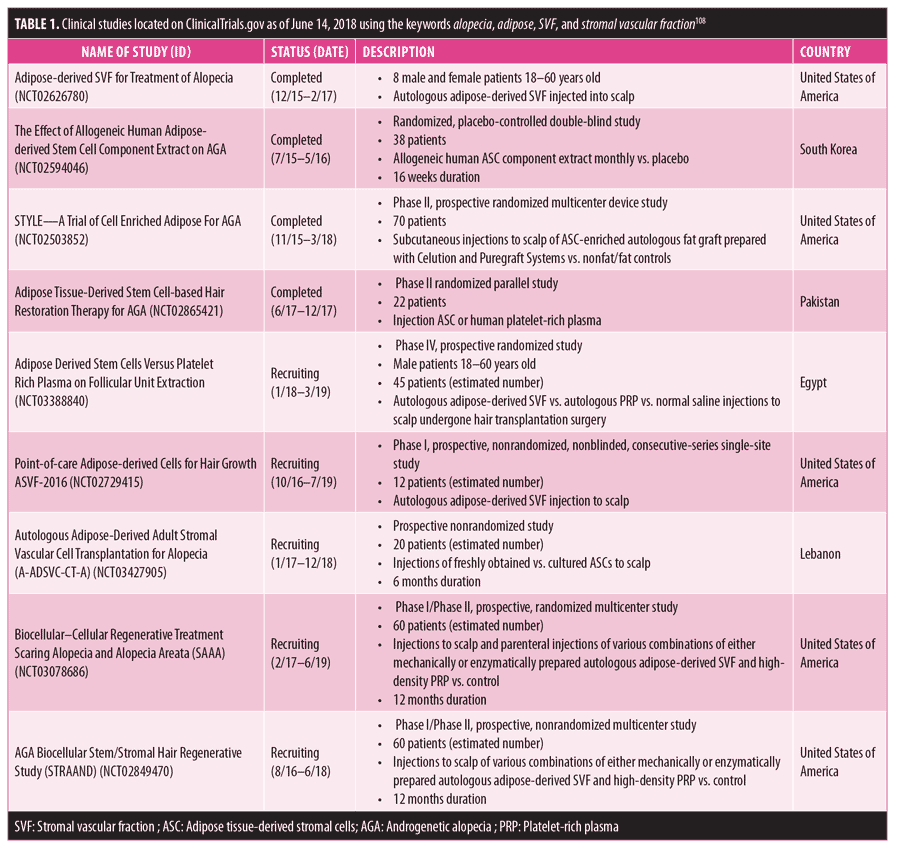
Ongoing clinical trials. Although the beneficial effects of SVF and its products on hair growth have been demonstrated, large controlled clinical studies examining the subjects of efficacy and possible side effects have not yet been reported. As of June 14, 2018, there were nine studies published on ClinicalTrials.gov dealing with the treatment of alopecia using SVF or ASC-based products.108 Eight of them were involved with androgenic alopecia and one covered scarring alopecias/alopecia areata. The status and basic characteristics of these trials are provided in Table 1.
Discussion
All of the aforementioned preclinical and clinical studies have supported the beneficial role of SVF and ASCs in promoting hair growth. The application of ASC-CM in hDPC cultures in vitro and hair follicles ex vivo led to their growth. In addition, pretreatment of hair follicles with ASC-CM had a protective effect against the growth-suppressing function of DHT and H2O2. The injection of ASC-CM or ASC/DPC-spheres in nude mice also induced the appearance of new hair follicles with superior results in mice injected with ASC-CM obtained from ASCs cultured in hypoxic conditions or irradiated with UVB light. Similarly, the application of ASC-CM or SVF-enriched autologous fat in patients with androgenic alopecia led to an increase of 14 to 23 percent in hair density and 11 to 24 percent in hair thickness as measured by trichoscopy or other photographic methods, usually after a six-month follow-up period. When measured, the rise in anagen hair count varied between 34 to 93 percent.
