 by Chaitra Prakash, MD; Puneet Bhargava, MD; Siddhi Tiwari, MD; Banashree Majumdar, MD; and Rishi Kumar Bhargava, MD
by Chaitra Prakash, MD; Puneet Bhargava, MD; Siddhi Tiwari, MD; Banashree Majumdar, MD; and Rishi Kumar Bhargava, MD
Drs. Prakash, P. Bhargava, Tiwari, and Majumdar are with the Department of Dermatology, Venereology and Leprosy, Sawai Man Singh Medical College and Attached Hospitals, Jaipur, Rajasthan, India. Dr. R.K. Bhargava is with Girdhar Hospital and Research Centre, Jaipur, Rajasthan, India.
J Clin Aesthet Dermatol. 2017;10(7):33–39
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Keywords: Acne vulgaris, acid mantle, pH meter, skin pH, stratum corneum
Abstract: Objective: Recurrent and chronic course of acne vulgaris, despite effect-proven therapies, point to an underfocused aspect in its pathogenesis and management. This study aims to assess in subjects with and without acne, the skin surface pH, a parameter that cumulatively represents functioning of various units of skin, including the barrier.
Methods: A total of 200 patients with acne and 200 age- and sex-matched controls were included. Under basal conditions, facial skin pH was derived from five sites using a skin pH-meter. The relation between skin pH and acne was evaluated according to sex.
Results: There were more subjects with normal skin pH in the control group compared to the case group, and the majority of acne occurrences in the case group were related to high skin pH (p=0.000). Mean pH among cases was higher than normal reference value (pH 4.5–5.5 for women, 4–5.5 for men) and that of controls (p<0.001). No significant association was observed between sex and skin pH in either cases or controls (p>0.05).
Conclusion: Increased facial skin pH in patients with acne at basal conditions mirrors a chronic state of stratum corneum instability, which could be predisposing individuals to acne occurrence and/or recurrences. It could possibly be a common domain via which the classical pathomechanisms might be acting in acne. Integrating measures that maintain stratum corneum pH during therapy might prove worthwhile.
Introduction
Etiopathogenesis of acne vulgaris is believed to be multifactorial; thus, treatment is multimodal. Most experts agree that hyperproliferation and disordered keratinization of follicular epithelium coupled with sebaceous gland overactivity and Propionibacterium acnes (P. acnes) colonization leads to the formation of clogged pores, which can be accompanied by varying degrees of inflammation.[1] The field has seen developments in acne management with the use of antibiotics, retinoids, hormonal, and anti-inflammatory agents. Despite all the available, effect-proven therapies, acne is inevitably recurrent and chronic. Hence, it is now recognized as a primary inflammatory disorder where the other believed mechanisms play a secondary role.[2] Furthermore, there is also consensus that acne-predisposed skin is in a constant state of subclinical inflammation, which might evolve in severity when there is superimposition of one or more of the previously mentioned pathomechanisms.[3] Alteration in skin barrier function and integrity have also been reported in patients with acne vulgaris.[4,5] Whether this alteration is a sequelae of the disease process or a predisposition to the inflammatory disease itself is not clear. Among the different biophysical parameters that are used to assess barrier function of skin, stratum corneum pH seems to cumulatively represent many aspects of skin function, including the barrier. It has also played a critical role in many inflammatory as well as infective skin conditions.[6] These observations make skin pH an interesting, “out of the box” domain in re-exploring acne vulgaris.
Materials and Methods
We conducted a prospective, observational study in the dermatology department of our institute from January 2014 to December 2015. A total of 200 cases of acne vulgaris and 200 age- and sex-matched healthy controls between ages of 15 and 30 years were included. Individuals that met the following criteria were included in the case group: 1) presence of mild-to-moderate-grade acne involving the face and 2) not taken any oral anti-acne medication in the last three months. Both case and control groups were instructed to refrain from using cleansers and topical applications to the face for a period of 24 hours prior to the pH test. Subjects were acclimated for 20 minutes at an ambient temperature of 21 degrees Celsius and relative humidity of 50 percent.[7] The face was gently dabbed with a tissue paper to remove excess sweat and sebum on the surface of skin. Skin pH was measured late in the afternoon using Skin pH Meter® 905 (Courage-Khazaka Electronic, Germany).
