J Clin Aesthet Dermatol. 2019;12(11):E53–E62
 by David M. Pariser, MD, FACP, FAAD; Lawrence J. Green, MD; Edward L. Lain, MD; Carsten Schmitz, MD, PhD; Amy S. Chinigo, MD; Brian McNamee, PhD; and David R. Berk, MD
by David M. Pariser, MD, FACP, FAAD; Lawrence J. Green, MD; Edward L. Lain, MD; Carsten Schmitz, MD, PhD; Amy S. Chinigo, MD; Brian McNamee, PhD; and David R. Berk, MD
Dr. Pariser is with the Eastern Virginia Medical School and Virginia Clinical Research, Inc. in Norfolk, Virginia. Dr. Green is with the George Washington University School of Medicine in Washington, DC. Dr. Lain is with the Austin Institute for Clinical Research in Pflugerville, Texas. Drs. Schmitz, Chinigo, and Berk are with Allergan plc in Irvine, California. Dr. McNamee is with Allergan Biologics Ltd. in Liverpool, United Kingdom.
FUNDING: This study was sponsored by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Regina Kelly, MA, of Peloton Advantage, LLC, an OPEN Health company, in Parsippany, New Jersey, and was funded by Allergan plc and Almirall, LLC.
DISCLOSURES: Dr. Pariser has been a consultant for Atacama Therapeutics, Bickel Biotechnology, Biofrontera AG, Celgene Corporation, Dermira, LeoPharma, Novartis, Regeneron, Sanofi, TDM SurgiTech, Inc., TheraVida, and Valeant Pharmaceutical International; a principal investigator for LeoPharma, Abbott Laboratories, Almirall, Amgen, AOBiome, LLC, Asana Biosciences, LLC, Bickel Biotechnology, Celgene Corporation, Dermavant Sciences, Dermira, Eli Lilly and Company, Menlo Therapeutics, Merck & Co., Inc., Novo Nordisk A/S, Ortho Dermatologics, Pfizer Inc., Regeneron, Stiefel, and Valeant Pharmaceuticals International; on a data safety monitoring board for BMS; and on an advisory board for Pfizer. Dr. Green has been an investigator, consultant, and speaker for Almirall, Kamedis, Ortho Derm and an investigator for Sol-Gel. Dr. McNamee is employed by Allergan plc and owns stock in Allergan plc. Dr. Berk was an employee of Allergan plc at the time the study was conducted and owns stock in Allergan plc. The other authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. We sought to evaluate the safety, tolerability, and patterns of use for the once-daily oral, narrow-spectrum antibiotic sarecycline in patients with moderate-to-severe acne vulgaris during a 40-week Phase III, multicenter, open-label extension study.
Participants.Patients aged nine years or older with moderate-to-severe acne who completed one of two prior Phase III, double-blind, placebo-controlled, 12-week trials in which they received sarecycline 1.5mg/kg/day or placebo were included.
Measurements. The primary assessment was the safety of sarecycline 1.5mg/kg/day for 40 weeks as indicated by adverse events (AEs), vital signs, electrocardiograms, clinical laboratory tests, and physical examinations. Patterns of sarecycline use were a secondary assessment.
Results. The safety population included 483 patients; 354 patients (73.3%) completed the study. The most common reasons for premature discontinuation were withdrawal by the patient (14.5%), lost to follow-up (7.9%), and AEs (2.5%). The most common treatment-emergent AEs (TEAEs) were nasopharyngitis (3.7%), upper-respiratory-tract infection (3.3%), headache (2.9%), and nausea (2.1%). Clinical laboratory evaluations suggested no clinically meaningful differences between the treatment sequences. Rates of TEAEs commonly associated with other tetracycline antibiotics include dizziness (0.4%) and sunburn (0.2%), and for gastrointestinal TEAEs, nausea (2.1%), vomiting (1.9%), and diarrhea (1.0%). Also reported herein are the results of a Phase I phototoxicity study.
Conclusion.Patients aged nine years or older with moderate-to-severe acne vulgaris who received sarecycline once daily for up to 40 weeks showed low rates of TEAEs, with nasopharyngitis, upper-respiratory-tract infection, headache, and nausea being the only TEAEs reported by 2% or more of patients. No clinically meaningful safety findings were noted.