As described above, the regenerative action of SVF is attributed mainly to its paracrine effect to neighboring cells and tissues via the secretion of various growth factors and cytokines of cells comprising the SVF including, in particular, ASCs. According to the literature, IGF-1 and PDGF stimulate the proliferation and inhibit the apoptosis of hair follicular cells by modulating their cell cycle via ERK and Akt or Sonic hedgehog, Lef-1, and Wnt signaling pathways, respectively.109–111 In addition, IGF-1 inhibits TGF-beta1, which is secreted by DPCs in response to androgens and reactive oxygen species and is a well-known suppressor of hair growth.112,113 Furthermore, bFGF induces the telogen-to-anagen phase transition of hair follicular cells and increases hair follicular size through Sonic hedgehog and Wnt signaling pathways.114,115 HGF alters the hair growth cycle by prolonging the anagen phase via the HFG–Met signaling pathway.116,117 VEGF exerts its growth-inducing action by stimulating angiogenesis, which nourishes the hair follicles and promotes hDPC proliferation via the VEGF receptor 2–ERK pathway.118,119 Apart from their paracrine effect, ASCs might exert their regenerative action by differentiating into follicular progenitor cells and even fusing with present follicular cells, thus reinforcing their self-dividing potential.120,121
When analyzing the referred studies with a more critical view, several drawbacks were detected that might limit the validity and significance of the respective results. First and foremost, all clinical studies were carried out involving a small number of participants, being therefore prone to sampling errors.122 In addition, in some studies, a percentage of patients were not followed up with until the end of the trial.123 Nevertheless, not all studies were performed with a control group and the controlled trials were either single-blinded or open-label, introducing additional types of bias such as performance bias.124 Another factor affecting the consistency of findings is the lack of standardization of the applied injection solution. Some studies used ASC-CM while others used fat enriched with either ASC-CM or SVF, and no study to date has investigated the effects of isolated SVF, which, as mentioned above, includes several types of cells besides ASCs. Furthermore, the enzymatic isolation of SVF and ASCs was used in all cases, and, when administering only the ASC-CM,102,104,106 the solution was not obtained from autologous ASCs. Also, there were no reports on the concentration of ASCs included in the SVF and, so far, there has been no comparison performed between the regenerative potential of SVF on hair follicles isolated either by mechanical or enzymatic methods. Of note, the use of ASC-CM has several advantages as compared with ASCs, including easier preparation and handling as well as the possibility of freeze-drying and long-term storage. This is also important for patients who have various conditions preventing them from being subjects for obtaining and applying autologous materials in whom alopecia treatment using allogeneic ASC-derived medicinal products could be a suitable strategy.
Regarding the results, there is a discrepancy in the data collected, hindering their objective comparison and evaluation. Not all studies measured hair density, anagen/telogen ratio or percentage, and hair density. One study even used only the VAS, which is a subjective means of measuring the efficacy of treatment. Finally, it is important to note that in a report pertaining to the STYLE trial, Aronowitz et al107 pointed out that SVF-enriched fat was applied to the patients, whereas, in the clinical trial description page, it is stated that ASC-enriched fat is used. This case shows that there are still misconceptions regarding the terminology affecting the correct evaluation of study results.
Conclusion
SVF is the cellular remnant of lipoaspirate left behind after mechanical or enzymatic manipulation and which possesses a proven regenerative potential attributed to the excretory and differentiating capacities of its components, including mainly of the ASCs. Apart from highlighting the potential mechanisms of action of SVF and the current methods of isolation, this review focused on covering the therapeutic possibilities of SVF in the treatment of hair loss. According to the cited literature, SVF could represent a well-promising option; however, better-designed and larger clinical studies are needed to better demonstrate and define more objectively the real therapeutic potential of the SVF in the ongoing battle against alopecia.
References
- Avram AS, Avram MM, James WD. Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. J Am Acad Dermatol. 2005;53(4):671–683.
- Gentile P, Orlandi A, Scioli MG, et al. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med. 2012;1(3):230–236.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228.
- Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–648.
- Cohen SR, Hewett S, Ross L, et al. Regenerative cells for facial surgery: biofilling and biocontouring. Aesthet Surg J. 2017;37(Suppl 3):S16–S32.
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298.
- Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22(3):313–325.
- Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001;226(6): 507–520.
- In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345.
- Young HE, Steele TA, Bray RA, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264(1):51–62.
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2(6): 477–488.
- Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14.
- Mazo M, Cemborain A, Gavira JJ, et al. Adipose stromal vascular fraction improves cardiac function in chronic myocardial infarction through differentiation and paracrine activity. Cell Transplant. 2012;21(5):1023–1037.
- Premaratne GU, Ma LP, Fujita M, et al. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg. 2011;6:43.
- Rajashekhar G, Ramadan A, Abburi C, et al. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One. 2014;9(1):e84671.
- Pers YM, Rackwitz L, Ferreira R, et al. Adipose Mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–856.
- Charles-de-Sa L, Gontijo-de-Amorim NF, Maeda Takiya C, et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg. 2015;135(4):999–1009.