The principle of the Skin pH Meter is based on a high-quality combined electrode, where a glass electrode and an additional reference electrode are placed in one housing that is connected to a handheld probe. When placed on the skin, the glass electrode measures the electric potential on the surface by sensing the hydrogen ion concentration and compares it with the voltage of a known solution in the reference electrode. The potential difference between two electrodes is deduced as the pH of skin and is calibrated on the digital screen. The pH of facial skin in each individual was derived by the average of readings taken at five sites: forehead, nose, both cheeks, and chin. A reference pH range of 4.5 to 5.5 was considered normal for women, and 4 to 5.5 was considered normal for men.[8,9] A value below and above the reference range was recorded as low and high, respectively. The difference in pH between cases and controls were compared. Data were analyzed using SPSS version 23.0 (SPSS Inc., Armonk, New York) and significance was measured using Chi square test and p value.
Results
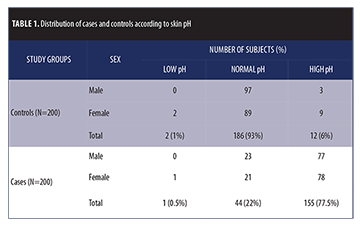 The study population comprised 200 cases and 200 age- and sex-matched controls. The male to female ratio was 1:1. Distribution of cases and controls across skin pH categories are shown in Table 1. Proportion of subjects with normal skin pH were significantly higher in the control group (186/200, 93%) as compared to the case group (44/200, 22%). The reverse was observed with high skin pH: individuals in the case group were found to have higher pH (155/200, 77.5%) compared to the control group (12/200, 6%). Low skin pH was observed in one patient in the case group (0.5%) and two patients in the control group (1%). The differences in proportions were statistically highly significant (Chi square= 210.452 with 2 degrees of freedom; p<0.001).
The study population comprised 200 cases and 200 age- and sex-matched controls. The male to female ratio was 1:1. Distribution of cases and controls across skin pH categories are shown in Table 1. Proportion of subjects with normal skin pH were significantly higher in the control group (186/200, 93%) as compared to the case group (44/200, 22%). The reverse was observed with high skin pH: individuals in the case group were found to have higher pH (155/200, 77.5%) compared to the control group (12/200, 6%). Low skin pH was observed in one patient in the case group (0.5%) and two patients in the control group (1%). The differences in proportions were statistically highly significant (Chi square= 210.452 with 2 degrees of freedom; p<0.001).
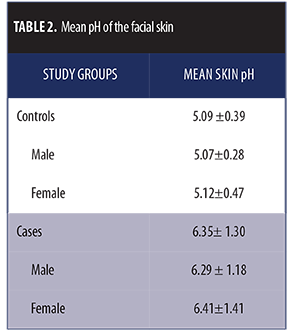
Mean pH in the control group was 5.09±0.39, which was mostly within the reference range for both sexes, and mean pH in the case group was 6.35±1.30 (Figures 1 and 2).The pH difference seen between both groups was statistically significant (p<0.001).
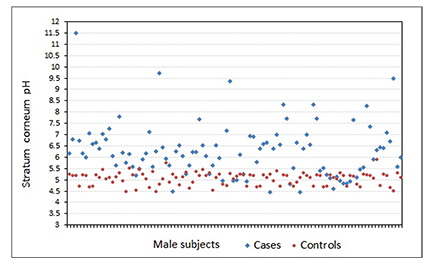
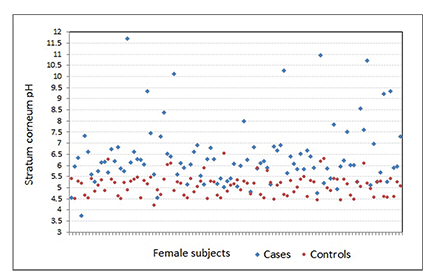
The pH difference seen between both groups was statistically significant (p<0.001).When we associated the groups with sex, mean pH among male and female participants in the control group was 5.07±0.28 and 5.12±0.47, respectively. Mean pH among male and female participants in the case group was 6.29±1.18 and 6.41±1.41, respectively (Table 2). No significant association was observed between sex and skin pH in either the control or case groups (p>0.05).