ClinicalTrials.gov Registration: NCT02413346
KEYWORDS: Acne vulgaris, drug-related adverse reactions, drug-related side effects, sarecycline, tetracycline
The tetracycline class of antibiotics has been a mainstay of oral therapy for acne vulgaris for more than a half-century.1–5 In combination with topical therapy, tetracycline-class antibiotics are considered first-line treatment for inflammatory lesions of moderate-to-severe acne.6 These antibiotics exert their antibacterial effects by inhibiting bacterial protein synthesis.7,8 They exhibit antimicrobial activity against Gram-positive organisms, such as Cutibacterium acnes (formerly known as Propionibacterium acnes)9 as well as many Gram-negative organisms not implicated in acne.2,7,8 Additionally, tetracycline-class antibiotics possess anti-inflammatory properties that might enhance their effectiveness for acne.1,10–13
However, current oral tetracycline-class antibiotics for acne, which include doxycycline and minocycline, have significant limitations. Side effects can include vestibular adverse events (AEs) such as dizziness, light-headedness, tinnitus, and vertigo with minocycline; photosensitivity with doxycycline; and gastrointestinal (GI) events, such as anorexia, nausea, vomiting, and diarrhea (which are more common with doxycycline).6,14,15 Other potential tetracycline side effects include urticaria, hepatotoxicity, and potentially serious autoimmune phenomena.6 Further limitations of doxycycline and minocycline are their broad spectrum of antimicrobial activity and the potential risks of disrupting the microbial flora and prompting the development of antimicrobial resistance, factors that have spurred directives in the medical community, encouraging more responsible usage of systemic antibiotics for acne.6,7,14,17–20
Sarecycline (Seysara™; Almirall, Barcelona, Spain) is a novel, narrow-spectrum, once-daily, oral tetracycline-class antibiotic indicated for the treatment of inflammatory lesions of non-nodular moderate-to-severe acne.21 In a nonclinical study, sarecycline showed limited activity against enteric Gram-negative bacteria, yet potent activity against a range of clinical isolates of C. acnes, including tetracycline-resistant organisms.22 If confirmed in clinical studies, the narrow (more selective) antibacterial spectrum of sarecycline might have the potential to ensure less disruption of the GI microbiome. Two identically designed, pivotal, randomized, double-blind, placebo-controlled, Phase III trials (SC1401 and SC1402) in patients aged nine years and older with moderate-to-severe acne demonstrated that sarecycline 1.5mg/kg/day significantly reduced acne severity and inflammatory and noninflammatory lesion counts compared to placebo at Week 12 and was safe and well-tolerated.23 In particular, the onset of efficacy for inflammatory lesions occurred by the first follow-up visit (Week 3). For noninflammatory lesions, efficacy was also observed beginning at Week 6 in study SC1401 and Week 9 in study SC1402. Treatment-emergent AEs (TEAEs) reported by two percent or more of patients in either the sarecycline or placebo group included nausea, nasopharyngitis, and headache, although only nausea occurred with at least a one-percent higher incidence in the sarecycline group than in the placebo group (pooled rates of nausea were 3.2% vs. 1.7%), and headache was more common with placebo than sarecycline (pooled rates of 3.8% vs. 2.8%).24 Notably, rates of TEAEs reported with other tetracycline-class antibiotics—i.e., vestibular events (0.5% in the sarecycline group and 1.1% in the placebo group), phototoxic effects (0.8% and 0.3%), and GI events (6.1% and 4.3%)—were low.
As an extension of studies SC1401 and SC1402, a Phase III, multicenter, open-label study (SC1403) was conducted to evaluate the long-term safety of sarecycline 1.5mg/kg/day in patients with moderate-to-severe acne vulgaris. In addition, a Phase I, single-center, single-dose, placebo-controlled, randomized crossover study investigated the potential for sarecycline to cause phototoxicity. This paper reports the results from both studies.
Methods
Phase III open-label study. A Phase III, open-label, 40-week study (ClinicalTrials.gov identifier NCT02413346) was conducted at 52 centers in the United States involving patients who had completed 12 weeks of study treatment (in either treatment group, sarecycline or placebo) in SC1401 or SC1402 (Figure 1). Study visits occurred at Weeks 2, 6, 12, 18, 24, 32, and 40 or at the point of early termination.
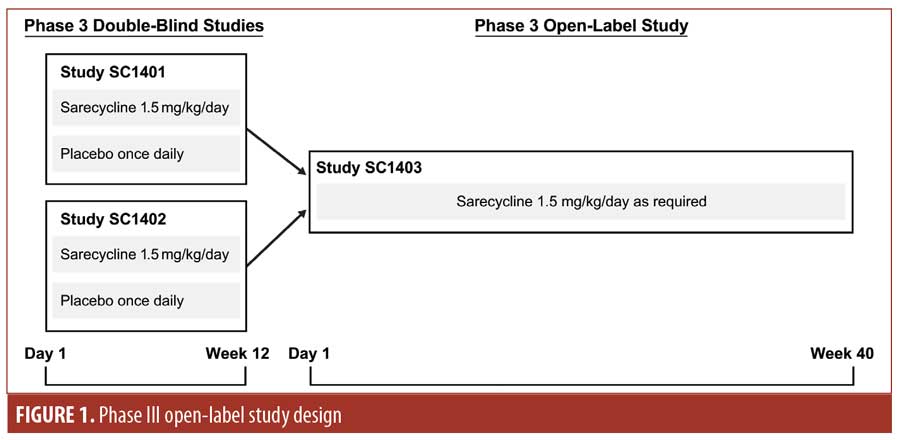
The study was conducted in accordance with Good Clinical Practice (GCP) guidelines and approved by an institutional review board (IRB). All patients and/or their legally authorized representative provided written informed consent or assent for study inclusion.
The target study enrollment size was 500 patients to allow for adequate numbers of patients to be exposed to 40 weeks of sarecycline treatment (up to 52 weeks for patients who received sarecycline in the double-blind studies). Eligible patients were those who completed either study SC1401 or SC1402. Patients enrolled in these double-blind, lead-in studies were aged 9 to 45 years, weighed 33 to 136kg, and had 20 to 50 inflammatory lesions, up to 100 noninflammatory lesions, up to two nodules, and an Investigator’s Global Assessment (IGA) score of three (moderate) or four (severe) points.23 Poor adherence with the SC1401 or SC1402 study protocols; use or planned use of systemic acne medications, retinoids, corticosteroids, androgens, or anti-androgens; significant illness; and pregnancy were among the exclusion criteria for the open-label extension study. During the study, patients were to refrain from using other systemic acne medications or medicated cleansers and to avoid tanning booths and excessive sun exposure. Concomitant topical antiacne medications were permitted.
The study population included both those who received sarecycline in the double-blind lead-in studies and in the open-label extension study (i.e., the sarecycline/sarecycline group) and those who received placebo in the double-blind studies and sarecycline in the open-label study (i.e., the placebo/sarecycline group). Patients who consented to participate in the open-label extension study constituted the screened population for SC1403. Patients from the screened population who received sarecycline either in the open-label study or in the double-blind studies composed the safety population.
The treatment regimen was one sarecycline tablet per day (about 1.5mg/kg/day) for up to 40 weeks, administered orally as 60mg, 100mg, or 150mg of sarecycline based on body weight category (33–54kg, 55–84kg, or 85–136kg, respectively). Patients were instructed to ingest the tablet at the same time each day at least one hour before or two hours after eating.
