 by Hitomi Sano, MD, PhD; Yu Hokazono, MD; and Rei Ogawa, MD PhD, FACS
by Hitomi Sano, MD, PhD; Yu Hokazono, MD; and Rei Ogawa, MD PhD, FACS
Drs. Hokazono, Sano, and Ogawa are with the Department of Plastic, Reconstructive, and Aesthetic Surgery at Nippon Medical School in Tokyo, Japan.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Objective.Pathological scars, including hypertrophic scars and keloids, have a strong predilection for specific regions of the body. Such site specificity might reflect regional differences in skin properties. Greater knowledge about the characteristics of the skin at various body regions can promote the development of clinical approaches to skin incision and flap design and reduce the formation of cutaneous scars. It could also help elucidate the etiology of pathological scar development and progression. Thus, we measured the distensibility and gross elasticity of the skin at various body sites.
Methods.Five healthy adult volunteers were enrolled. In each, the cutaneous viscoelasticity at 16 sites (forehead, superior eyelid, lower jaw, earlobe, deltoid, outside and medial side of the upper arm, palm, scapular region, anterior chest, upper abdomen, lateral abdomen, lower abdomen, lateral thigh, anterior lower leg, and planta) was examined using a Cutometer MPA 580® (Courage Khazaka electronic GmbH, Cologne, Germany).
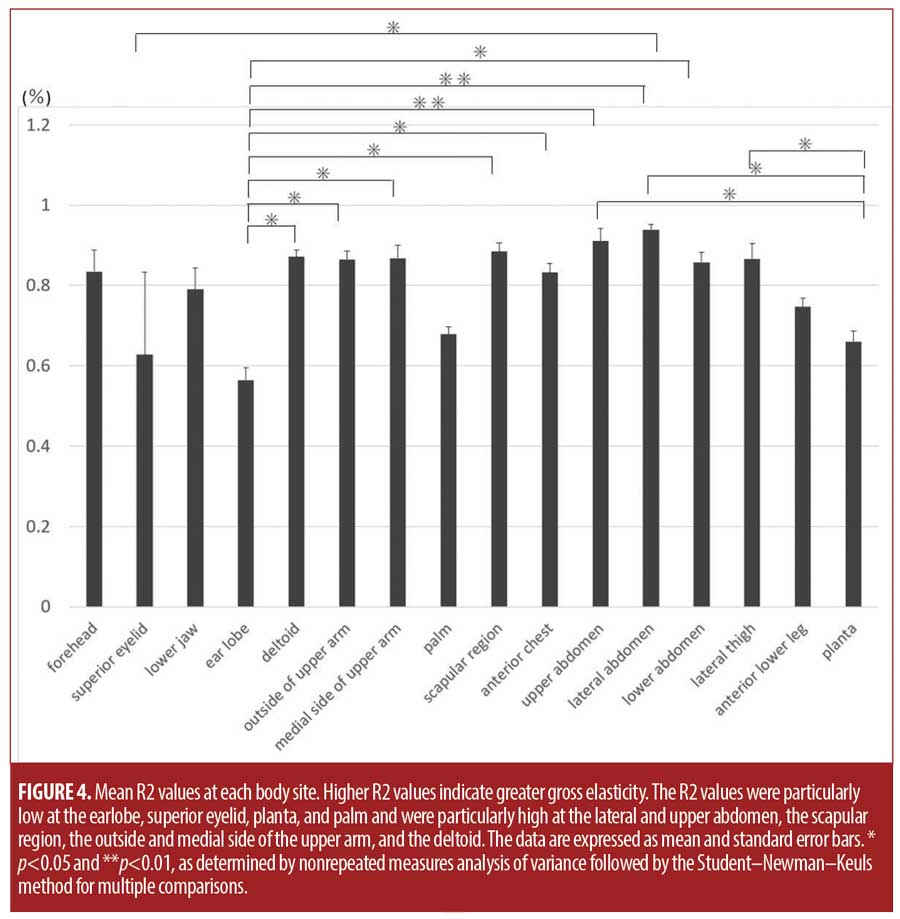
Results. The skin was particularly distensible at the medial side of the upper arm, followed by the earlobe, lower jaw, upper abdomen, lateral abdomen, lower abdomen, and superior eyelid. It was poorly distensible at the planta, followed by the anterior lower leg, palm, and forehead. The skin was poorly elastic at the earlobe, superior eyelid, planta, and palm and highly elastic at the lateral and upper abdomen, scapular region, and deltoid.