Discussion
The term pH is an acronym for “potential hydrogen” and is used to describe the acid-alkaline ratio of a substance ranging from zero (the most acidic) to 14 (the most alkaline) with 7 as its neutral phenomenon.[10] Skin pH is normally acidic, ranging between 4 and 6, while the body’s internal environment maintains a neutral to slightly alkaline pH (~7.4).[11,12] This creates a steep pH gradient of 2 to 3 units between the outermost layer of skin (i.e., the stratum corneum) and the systemic milieu. In fact, the first viable epidermal layer below the stratum corneum (stratum granulosum), which is approximately 10µm below the skin surface, reaches neutrality.[13] There is also evidence that pH increases with each deeper corneocyte layer.[14]
Formation of the acid mantle. The acidic nature of the whole skin surface was first claimed by Heuss in 1892; however, the first scientific study relating to skin surface pH appears to have been carried out by Schade and Marchionini in 1928, who called it the “acid mantle.”[6] The process of formation of the acidic cover is complex, yet interesting. Eccrine sweat coat containing lactic acid was thought to be the major determinant of acidic pH of the stratum corneum previously.[15] However, additional mechanisms have been proposed. Proton-donating free fatty acids generated from the bulk hydrolysis of epidermal phospholipids and other complex lipids in the late stages of differentiation play an important role in acidification of the surface.[16] Lipase activity of resident bacterial flora and sebaceous glands also produce free fatty acids that accumulate on the skin surface, contributing to acidity.[17] Additionally, urocanic acid generated from histidine metabolism might add significantly to the acidic pool.[18] Apart from the previously mentioned passive mechanisms, active energy-requiring pathways like the sodium/hydrogen antiporter system influences the skin pH by regulating the hydrogen ion concentration.[12,19] Thus, skin surface pH is a parameter that cumulatively represents functioning of various units of skin, including the stratum corneum.
There was no significant difference observed between the pH of a follicular comedone and its adjacent skin surface.[20] In consensus with this observation, the present study was designed to measure stratum corneum pH on the facial skin surface, which is analogous to that of pilosebaceous unit, the “crime site” in acne.
Factors influencing skin pH. A large number of variables are known to affect skin pH, which are classically categorized as exogenous and endogenous.[21] However, with reference to the disease under discussion, we considered them as modifiable and non-modifiable.
Non-modifiable factors. Non-modifiable variables are primarily the ethnic origin, age, sex, and anatomic site. The pH of pigmented skin is found to be lower than that of white skin.[22] Skin of a full-term newborn begins with a neutral pH, and the transition to acidity occurs during the first year of life, with larger incremental changes occurring in the first two months. Under physiological conditions, skin pH is maintained in the acidic range (4–5.5) throughout adult life, until old age when it rises again.[23–25] There exists a difference in physiology of skin between men and women across all age groups, mostly with a pH value below 5 in men and above 5 in women.[8] Body site plays a role in skin pH as well, with infants experiencing higher pH on extensor surfaces and prominences, such as the cheeks and buttocks.[26] In the intertrigenous areas and regions supplied by apocrine glands in the normal adult skin, the pH is found to be less acidic or even neutral and are termed physiologic holes of the acid mantle.[27] However, the pH of different areas on the face does not seem to vary considerably.[28] There could also be influence of surrounding environment and hormones underlying circadian rhythm on skin pH.[29,30]
Modifiable factors. Modifiable factors of skin pH include cleansers, topicals, sweat, sebum, and diet. Cleansers can be classified according to the type of surfactant used. Cleansers with detergent-based surfactants are known as syndets, which are generally neutral or acidic compared to the soap base, which are typically alkaline in nature.[31] There is both a short-term effect on skin pH lasting about two hours after a washing procedure and a long-term effect, with as few as two washing procedures of one minute each a day. The change in skin pH is directly proportional to the pH of the cleanser, and the effect is mutually reversible.[32] Not only does the use of cleansers and cosmetics have profound influence on skin surface pH, the use of plain tap water with a pH value generally around 8 can also increase skin pH up to six hours after application before returning to its natural value.[33]
Excessive perspiration due to heavy exercise and prolonged exposure to thermal environment could increase skin pH. However, sweat, because of moderate muscular exercise, does not alter the acidity of skin. Furthermore, muscular activity is beneficial in acne by reducing associated stress and insulin resistance.[34–36]
Nutritional factors exert promising actions on the skin, but only scant information is available on their modulating effects on the skin condition of humans. There are reports that have concluded that intake of vitamin A and monounsaturated fat can decrease skin surface pH in both sexes.[37] A positive association between acidic skin pH and increased fluid and calcium intake are also noted in the male sex.[37] Diets rich in histidine can increase urocanic acid content on skin and thereby decrease skin pH.[38]