Stopping and reinitiating treatment was permitted based on the investigator’s judgment of the patient’s acne condition, which is consistent with current clinical practice.
The primary assessment was the safety of once-daily sarecycline for moderate-to-severe acne over 40 weeks as measured by AEs, vital signs, electrocardiograms (ECGs), clinical laboratory tests, and physical examinations. Patterns of sarecycline use based on the circumstances for stopping and reinitiating treatment were a secondary assessment, and IGA for inflammatory acne was an informal efficacy analysis. IGA scores (0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe) reflected the investigator’s overall general assessment of the quantity and quality of inflammatory lesions.
AEs, vital signs, and IGA scores for the severity of facial acne and, separately, of the back, neck, and chest acne were evaluated at each visit. Also performed were clinical laboratory tests at Weeks 2, 12, 24, and 40 or the point of early termination; physical examinations at Weeks 12, 24, and 40 or the point of early termination; and ECGs at Week 40 or the point of early termination. TEAEs were defined as AEs occurring on or after the date of informed consent for this open-label study and were classified using the Medical Dictionary for Regulatory Activities (MedDRA). GI side effects were also classified by preferred term for analyzing GI TEAEs based on relevant Standardized MedDRA Queries (SMQs). The study investigator assessed TEAEs as mild, moderate, or severe and as not related, possibly related, or related to study treatment.
IGA success was defined as at least a two-point decrease from baseline in the IGA score and an IGA score of clear (zero points) or almost clear (one point) for patients whose baseline IGA score was three points or more.
Data reported are from the 40-week, open-label extension study only and do not include data from the double-blind, placebo-controlled studies. Baseline values from the relevant double-blind, lead-in study were used as the baseline for all safety and efficacy parameters in this study. Statistical analyses were conducted on the screened and safety populations. Descriptive statistics were used for all continuous variables and included mean, median, 25th percentile, 75th percentile, standard deviation (SD), standard error of the mean, minimum, maximum, and number of patients. Change from baseline and percentage change from baseline were calculated for continuous endpoints. Frequency distributions for categorical variables were presented as counts and percentages.
TEAEs were summarized by the number and percentage of patients reporting a TEAE overall and by treatment group (sarecycline/sarecycline and placebo/sarecycline). The number and percentage of patients who stopped and started therapy, the reasons for stopping the first treatment course, and the average treatment duration for patients with at least one treatment course and for the overall safety population were also summarized.
Phase I phototoxicity study. A Phase I, single-center, randomized, double-blind, placebo-controlled, crossover study was conducted to evaluate the potential of sarecycline to cause phototoxicity. The study was conducted in accordance with GCP guidelines and approved by an IRB. All subjects provided written informed consent.
The study featured a two-treatment, two-period, two-sequence crossover design. Subjects received single oral doses of placebo or 240mg of sarecycline in a random order in each of the two treatment periods. Sarecycline was not dosed on the basis of body weight. Treatment periods were separated by at least nine days. A single dose of 240mg of sarecycline would be expected to provide comparable exposure to a 150-mg dose at steady state.
Between two and eight days before study treatment administration in the first period, the minimum erythemal dose (MED) of ultraviolet (UV) radiation was determined for each subject at 24 hours after exposure of an unprotected area of skin from each subject’s back to increasing doses of UVA/UVB from a solar simulator.
At three hours after administration of the study treatment, a previously unexposed area of each subject’s back was irradiated with 16J/cm2 of UVA, after which point, another area was irradiated with UVA/UVB at 50 percent of the subject’s MED.
The planned study enrollment was 20 subjects. Eligible subjects were healthy, nonsmoking men aged 18 to 45 years, weighing at least 45kg, with a body mass index of 18.5 to 29.9kg/m2 and a Fitzpatrick Skin Phototype of I (ivory white [pale-white] skin), II (white or fair skin), or III (medium-white skin). Exclusion criteria included an investigator-assessed, inadequate response to UV irradiation during MED determination; history of conditions or surgeries with the potential to impact drug absorption, metabolism, or excretion; and hypersensitivity or idiosyncratic reaction to or intolerance of tetracycline. Concomitant prescription or nonprescription medications or formulations were prohibited within 16 days of the first period and during the study.
UV-exposed skin was assessed visually at 24, 48, and 72 hours after irradiation, and UV-induced skin reaction was evaluated using the following dermal response score scale: -/0=no reaction; +/1.0=mild but definite erythema; ++/2.0=moderate erythema; +++/3.0=marked/severe erythema; Hr=hyperpigmentation; **/1.0=mild but definite edema; and ***/1.5=definite edema with erosion/vesiculation. Clinical laboratory tests, physical examinations, vital signs, and ECGs were conducted.
Mean and maximum numerical UV-induced dermal response scores were determined for sarecycline and placebo. Differences between dermal response scores for sarecycline and placebo were analyzed using analysis of variance, with sequence, subject within sequence, period, and treatment as effects. The effect of sequence was tested using the mean square of subject within sequence as an error term.
Results
Phase III open-label study. The screened population of the Phase III open-label study consisted of 490 patients, 483 of whom composed the safety population. The sarecycline/sarecycline group (i.e., patients who received sarecycline in the placebo-controlled, double-blind lead-in trials) comprised 247 patients, and the placebo/sarecycline group (i.e., patients who received placebo in the placebo-controlled, double-blind lead-in trials) included 236 patients; of these individuals, 472 (i.e., 97.7% of the safety population) received at least one treatment course of sarecycline in SC1403. The remaining 11 patients, assessed at the beginning of the study as not requiring further treatment with sarecycline, received sarecycline only in study SC1401 or SC1402 and not in SC1403.