Conclusions.Except for the earlobe, all regions with poorly distensible and hard skin are not prone to pathological scarring. This association between these skin properties and abnormal scarring could be useful for skin surgeons.
Keywords: Skin extension ability, skin elasticity, keloid, scar formation, hypertrophic scar
J Clin Aesthet Dermatol. 2018;11(6):15–18
Introduction
Pathological scars, including hypertrophic scars and keloids, are strongly predisposed to occur on specific regions of the body.1,2 Such site specificity could be due to regional differences in skin thickness and hardness, the presence or absence of mechanical forces (e.g., skin tension), vascular flow, and innervation.1–3
A better understanding of skin characteristics at various body regions could help to develop clinical approaches to skin incision and flap design and reduce skin scarring. It could also help elucidate the etiology of pathological scars. However, there is little published work on the characteristics of the skin at the various regions of the whole body to date.
The skin is viscoelastic because it contains both fluidic and fibrous components. The Cutometer MPA 580® (Courage Khazaka electronic GmbH, Cologne, Germany) is a noninvasive device that measures the viscoelastic properties of skin by using the suction method. It has been used widely to evaluate skin distensibility and gross elasticity and how these properties change in aging, edema, and various skin diseases.4–7
In the present study, we measured the distensibility and gross elasticity of the skin at various body sites of healthy volunteers by using the viscoelasticity device.
Methods
Five adult Japanese volunteers were enrolled in this study. The study was reviewed and approved by the institutional review board of Nippon Medical School in Tokyo and was conducted according to the tenets of the Declaration of Helsinki and its revisions.
On each volunteer, the viscoelasticity of the skin was measured at 16 body sites, namely, the forehead, superior eyelid, lower jaw, earlobe, deltoid, outside and medial side of the upper arm, palm, scapular region, anterior chest, upper, lateral, and lower abdomen, lateral thigh, anterior lower leg, and planta (Figure 1).
Measurement of skin viscoelasticity. The Cutometer MPA 580 ® measures skin viscoelasticity8 (Figure 1) by sucking the skin into a hollow aperture in the center of the probe and then releasing it. The device has four different measurement modes that feature preprogrammed sequences of “on/off” pressure cycles. For this study, Mode 1 was used because it delivers a two-second cycle of negative air pressure (400mbar) followed by a two-second cycle of no pressure. The probe that has a 6mm, hollow aperture most efficiently measures the viscoelasticity of the dermis9,10 and thus was chosen for this study.
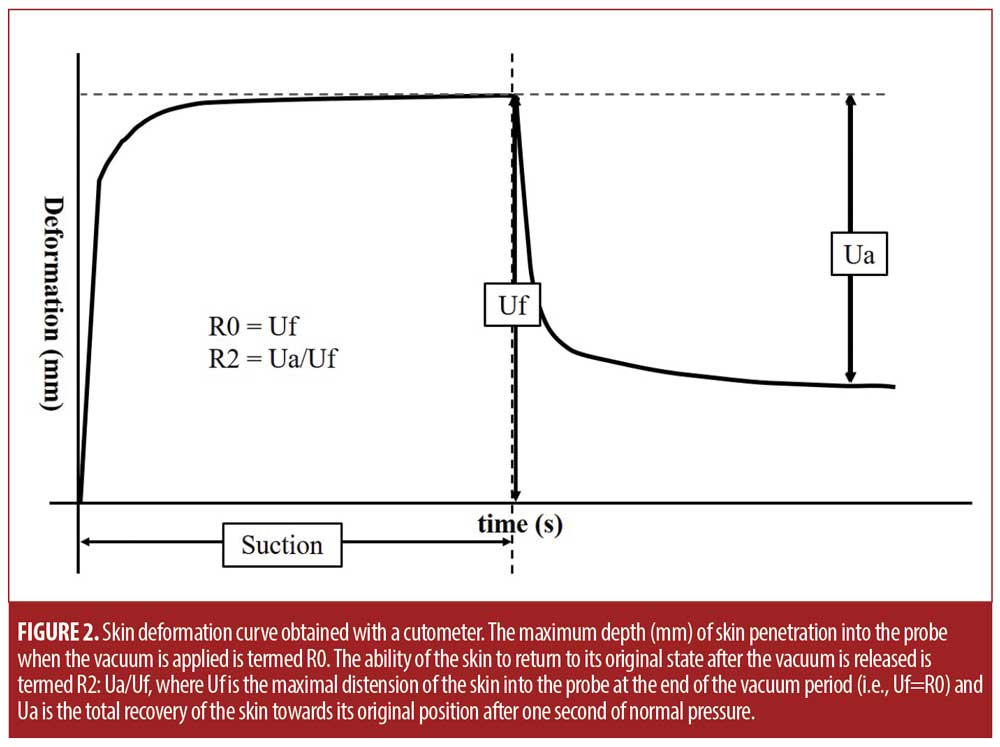