- Moustaki M, Papadopoulos O, Verikokos C, et al. Application of adipose-derived stromal cells in fat grafting: basic science and literature review. Exp Ther Med. 2017;14(3):2415–2423.
- Nseir I, Delaunay F, Latrobe C, et al. Use of adipose tissue and stromal vascular fraction in hand surgery. Orthop Traumatol Surg Res. 2017;103(6):927–932.
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13(4):247–260.
- Qi J, Garza LA. An overview of alopecias. Cold Spring Harb Perspect Med. 2014;4(3). pii: a013615.
- Piraccini BM, Alessandrini A. Androgenetic alopecia. G Ital Dermatol Venereol. 2014;149(1):15–24.
- Varothai S, Bergfeld WF. Androgenetic alopecia: an evidence-based treatment update. Am J Clin Dermatol. 2014;15(3):217–230.
- Ramos PM, Miot HA. Female pattern hair loss: a clinical and pathophysiological review. Anais Brasileiros de Dermatologia. 2015;90(4):529–543.
- Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4:1–11.
- Agarwal AK, Garg A. Genetic disorders of adipose tissue development, differentiation, and death. Annu Rev Genomics Hum Genet. 2006;7:175–199.
- Goltz RW. Focal dermal hypoplasia syndrome. An update. Arch Dermatol. 1992;128(8):1108–1111.
- Hausman GJ, Martin RJ. The development of adipocytes located around hair follicles in the fetal pig. J Anim Sci. 1982;54(6):1286–1296.
- Schmidt B, Horsley V. Unravelling hair follicle-adipocyte communication. Exp Dermatol. 2012;21(11):827–830.
- Conde-Green A, Kotamarti VS, Sherman LS, et al. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: review of upcoming techniques. Plast Reconstr Surg Glob Open. 2016;4(9):e1017.
- Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713.
- Levi B, James AW, Glotzbach JP, et al. Depot-specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2010;126(3):822–834.
- Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60(3):306–322.
- De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109.
- Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60(5):538–544.
- Varghese J, Griffin M, Mosahebi A, Butler P. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther. 2017;8(1):45.
- Oberbauer E, Steffenhagen C, Wurzer C, et al. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen (Lond). 2015;4:7.
- Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8(1):145.
- Dykstra JA, Facile T, Patrick RJ, et al. Concise review: fat and furious: harnessing the full potential of adipose-derived stromal vascular fraction. Stem Cells Transl Med. 2017;6(4): 1096–1108.
- Lockhart RA, Aronowitz JA, Dos-Anjos Vilaboa S. Use of freshly isolated human adipose stromal cells for clinical applications. Aesthet Surg J. 2017;37(Suppl 3):S4–S8.
- Carvalho PP, Gimble JM, Dias IR, et al. Xenofree enzymatic products for the isolation of human adipose-derived stromal/stem cells. Tissue Eng Part C Methods. 2013;19(6):473–478.
- Kirkpatrick CJ, Melzner I, Goller T. Comparative effects of trypsin, collagenase and mechanical harvesting on cell membrane lipids studied in monolayer-cultured endothelial cells and a green monkey kidney cell line. Biochim Biophys Acta. 1985;846(1):120–126.
- Baptista LS, do Amaral RJ, Carias RB, et al. An alternative method for the isolation of mesenchymal stromal cells derived from lipoaspirate samples. Cytotherapy. 2009;11(6):706–715.
- Francis MP, Sachs PC, Elmore LW, Holt SE. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis. 2010;6(1):11–14.
- Markarian CF, Frey GZ, Silveira MD, et al. Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett. 2014;36(4):693–702.
- Shah FS, Wu X, Dietrich M, Rood J, Gimble JM. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy. 2013;15(8):979–985.
- Raposio E, Caruana G, Bonomini S, Libondi G. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg. 2014;133(6):1406–1409.
- Guo J, Nguyen A, Banyard DA, et al. Stromal vascular fraction: a regenerative reality? Part 2. Mechanisms of regenerative action. J Plast Reconstr Aesthet Surg. 2016;69(2):180–188.
- SundarRaj S, Deshmukh A, Priya N, et al. Development of a system and method for automated isolation of stromal vascular fraction from adipose tissue lipoaspirate. Stem Cells Int. 2015;2015:109353.
- Bagno LL, Carvalho D, Mesquita F, et al. Sustained IGF-1 secretion by adipose-derived stem cells improves infarcted heart function. Cell Transplant. 2016;25(9):1609–1622.
- Manferdini C, Maumus M, Gabusi E, et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013;65(5):1271–1281.