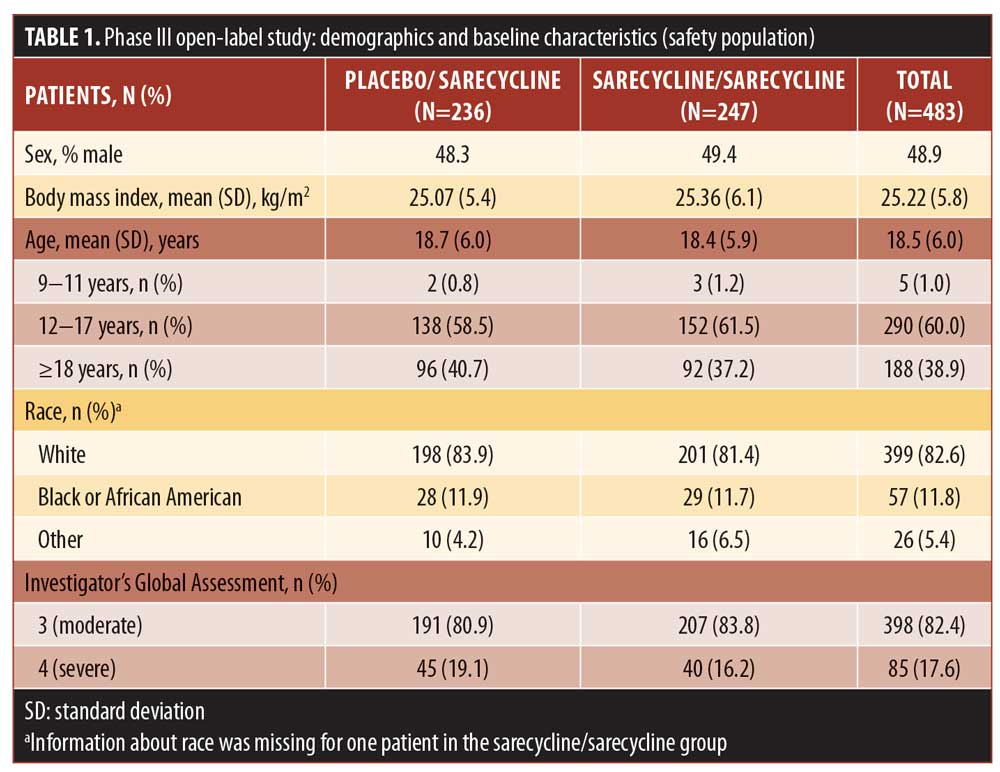
Most patients in the safety population were white (82.6%) and female (51.1%); mean (SD) age was 18.5 (6.0) years (range: 9–44 years). Demographic characteristics for the sarecycline/sarecycline and placebo/sarecycline groups were similar (Table 1).
A total of 354 patients (73.3% of the safety population) completed the study. Table 2 shows discontinuation rates and the reasons for discontinuation, the most common of which were withdrawal by the patient (14.5%), lost to follow-up (7.9%), and AEs (2.5%).
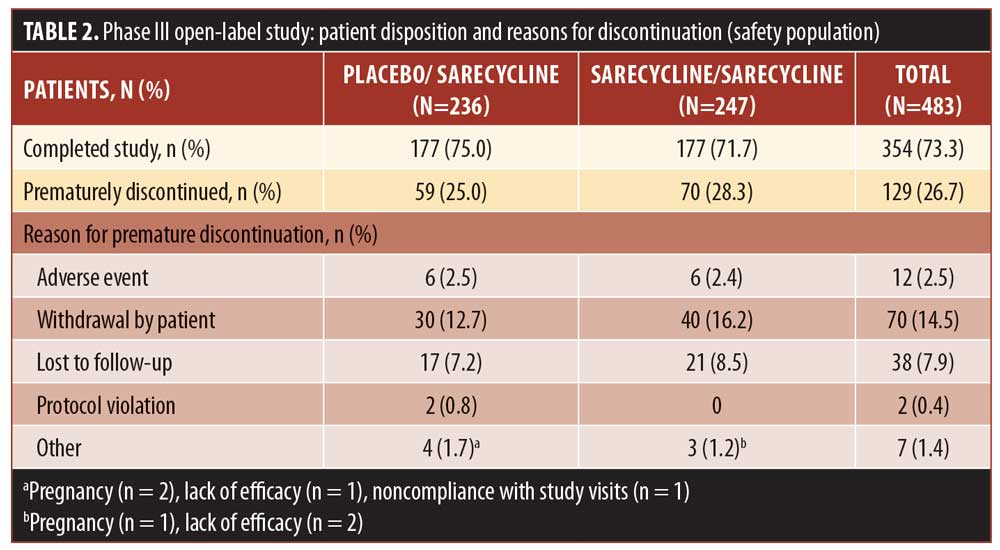
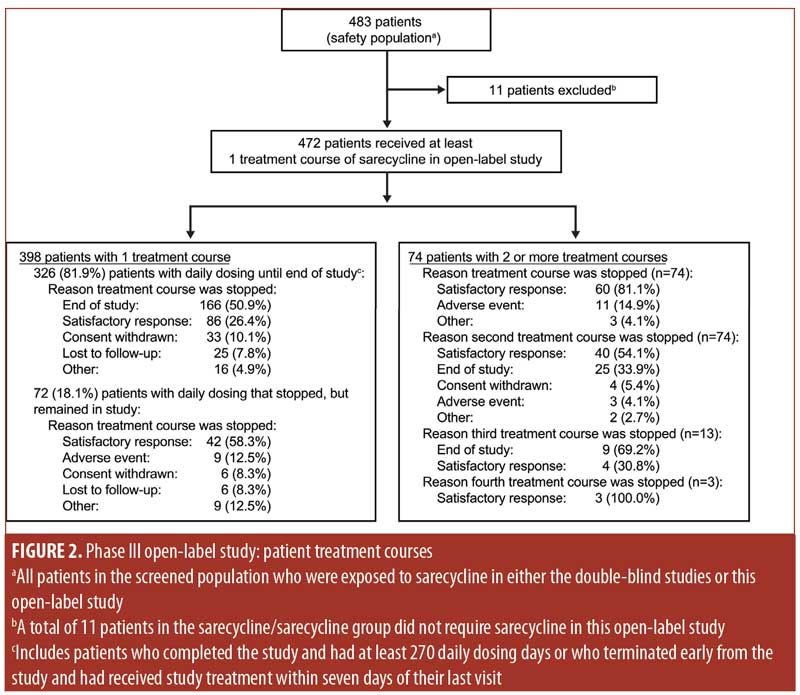
Figure 2 shows the treatment courses of patients in the safety population in the phase III open-label study. Among patients who had one treatment course (n=398 patients; 84.3%) and remained on daily sarecycline therapy until the end of the study or early termination (n=326), the most common reasons for stopping treatment were reaching the end of the study and having a satisfactory response. Most of the patients who had one treatment course but who stopped treatment and remained in the study, or who had two or more treatment courses, stopped the first treatment course because they experienced a satisfactory response.