The cutometer generates a graph that depicts the skin deformation over the four-second test (Figure 2). The maximum depth (mm) of skin penetration into the probe when the vacuum is applied is termed R0: it measures skin distensibility and reflects skin firmness. The ability of the skin to return to its original state after the vacuum is released is termed R2: it reflects gross skin elasticity and is calculated by using the following formula: R2=Ua/Uf, where Uf is the maximal distension of the skin into the probe at the end of the vacuum period (i.e., Uf=R0) and Ua is the total recovery of the skin toward its original position after one second of normal pressure. Higher R0 values indicate skin that has greater capacity to deform, while higher R2 values indicate greater elasticity. Each region of the body of the right side was subjected to three consecutive measurements. All tests were performed while the subject was in a state of bequeme Stellung. Subjects were either sitting upright or laying in a supine position while the tests were performed.
Statistical analysis. The triplicate R0 and R2 measurements were expressed as means ± standard deviations (SD). Differences between body sites in terms of R0 and R2 were assessed by nonrepeated measures analysis of variance followed by the Student–Newman–Keuls method for multiple comparisons. All statistical analyses were performed by using Microsoft Excel 97?2003 (Microsoft Corp., Redmond, WA, USA). P values less than 0.05 were considered to indicate statistical significance.
Results
Two healthy men and three healthy women volunteered to participate in the present study. The average age (SD) of the volunteers was 34.6 (12.0) years. Their average height, weight, and body mass index (BMI) were 167.2 (3.4)cm, 62.4 (7.3)kg, and 22.3 (2.7)kg/m2, respectively.
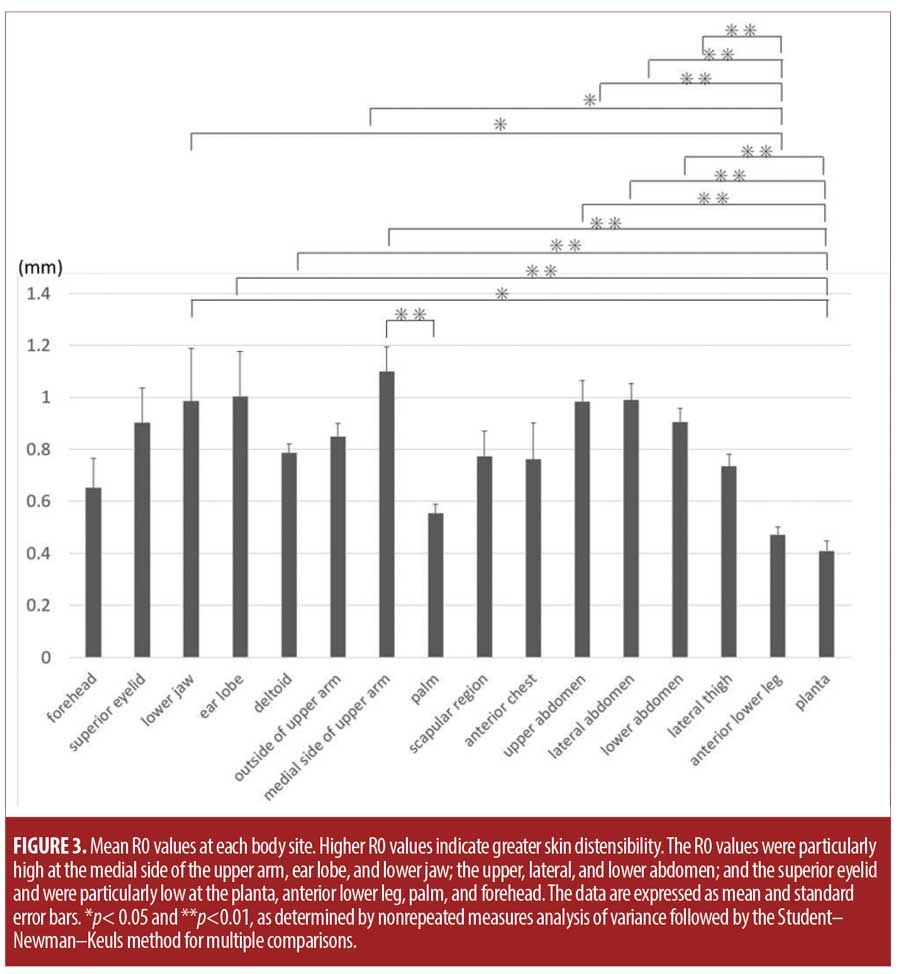
The average R0 and R2 values at the 16 body sites are shown in Figures 3 and 4, respectively. The medial side of the upper arm, followed by the earlobe; lower jaw; the upper, lateral, and lower abdomen; and the superior eyelid had particularly high R0 values. The planta, followed by the anterior lower leg, palm, and forehead, had particularly low R0 values (Figure 3). Other sites had intermediate R0 values. The planta had significantly lower R0 values than did the lower jaw; earlobe; deltoid; medial side of the upper arm; and the upper, lateral, and lower abdomen. Moreover, the anterior lower leg had significantly lower R0 values than did the lower jaw; the medial side of the upper arm; and the upper, lateral, and lower abdomen. The palm had significantly lower R0 values than did the medial side of the upper arm (Figure 3).
The lateral abdomen, followed by the upper abdomen, scapular region, deltoid, and the outer and medial sides of the upper arm, had particularly high R2 values. The earlobe, followed by the superior eyelid, planta, and palm, had particularly low R2 values (Figure 4). Other sites had intermediate values. The earlobe had significantly lower R2 values than did the deltoid; the outside and medial side of the upper arm; the scapular region; the anterior chest; and the upper, lateral, and lower abdomen. The planta had significantly lower R2 values than did the upper and lateral abdomen and the lateral thigh. The superior eyelid had significantly lower R2 values than did the lateral abdomen (Figure 4).