Role of alkaline skin pH in the pathogenesis of acne. Acidic pH of stratum corneum is essential for optimal functioning of the natural barrier system of the skin. A shift in the normal skin pH and, in turn the barrier dysfunction, predisposes the skin to several inflammatory and infectious dermatoses, including acne vulgaris.[39–43]
Skin pH and follicular keratinization. Epidermal keratinization involves the formation and recycling of an effective barrier strata made of protein-rich corneocytes and lipid-rich cementing substance. The process requires several enzymes that are pH-dependent. beta-glucocerebrosidase and acidic sphingomyelinase are involved in ceramide synthesis and require a pH of 5.6 and 4.5, respectively. Formation and processing of lamellar structures in the epidermis occurs in an acidic environment.[44] A transient increase in pH toward neutrality is linked to activation of serine proteases involved in physiological desquamation by degradation of desmoglein 1.[45,46] A sustained serine protease activity, because of persistently elevated pH, can inhibit lamellar body secretion and stimulate epidermal hyperproliferation.[47] Follicular parakeratosis seen in acne-prone areas of the adult face occurring secondary to epidermal hyperplasia could be a potential consequence of deranged pH.[48]
Skin pH and bacterial growth. Bactericidal activity, because of dermicidin and nitrites in sweat, occurs optimally at pH 5.5.49,50 As skin pH rises, resident bacterial flora undergo a change: population and activity of P. acnes and Staphylococcus aureus increases as the action of antimicrobial peptides diminishes, predisposing the epithelium to infection.[32,48,51]
Skin pH and sebaceous gland activity. Seborrhea associated with acne is found to have reduced free fatty acid content that has a share in the acid mantle.[52] The resultant effect could be adverse on the skin pH.
Skin pH and testosterone. Androgens, particularly testosterone, apart from causing sebaceous gland hyperplasia and hyperactivity are also shown to slow down barrier recovery, while the estrogens and antiandrogens, such as flutamide, have the converse effect.[53] Testosterone can inhibit lamellar body formation and secretion into extracellular space. This might have implications on the skin’s acidic pH, as exocytosis of lamellar bodies is a source of protons for stratum corneum acidification.[54]
Skin pH and exogenous agents. Skin that is alkaline-pH adapted can show severe irritation to ingredients such as sodium lauryl sulfate, which is often found in skin care cosmetics and camouflaging products that are often used for “clearing” acne, explaining induced acne.[55]
Acidifying the stratum corneum in acne. Evidence in the literature shows benefits of acidifying skin surface in acne. Studies have shown that lowering the pH reduces the inflammatory TH2 response and quickens barrier function recovery, thereby preventing epidermal hyperproliferation.[56,57]
When a syndet bar of the acidic type is regularly used for cleansing the face in pre-acne conditions, the symptoms and signs of skin irritation and the occurrence of inflammatory lesions of acne were found to be lower than those of an alkaline soap.[58] The phenomena of acidifying the skin surface can help reduce the number of inflammatory papulopustules in skin affected with acne.[59] Research has shown that regular use of acidic syndets lower skin surface pH and propionibacterial count compared to the neutral preparations.[50,60] Lipids added to cleansers reduce the interactions between surfactants and skin lipids and also partially replace the skin lipid barrier removed by washing. Super fatted syndets are the mildest cleansing products, as they do not raise the pH values above normality. Citric acid acts as a pH regulator both in soap-based and nonsoap-based surfactants, reducing their alkalinity.[61]
Several studies have shown that application of antibiotics like erythromycin and other anti-acne topicals benefit acne treatment by decreasing skin surface pH.[56,62,63] Rendon et al[64] report that beta-lipohydroxy acid (LHA), a derivative of salicylic acid, reverts keratinization of pilosebaceous unit in acne to normal skin turnover. By the virtue of its pH (5.5) being similar to the normal skin, it cleanly detaches individual corneocytes rather than uneven exfoliation of cells in clumps as seen with salicylic and glycolic acid peels.[64]
In the present study, we measured facial skin pH in an Indian adult population and found that the mean pH value in the healthy control group was within the normal pH range for the stratum corneum. The sex difference in pH that we observed in both the control and case groups was insignificant. Increased skin pH was a significant feature of patients with acne compared to the controls in our study. A similar observation was made by Youn et al, who also noted a sex difference.[65] Furthermore, to validate the feature, we incorporated criteria to maintain the stratum corneum in the basal functional state and eliminate possible confounding factors that affect the results of in-vivo skin pH tests. The outcome of our study implies that affected skin of patients with acne is chronically in a state of stratum corneum instability, which could explain the underlying pathology. This is simply represented by increased skin pH at basal conditions. Since skin pH is a multifaceted index that is influenced by various factors, its distinct role in acne pathogenesis is often overlooked. However, on systematic review of the literature, it is now evident that disturbed stratum corneum pH could be pivotal to occurrence and recurrence of acne. Integrating measures that maintain skin pH while treating the condition could cut short the long tale of acne. Future research should be directed to test this hypothesis.