Overall, 38.9 percent of patients in the safety population (188/483), which included patients who stopped and restarted treatment, reported at least one TEAE, including 94 patients in each group (placebo/sarecycline and sarecycline/sarecycline) (Table 3). Of 404 total TEAEs reported, most were assessed by the investigator as mild or moderate in severity (n=396; 97.5%) and not related to the study treatment (n=354; 87.6%). In the sarecycline/sarecycline group, the investigator assessed 98.5 percent (193/196) of TEAEs as being mild or moderate in severity and 89.3 percent (175/196) as not related to the study treatment, while, in the placebo/sarecycline group, the investigator considered 97.6 percent (203/208) and 86.1 percent (179/208) of TEAEs, respectively, to be mild or moderate and not related to study treatment. Table 4 details TEAEs assessed by the investigator as related or possibly related to treatment.
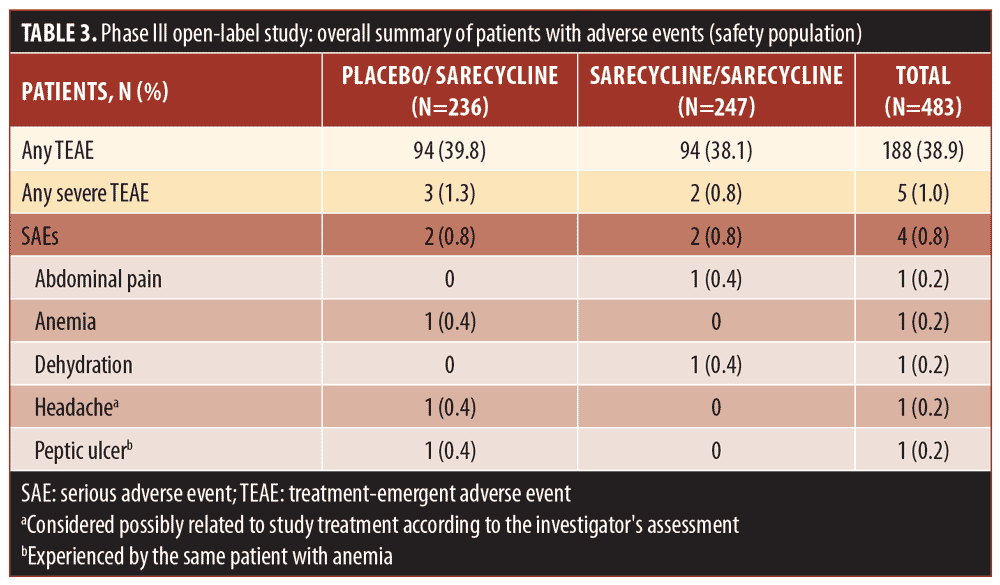
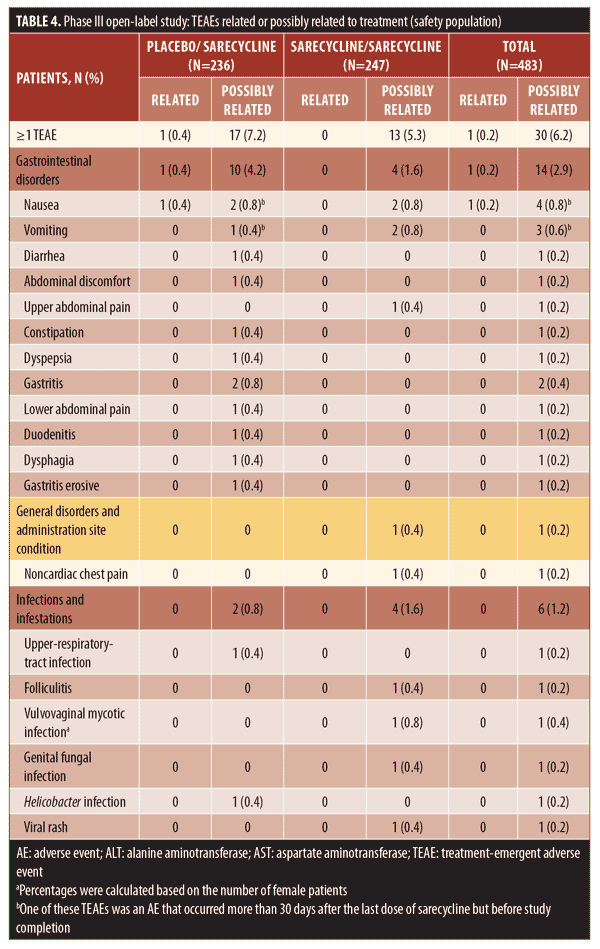
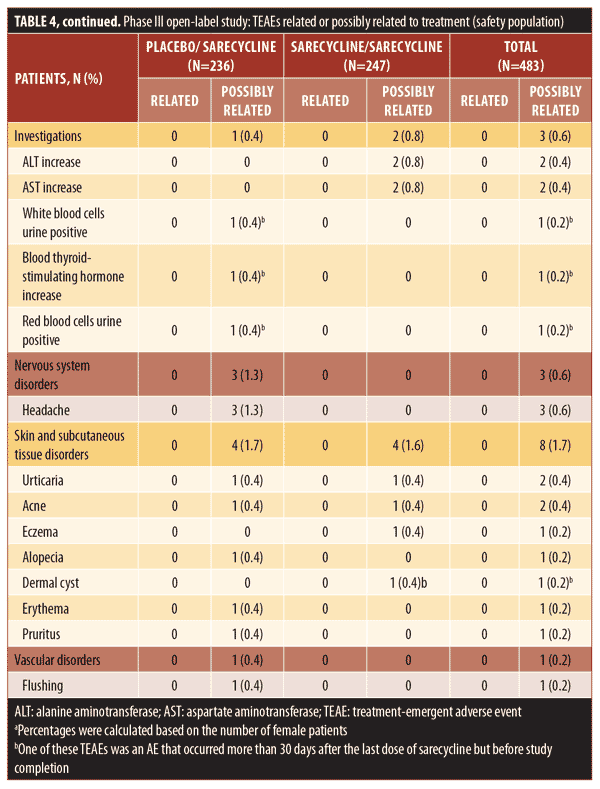
No deaths were reported, and the rate of treatment-emergent serious AEs (TESAEs) was 0.8 percent (Table 3). Five TESAEs occurred in four patients, including abdominal pain, anemia, dehydration, headache, and peptic ulcer, with anemia and peptic ulcer occurring in the same patient. The case of abdominal pain was investigator-assessed as moderate, whereas the other TESAEs were severe. However, no TESAE was deemed by the investigator