- Penuelas O, Melo E, Sanchez C, et al. Antioxidant effect of human adult adipose-derived stromal stem cells in alveolar epithelial cells undergoing stretch. Respir Physiol Neurobiol. 2013;188(1):1–8.
- Kim WS, Park BS, Kim HK, et al. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49(2):133–142.
- Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2(7):a006692.
- Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77(1):22–30.
- Hager G, Holnthoner W, Wolbank S, et al. Three specific antigens to isolate endothelial progenitor cells from human liposuction material. Cytotherapy. 2013;15(11):1426–1435.
- Rosell A, Morancho A, Navarro-Sobrino M, et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS One. 2013;8(9):e73244.
- Corselli M, Crisan M, Murray IR, et al. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytometry A. 2013;83(8): 714–720.
- Gokcinar-Yagci B, Uckan-Cetinkaya D, Celebi-Saltik B. Pericytes: properties, functions and applications in tissue engineering. Stem Cell Rev. 2015;11(4):549–559.
- Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153(3):543–553.
- Dohgu S, Takata F, Yamauchi A, et al. Brain pericytes contribute to the induction and up-regulation of blood–brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038(2):208–215.
- Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561.
- Kim JA, Tran ND, Li Z, et al. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab. 2006;26(2):209–217.
- Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28(2):105–112.
- Tu Z, Li Y, Smith DS, et al. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci. 2011;52(12):9005–9010.
- Castejon OJ. Ultrastructural pathology of cortical capillary pericytes in human traumatic brain oedema. Folia Neuropathol. 2011;49(3):162–173.
- Farrington-Rock C, Crofts NJ, Doherty MJ, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110(15):2226–2232.
- James AW, Zara JN, Zhang X, et al. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. 2012;1(6):510–519.
- Astori G, Vignati F, Bardelli S, et al. “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med. 2007;5:55.
- Navarro A, Marin S, Riol N, et al. Human adipose tissue-resident monocytes exhibit an endothelial-like phenotype and display angiogenic properties. Stem Cell Res Ther. 2014;5(2):50.
- Koh YJ, Koh BI, Kim H, et al. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31(5):1141–1150.
- Morris ME, Beare JE, Reed RM, et al. Systemically delivered adipose stromal vascular fraction cells disseminate to peripheral artery walls and reduce vasomotor tone through a CD11b+ cell-dependent mechanism. Stem Cells Transl Med. 2015;4(4):369–380.
- Fabriek BO, van Bruggen R, Deng DM, et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113(4):887–892.
- Poitevin S, Garnotel R, Antonicelli F, et al. Type I collagen induces tissue factor expression and matrix metalloproteinase 9 production in human primary monocytes through a redox-sensitive pathway. J Thromb Haemost. 2008;6(9): 1586–1594.
- Hameedaldeen A, Liu J, Batres A, et al. FOXO1, TGF-beta regulation and wound healing. Int J Mol Sci. 2014;15(9):16257–16269.
- Zeyda M, Farmer D, Todoric J, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond). 2007;31(9):1420–1428.
- Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652.
- Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840.
- Eto H, Ishimine H, Kinoshita K, et al. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev. 2013;22(6):985–997.
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302.
- Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939.
- Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569.
- Gondek DC, Lu LF, Quezada SA, et al. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174(4):1783–1786.
- Tiemessen MM, Jagger AL, Evans HG, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104(49):19446–19451.
- Kondelkova K, Vokurkova D, Krejsek J, et al. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove). 2010;53(2):73–77.
- Ramakrishnan VM, Boyd NL. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng Part B Rev. 2018;24(4):289–299.
- Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201.
- Herdrich BJ, Lind RC, Liechty KW. Multipotent adult progenitor cells: their role in wound healing and the treatment of dermal wounds. Cytotherapy. 2008;10(6):543–550.
- Cai L, Johnstone BH, Cook TG, et al. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells. 2007;25(12):3234–3243.
- Bluher S, Kratzsch J, Kiess W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Pract Res Clin Endocrinol Metab. 2005;19(4):577–587.
- Bauer AL, Jackson TL, Jiang Y. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput Biol. 2009;5(7):e1000445.
- Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625–637.
- Sun M, He Y, Zhou T, et al. Adipose extracellular matrix/stromal vascular fraction gel secretes angiogenic factors and enhances skin wound healing in a murine model. Biomed Res Int. 2017;2017:3105780.
- Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns. 2014;40(7):1375–1383.
- Guillaume-Jugnot P, Daumas A, Magalon J, et al. Autologous adipose-derived stromal vascular fraction in patients with systemic sclerosis: 12-month follow-up. Rheumatology (Oxford). 2016;55(2):301–306.