Conclusion
Our study clearly demonstrated the presence of significant skin pH alteration in patients with acne vulgaris of both sexes when tested under basal conditions. The increase in skin pH mirrors either an underlying disturbed stratum corneum predisposing individuals to acne or a lacuna in acne management leading to frequent recurrences. Moreover, stratum corneum pH could be a common domain through which the classical pathomechanisms might be acting in acne. We propose that skin pH could possibly be a determining element in causation of acne. Sustaining the acidic mantle during therapy by taking into account the modifiable factors and various possible interventions can pave the way for further advances in acne treatment.
References
- Bergfeld WF. The pathophysiology of acne vulgaris in children and adolescents, Part 1. Cutis. 2004;74(2):92–97.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13(6):s57–60.
- Rocha MA, Costa CS, Bagatin E. Acne vulgaris: an inflammatory disease even before the onset of clinical lesions. Inflamm Allergy Drug Targets. 2014;13(3):162–167.
- Yamamoto A, Takenouchi K, Ito M. Impaired water barrier function in acne vulgaris. Arch Dermatol Res. 1995;287(2):214–218.
- Stalder JF, Tennstedt D, Deleuran M, et al. Fragility of epidermis and its consequence in dermatology. J Eur Acad Dermatol Venereol. 2014;28 Suppl 4:1–18.
- Chikakane K, Takahashi H. Measurement of skin pH and its significance in cutaneous diseases. Clin Dermatol. 1995;13:299–306.
- Parra JL, Paye M; EEMCO Group. EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol Appl Skin Physiol. 2003;16(3):188–202.
- Luebberding S, Krueger N, Kerscher M. Skin physiology in men and women: in vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int J Cosmet Sci. 2013;35(5):477–483.
- Blank HI. Measurement of pH of the skin surface. J Invest Dermatol. 1939;2:67–79.
- Ferry RJ. A short review of the pH scale. The McAllen International Orchid Society Journal. 2004;5(8):3.
- Zlotogorski A. Distribution of skin surface pH on the forehead and cheek of adults. Arch Dermatol Res. 1987;279(6):398–401.
- Raphael KL, Murphy RA, Shlipak MG, et al.; Health ABC Study. Bicarbonate Concentration, Acid-Base Status, and Mortality in the Health, Aging, and Body Composition Study. Clin J Am Soc Nephrol. 2016 Jan 14. pii:CJN.06200615. [Epub ahead of print]
- Hanson KM, Behne MJ, Barry NP, Mauro TM, Gratton E, Clegg RM. Two-photon fluorescence lifetime imaging of the skin stratum corneum pH gradient. Biophys J. 2002;83(3):1682–1690.
- Ohman H, Vahlquist A. In vivo studies concerning a pH gradient in human stratum corneum and upper epidermis. Acta Dermatol Venereol (Stockh). 1994;74:375–379.
- Ament W, Huizenga JR, Mook GA, Gips CH, Verkerke GJ. Lactate and ammonia concentration in blood and sweat during incremental cycle ergometer exercise. Int J Sports Med. 1997;18:35–39.
- Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117(1):44–51.
- Puhvel SM, Reisner RM, Amirian DA. Quantification of bacteria in isolated pilosebaceous follicles in normal skin. J Invest Dermatol. 1975;65(6):525–531.