as related to the study treatment, except for the case of headache, which was considered as possibly related. Ultimately, all TESAEs resolved, with study treatment withdrawn in the patients with abdominal pain, anemia, and peptic ulcer, interrupted in the patient with dehydration, and unchanged in the patient with headache.
Table 5 presents TEAEs occurring in at least two percent of patients in either the sarecycline/sarecycline group or the placebo/sarecycline group. Among all patients, the three most frequently occurring TEAEs were nasopharyngitis (18/483; 3.7%), upper-respiratory-tract infection (16/483; 3.3%), and headache (14/483; 2.9%). In the sarecycline/sarecycline group, the three most frequently occurring TEAEs were upper-respiratory-tract infection (9/247; 3.6%), nausea (6/247; 2.4%), and vomiting (6/247; 2.4%). In the placebo/sarecycline group, the three most frequently occurring TEAEs were nasopharyngitis (13/236; 5.5%), headache (9/236; 3.8%), and upper-respiratory-tract infection (7/236; 3.0%).
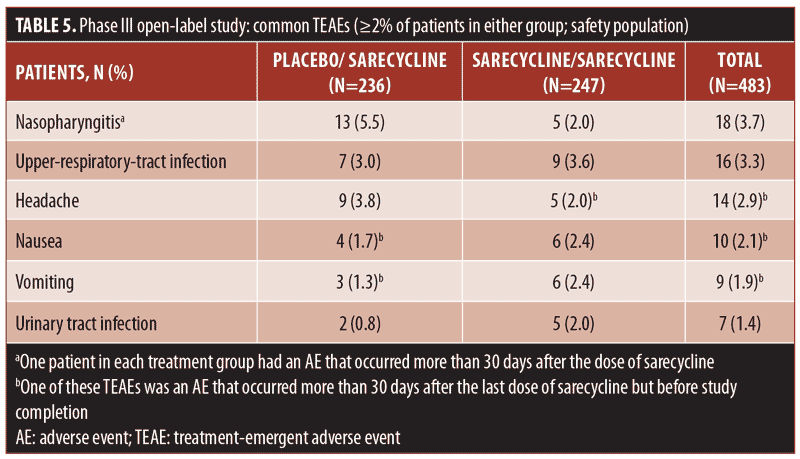
The overall rate for GI TEAEs, as reported by SMQ preferred term, was 6.6 percent (32/483), including 6.5 percent (16/247) in the sarecycline/sarecycline group and 6.8 percent (16/236) in the placebo/sarecycline group (Table 6). The most common GI TEAEs overall were nausea, vomiting, and diarrhea, occurring in 2.1 percent (10/483), 1.9 percent (9/483), and 1.0 percent (5/483) of patients, respectively.