- Won CH, Yoo HG, Kwon OS, et al. Hair growth promoting effects of adipose tissue-derived stem cells. J Dermatol Sci. 2010;57(2):134–137.
- Huang CF, Chang YJ, Hsueh YY, et al. Assembling composite dermal papilla spheres with adipose-derived stem cells to enhance hair follicle induction. Sci Rep. 2016;6:26436.
- Won CH, Park GH, Wu X, et al. The basic mechanism of hair growth stimulation by adipose-derived stem cells and their secretory factors. Curr Stem Cell Res Ther. 2017;12(7): 535–543.
- Park BS, Kim WS, Choi JS, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31(1):27–34.
- Jeong YM, Sung YK, Kim WK, et al. Ultraviolet B preconditioning enhances the hair growth-promoting effects of adipose-derived stem cells via generation of reactive oxygen species. Stem Cells Dev. 2013;22(1):158–168.
- Lee A, Bae S, Lee SH, et al. Hair growth promoting effect of dermal papilla like tissues from canine adipose-derived mesenchymal stem cells through vascular endothelial growth factor. J Vet Med Sci. 2017;78(12):1811–1818.
- Fukuoka H, Suga H, Narita K, et al. The latest advance in hair regeneration therapy using proteins secreted by adipose-derived stem cells. Am J Cosmetic Surg. 2012;29(4):273–282.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride male pattern hair loss study group. J Am Acad Dermatol. 1998;39(4 Pt 1):578–589.
- Fukuoka H, Suga H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: follow-up eith trichograms. Eplasty. 2015;15:e10.
- Perez-Meza D, Ziering C, Sforza M, et al. Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells Cloning. 2017;10:1–10.
- Shin H, Ryu HH, Kwon O, et al. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss. a retrospective case series study. Int J Dermatol. 2015;54(6): 730–735.
- Aronowitz JA, Lockhart RA, Birnbaum ZE, et al. Abstract. Stromal vascular fraction enhanced adipose transplantation in hair loss early experience & active phase II FDA investigation. Plast Reconstr Surg Glob Open. 2016;4(9 Suppl):50.
- ClinicalTrials.gov. Search of adipose | alopecia—list results. ClinicalTrials.gov. 2017.
- Weger N, Schlake T. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125(5):873–882.
- Su HY, Hickford JG, Bickerstaffe R, Palmer BR. Insulin-like growth factor 1 and hair growth. Dermatol Online J. 1999;5(2):1.
- Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci. 2006;43(2):105–115.
- Li J, Yang Z, Li Z, et al. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-beta1 in C57BL/6 mice in vivo. Growth Horm IGF Res. 2014;24(2-3):89–94.
- Shin H, Yoo HG, Inui S, et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013;46(9): 460–464.
- Lin WH, Xiang LJ, Shi HX, et al. Fibroblast growth factors stimulate hair growth through beta-catenin and Shh expression in C57BL/6 mice. Biomed Res Int. 2015;2015:730139.
- Ozeki M, Tabata Y. Promoted growth of murine hair follicles through controlled release of basic fibroblast growth factor. Tissue Eng. 2002;8(3):359–366.
- Lindner G, Menrad A, Gherardi E, et al. Involvement of hepatocyte growth factor/scatter factor and met receptor signaling in hair follicle morphogenesis and cycling. FASEB J. 2000;14(2):319–332.
- Jindo T, Tsuboi R, Takamori K, Ogawa H. Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J Invest Dermatol. 1998;110(4):338–342.
- Li W, Man XY, Li CM, et al. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp Cell Res. 2012;318(14):1633–1640.
- Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107(4):409–417.
- Ying W, Min-min D, Hai-bo Y, Song-tao G. In vitro differentiation of adipose-derived stem cells into hair cell-like cells in Guinea pigs. J Otol. 2007;2(2):97–101.
- Suzuki E, Fujita D, Takahashi M, et al. Adipose tissue-derived stem cells as a therapeutic tool for cardiovascular disease. World J Cardiol. 2015;7(8):454–465.
- Kianifard F, Islam MZ. A guide to the design and analysis of small clinical studies. Pharm Stat. 2011;10(4):363–368.
- Higgins J, Altman D, Sterne J, eds. Chapter 8. Assessing risk of bias in included studies. In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5.1.0 London, UK: The Cochrane Collaboration; 2011.
- Chow S, Liu J. Bias and variability. In. Design and Analysis of Clinical Trials. 2nd ed. Hoboken, NJ. Wiley; 2004: 47–53.