- Krien PM, Kermici M. Evidence for the existence of a self-regulated enzymatic process within the human stratum corneum- an unexpected role for urocanic acid. J Invest Dermatol. 2000;115:414–420.
- Reddy MM, Wang XF, Quinton PM. Effect of cytosolic pH on epithelial Na+ channel in normal and cystic fibrosis sweat ducts. J Membr Biol. 2008;225(1-3):1–11.
- Holland DB, Cunliffe WJ. Skin surface and open comedone pH in acne patients. Acta Derm Venereol. 1983;63(2):155–158.
- Yosipovitch G, Maibach HI. Skin surface pH: A protective acid mantle. Cosmet Toilet. 1996;111:101–102.
- Man MQ, Lin TK, Santiago JL, et al. Basis for enhanced barrier function of pigmented skin. J Invest Dermatol. 2014;134(9):2399–2407.
- Yosipovitch G, Maayan-Metzger A, Merlob P, Sirota L. Skin barrier properties in different body areas in neonates. Pediatrics. 2000;106:105–108.
- Fluhr JW, Pfistere S, Gloor M. Direct comparison of skin physiology in children and adults with bioengineering methods. Pediatr Dermatol. 2000;17:436–439.
- Thune P, Nilsen T, Hanstad IK, Gustavsen T, Lövig Dahl H. The water barrier function of the skin in relation to the water content of stratum corneum, pH and skin lipids. The effect of alkaline soap and syndet on dry skin in elderly, nonatopic patients. Acta Derm Venereol. 1988;68:277–283.
- Hoeger PH, Enzmann C. Skin physiology of the neonate and young infant: A prospective study of functional skin parameters during early infancy. Pediatr Dermatol. 2002;19:256–262.
- Schmid MH, Korting HC. The concept of the acid mantle of the skin: its relevance for the choice of skin cleansers. Dermatology. 1995;191(4):276–280.
- Kim MK, Choi SY, Byun HJ, et al. Comparison of sebum secretion, skin type, pH in humans with and without acne. Arch Dermatol Res. 2006;298(3):113–119.
- Abe T, Mayuzumi J, Kikuchi N, Arai S. Seasonal variations in skin temperature,skin pH, evaporative water loss and skin surface lipid values on human skin. Chem Pharm Bull (Tokyo). 1980;28(2):387–392.
- Le Fur I, Reinberg A, Lopez S, Morizot F, Mechkouri M, Tschachler E. Analysis of circadian and ultradian rhythms of skin surface properties of face and forearm of healthy women. J Invest Dermatol. 2001;117(3):718–724.
- Kirsner RS, Froelich CW. Soaps and detergents: understanding their composition and effect. Ostomy Wound Manage. 1998;44(3A):62S–70S.
- Korting HC, Braun-Falco O. The effect of detergents on skin pH and its consequences. Clin Dermatol. 1996;14(1):23–27.
- Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359–370.
- Herrmann F, Mandol L. Studies of pH of sweat produced by different forms of stimulation. J Invest Dermatol. 1955;24(3):225–246.
- Wang S, Zhang G, Meng H, Li L. Effect of Exercise-induced Sweating on facial sebum, stratum corneum hydration, and skin surface pH in normal population. Skin Res Technol. 2013;19(1):e312–317.
- Mercau ME, Repetto EM, Perez MN, et al. Moderate exercise prevents functional remodeling of the anterior pituitary gland in diet-induced insulin resistance in rats: role of oxidative stress and autophagy. Endocrinology. 2015 Dec 16:en20151777. [Epub ahead of print]
- Boelsma E, van de Vijver LP, Goldbohm RA, Klöpping-Ketelaars IA, Hendriks HF, Roza L. Human skin condition and its associations with nutrient concentrations in serum and diet. Am J Clin Nutr. 2003;77(2):348–355.
- Shibata K, Fukuwatari T, Zushi S, Sugimoto E. Effect of dietary histidine content on the change in content of skin urocanic acid isomers in hairless mice irradiated with ultraviolet B. Biosci Biotechnol Biochem. 2001;65(6):1415–1418.
- Runeman B, Faergemann J, Larkö O. Experimental Candida albicans lesions in healthy humans: Dependence on skin pH. Acta Derm Venereol. 2000;80:421–424.