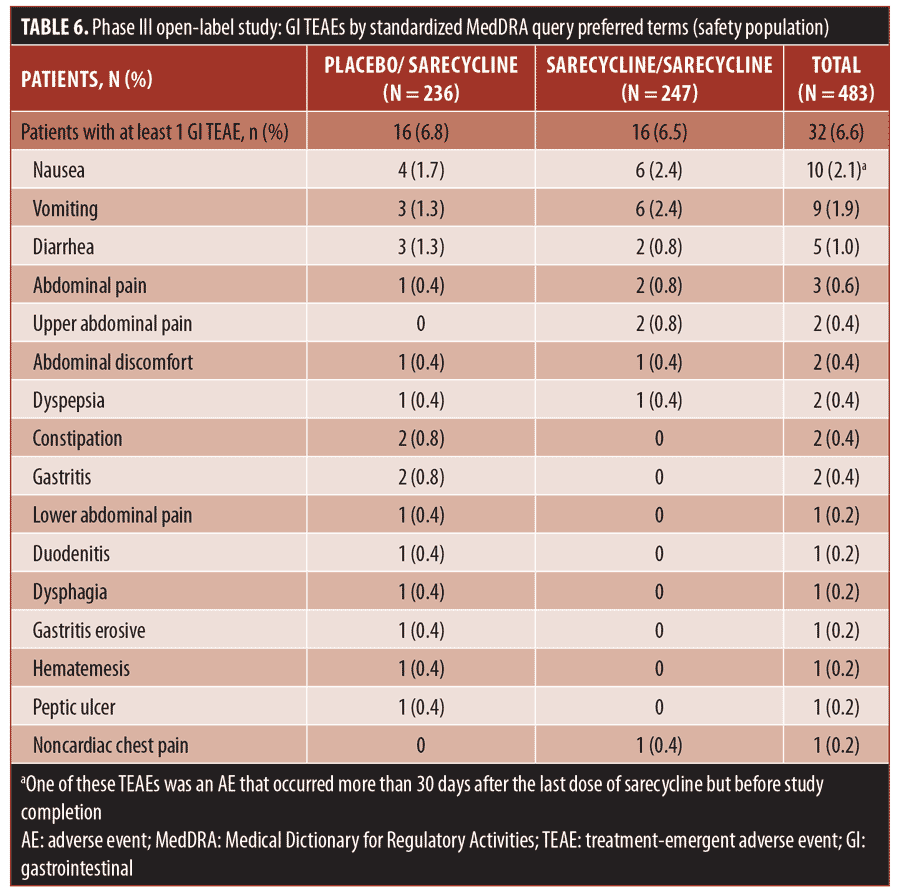
Regarding potentially phototoxic- and vestibular-related TEAEs, one patient (0.2%) in the sarecycline/sarecycline group reported sunburn, which was assessed by the investigator as mild and not treatment-related. Additionally, one patient (0.2%) in the placebo/sarecycline group reported hyperpigmentation on the upper forehead that occurred on Day 158, thought to be due to excessive sun exposure; the dose of sarecycline was unchanged due to this TEAE. The event was considered mild and not related to the study treatment per the judgment of the investigator, and the patient completed the study. The potentially vestibular-related events of nausea, vomiting, and dizziness occurred in 2.1 percent (10/483), 1.9 percent (9/483), and 0.4 percent (2/483) of patients, respectively, and there were no reports of vertigo or tinnitus.
The incidence of fungal infection TEAEs was 1.4 percent overall (7/483). Vulvovaginal mycotic infection, genital fungal infection, and genital candidiasis were reported in two patients in the sarecycline/sarecycline group (0.8% of women in the safety population of n=247 total), one patient in the sarecycline/sarecycline group (0.2% of the safety population of n=483 total), and one patient in the placebo/sarecycline group (0.2% of n=483 total), respectively. Only two fungal-infection TEAEs were considered by the investigator to be possibly related to study treatment; these included a genital fungal infection and a vulvovaginal mycotic infection, both of which were assessed by the investigator as mild, and the dose was not changed. These and other TEAEs of interest are summarized in Table 7.
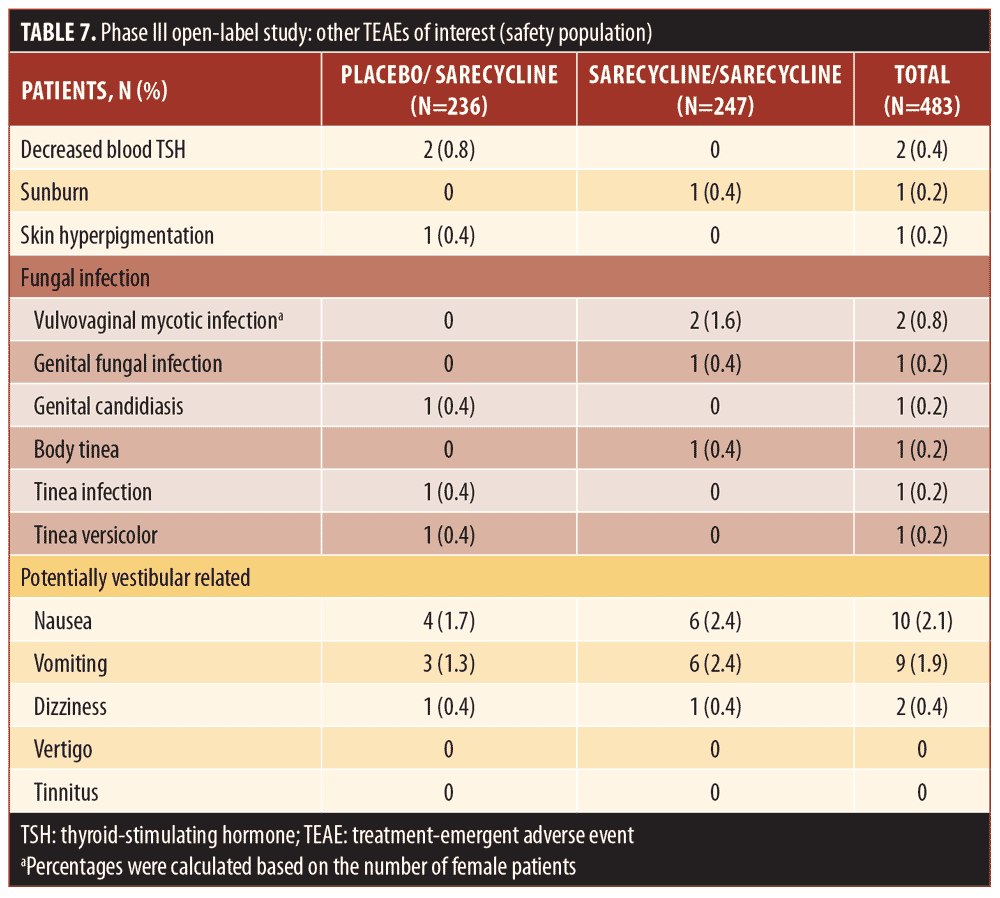
AEs leading to study discontinuation (Table 8) were reported for a total of 12 patients (2.5%), which included six (2.4%) in the sarecycline/sarecycline group and six (2.5%) in the placebo/sarecycline group. Eight of the AEs leading to study discontinuation (five among four patients in the sarecycline/sarecycline group and three among three patients in the placebo/sarecycline group) were investigator-assessed as possibly related to study treatment; the remaining six were considered by the investigator as not related. The only AE leading to the study discontinuation of more than one patient across the treatment groups was urticaria (i.e., one patient in the sarecycline/sarecycline group and one in the placebo/sarecycline group).