Discussion
Scar formation is a natural part of the healing process of skin wounds. Clinical experience shows that scars can heal differently depending on the location of the wound. For example, when a wound is located on a joint, it is more likely to result in a wide scar. This reflects the frequent joint movements that occur, which stretch the scar.
Pathological scars such as hypertrophic scars and keloids also tend to occur on sites that are highly mobile and subject to high tension, namely, the anterior chest, the shoulder, the suprapubic region, and the upper arm.1,11,12,13 Multiple recent studies suggest that mechanical force is a major driver of this pathological scar site specificity.2,3,14 It is thought that repeated and/or strong mechanical forces provoke and prolong inflammation at the wound site, which retards completion of wound healing and leads to the development of pathological scars. Indeed, the two sites that are particularly prone to pathological scarring, namely, the anterior chest and scapular area, are subject to both static tension and cyclic stretch.15 Conversely, the areas that only rarely develop pathological scars, namely, the eyelids, the palm, the anterior lower leg, the parietal region, the forehead, and the planta,16,17 are rarely subjected to skin stretching and/or contraction. In the case of eyelids, although they are highly mobile, the eyelid skin is always relaxed.
The properties of the skin at the different body regions might also influence predisposition to pathological scarring. In this study, we measured the distensibility (R0) and gross elasticity (R2) of various body sites. The medial side of the upper arm; the earlobe; the lower jaw; the upper, lateral, and lower abdomen; and the superior eyelid had pronounced skin distensibility. These observations are consistent with our clinical impressions. All of these sites are easy to operate on because the skin is relaxed and wounds are easily closed primarily. In terms of pathological scarring, the medial side of the upper arm and the superior eyelid rarely develop keloids or hypertrophic scars; indeed, wounds in these regions generally become relatively inconspicuous scars. The exception is when these regions are subject to unusual skin troubles, such as acne, or cosmetic wounding, such as skin piercing. In this case, repeated and/or prolonged inflammation could ensue, thus leading to pathological scars. Two other highly distensible sites, the lower jaw and the earlobe, are more prone to pathological scarring than are the superior eyelid and the medial side of the upper arm: this is because the earlobe is often pierced and subjected to inflammation caused by repeated infections and stretching by earrings, while the lower jaw is prone to repeated and/or prolonged acne or folliculitis.