- Seidenari S, Francomano M, Mantovani L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermatitis. 1998;38:311–315.
- Panther DJ, Jacob SE. The importance of acidification in atopic eczema: An underexplored avenue for treatment. J Clin Med. 2015;4(5):970–978.
- Ohman H, Vahlquist A. The pH gradient over the stratum corneum differs in x-linked recessive and autosomal dominant ichthyosis: a clue to the molecular origin of the “acid skin mantle”? J Invest Dermatol. 1998;111:674–677.
- Berg RW, Milligan MC, Sarbaugh FC. Association of skin wetness and pH with diaper dermatitis. Ped Dermatol. 1994;11:18–20.
- Bouwstra JA, Gooris GS, Dubbelaar FE, Weerheim AM, Ponec M. pH, cholesterol sulfate, and fatty acids affect the stratum corneum lipid organization. J Invest Dermatol. 1998;3:69–74.
- Zeeuwen PL. Epidermal differentiation: the role of proteases and their inhibitors. Eur J Cell Biol. 2004;83(11-12):761–773.
- Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013;93(3):261–267.
- Hachem JP, Man MQ, Crumrine D, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510–520.
- Tagami H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int J Cosmet Sci. 2008;30(6):413–434.
- Schittek B, Hipfel R, Sauer B, et al. Dermicidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–1137.
- Weller R. Price RJ. Ormerod AD. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. J Appl Microbiol. 2001;90:648–652.
- Korting HC, Hubner K, Greiner K, Hamm G, Braun- Falco O. Differences in the skin surface pH and bacterial microflora due to the long-term application of synthetic detergent preparations of pH 5.5 and pH 7.0. Results of a crossover trial in healthy volunteers. Acta Derm Venereol. 1990;70:429–431.
- Pappas A, Johnsen S, Liu JC, Eisinger M. Sebum analysis of individuals with and without acne. Dermatoendocrinol. 2009;1(3):157–161.
- Kao JS, Garg A, Mao-Qiang M, et al. Testosterone perturbs epidermal permeability barrier homeostasis. J Invest Dermatol. 2001;116(3):443–451.
- Chapman SJ, Walsh A. Membrane-coating granules areacidic organelles which possess proton pumps. J Invest Dermatol. 1989;93:466–470.
- Kim E, Kim S, Nam GW, Lee H, Moon S, Chang I. The alkaline pH-adapted skin barrier is disrupted severely by SLS-induced irritation. Int J Cosmet Sci. 2009;31(4):263–269.
- Mauro T, Holleran WM, Grayson S, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290(4):215–222.
- Hatano Y, Man MQ, Uchida Y, et al. Maintenance of an acidic stratum corneum prevents emergence of murine atopic dermatitis. J Invest Dermatol. 2009;129:1824–1835.
- Korting HC, Ponce-Pöschl E, Klövekorn W, Schmötzer G, Arens-Corell M, Braun-Falco O. The influence of the regular use of a soap or an acidic syndet bar on pre-acne. Infection. 1995;23(2):89–93.
- Schürer NY, Bock M. Lowering lesional surface pH in acne: a new treatment modality for Herpifix. J Dermatolog Treat. 2009;20(1):27–31.
- Korting HC, Kober M, Mueller M, Braun-Falco O. Influence of repeated washings with soap and synthetic detergents on pH and resident flora of the skin of forehead and forearm. Results of a cross-over trial in health probationers. Acta Derm Venereol. 1987;67(1):41–47.
- Moldovan M, Nanu A. Influence of cleansing product type on several skin parameters after single use. Farmacia. 2010;58(1):29–37.
- Korting HC, Kerscher M, Schäfer-Korting M, Berchtenbreiter U. Influence of topical erythromycin preparations for acne vulgaris on skin surface pH. Clin Investig. 1993;71(8):644–648.
- Sparavigna A, Tenconi B, De Ponti I, La Penna L. An innovative approach to the topical treatment of acne. Clin Cosmet Investig Dermatol. 2015;8:179–185.
- Rendon MI, Berson DS, Cohen JL, Roberts WE, Starker I, Wang B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3(7):32–43.
- Youn SH, Choi CW, Choi JW, Youn SW. The skin surface pH and its different influence on the development of acne lesion according to gender and age. Skin Res Technol. 2013;19(2):131–136.