By contrast, several other sites exhibited very low distensibility, namely, the planta, anterior lower leg, palm, and forehead. In particular, the anterior lower leg and planta exhibited significantly less distensibility versus many other regions of the body. As mentioned previously, these sites rarely develop pathological scars. These observations together suggest that low distensibility is associated with a lack of susceptibility to pathological scarring, while high distensibility is associated with pathological scarring when it is partnered with inflammatory insults, such as skin infections and piercing.
In terms of gross elasticity (R2), the earlobe, superior eyelid, planta, and palm were comparatively hard. Except for the earlobe, pathological scars are rarely observed at these sites. This could reflect the fact that pathological scars develop from the dermis, and the possibility that hard skin could attenuate the impact of mechanical forces on the dermis. Indeed, all of these hard areas have one thing in common: dermal sutures are not required during wound closure.
Limitations. One limitation of this study is the small sample size. We only measured the skin viscoelasticity of five subjects who were relatively homogeneous in terms of age and BMI. Both factors can markedly influence the thickness of skin and therefore the R0 and R2 values. Further analysis with a larger cohort of various ages and BMI values are warranted.
Conclusion
The distensibility and gross elasticity of the skin at various body sites were evaluated. Except for the earlobe, the sites with the least distensible and hardest skin appear to be less prone to pathological scarring. This information could be of interest for skin surgeons, but more research is needed to confirm our findings.
References
- Ogawa R, Mitsuhashi K, Hyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003; 111(2):547–553.
- Akaishi S, Akimoto M, Ogawa R, et al. The relationship between keloid growth pattern and stretching tension: visual analysis using the ?nite element method. Ann Plast Surg. 2008;60(4):445–451.
- Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20(2):149–157.
- Ryu HS, Joo YH, Kim SO, et al. In?uence of age and regional differences on skin elasticity as measured by the Cutometer. Skin Res Technol. 2008;14(3):354–358.
- Takema Y, Yorimoto Y, Kawai M, et al. Age-related changes in the elastic properties and thickness of human facial skin. Br J Dermatol. 1994;131(5):641–648.
- Dobrev H. In vivo study of skin mechanical properties in patients with systemic sclerosis. J Am Acad Dermatol. 1999;40(3):436–442.
- Dobrev H. Use of Cutometer to assess dermal edema in erysipelas of the lower legs. Skin Res Technol. 1998;4(3):155–159.
- Draaijers LJ, Botman YA, Tempelman FR, et al. Skin elasticity meter or subjective evaluation in scars: a reliability assessment. Burns. 2004;30(2):109–114.
- Barel AO, Courage W, Clarys P. Suction method for measurement of skin mechanical properties: the cutometer. In: Serup J, editors. Handbook of non-invasive methods and the skin. Vol. 106. Boca Raton, FL: CRC Press; 1995: 335–340.
- Pierard GE, Nikkels-Tassoudji N, Pierard-Franchimont C. Influence of the test area on the mechanical properties of skin. Dermatology. 1995;191(1):9–15.
- Hawkins HK. Pathophysiology of the burn scar. In: Herndon DN, editors. Total Burn Care. Philadelphia, PA: Saunders Elsevier; 2007: 608–619.
- From L, Assad D. Neoplasms, pseudoneoplasms, and hyperplasia of supporting tissue origin. In: Jeffers JD, Englis MR, editors. Dermatology in General Medicine. New York, NY: McGraw-Hill; 1993: 1198–1199.
- Muir IF. On the nature of keloid and hypertrophic scars. Br J Plast Surg. 1990;43(1):61–69.
- Akaishi S, Ogawa R, Hyakusoku H. Visual and pathologic analyses of keloid growth patterns. Ann Plast Surg. 2010;64(1):80–82.
- Ogawa R, Akaishi S, Kuribayashi S, Miyashita T. Keloids and hypertrophic scars can now be cured completely: recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J Nippon Med Sch. 2016;83(2):46–53.
- Niessen FB, Spauwen PH, Schalkwijk J. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104(5):1435–1458.
- Ohmori Y, Akaishi S, Ogawa R, et al. Analysis of regions where keloids tend to occur. Scar Management. 2010;4:112–115.