Laboratory testing revealed no clinically meaningful mean changes from baseline or differences between the sarecycline/sarecycline and placebo/sarecycline groups with regard to any hematology, chemistry, or urinalysis parameter. No patients had aspartate aminotransferase and/or alanine aminotransferase values on clinical laboratory testing during the study that were three or more times the upper limit of normal with concurrent total bilirubin values of two or more times the upper limit of normal, and, thus, no patient met the prespecified laboratory criteria for a possible Hy’s law case (indicative of potential drug-induced liver injury).
The percentage of patients who achieved IGA success for facial acne increased from the end of the double-blind studies to the end of this open-label trial, specifically from 18.6 percent (46/247) to 29.9 percent (72/241) in the sarecycline/sarecycline group and from 9.7 percent (23/236) to 29.1 percent (68/234) in the placebo/sarecycline group. Regarding nonfacial acne, comparable proportions of the sarecycline/sarecycline group and the placebo/sarecycline group who had baseline nonfacial IGA scores of more than zero points demonstrated at least a one-point improvement in IGA score by the end of this open-label trial.
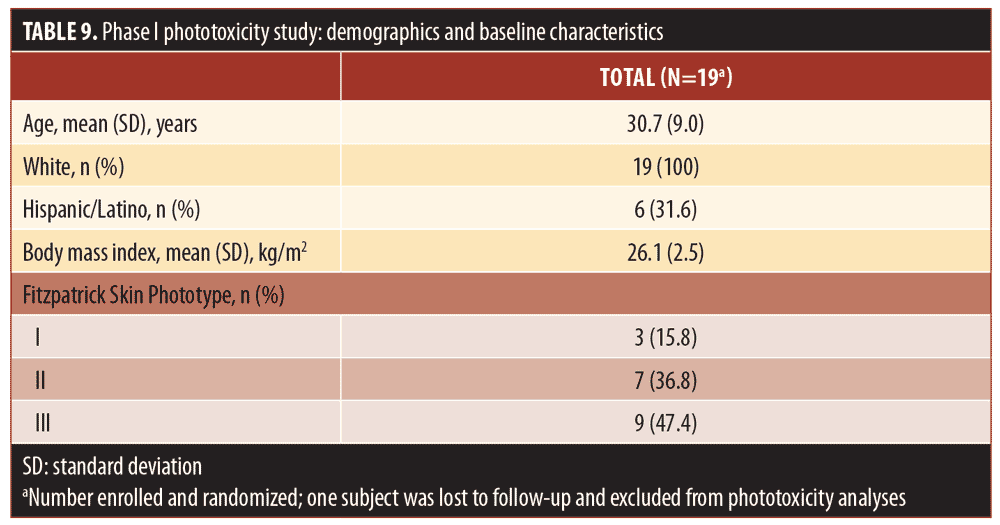
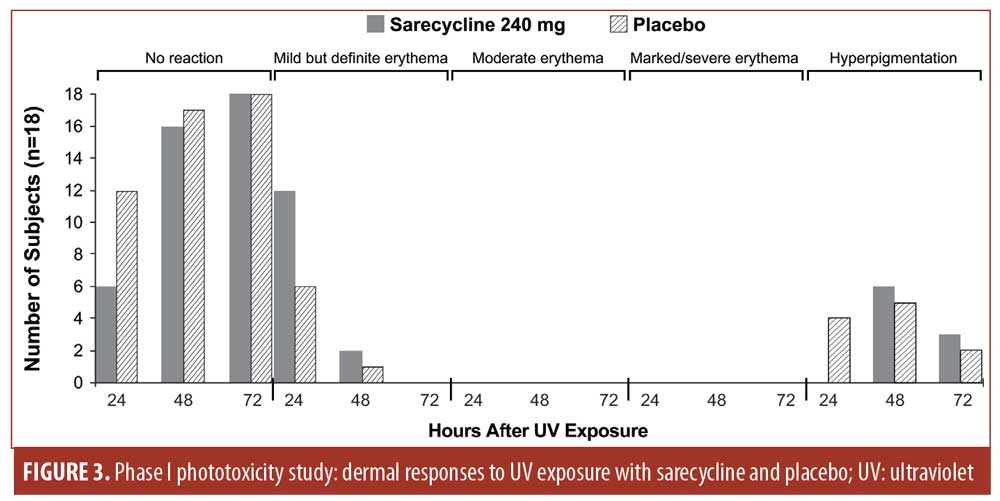
Phase I phototoxicity study. Nineteen subjects (median age: 30 years; range: 19–44 years) met all eligibility criteria and were enrolled in the Phase I phototoxicity study. In total, three, seven, and nine subjects had Fitzpatrick Skin Types I, II, and III, respectively. Table 9 summarizes the demographics and baseline characteristics of subjects in the Phase I study. One subject was lost to follow-up and a total of 18 subjects completed both treatment periods and were evaluable for UV-induced skin response.
No dermal response to UV exposure exceeding mild erythema (one point) was observed at any time point with either sarecycline 240mg or placebo (Figure 3). Sarecycline-treated subjects’ response to UV exposure at 50-percent MED was either no reaction (zero points) or mild but definite erythema (one point). Mild but definite erythema was observed in 12 sarecycline and six placebo subjects at 24 hours after UV exposure and in two sarecycline and one placebo subjects at 48 hours after UV exposure. At 72 hours postdose, there was no evidence of a dermal response to UV exposure in any subject with either sarecycline or placebo, nor was there evidence of edema at any time point in any subject.
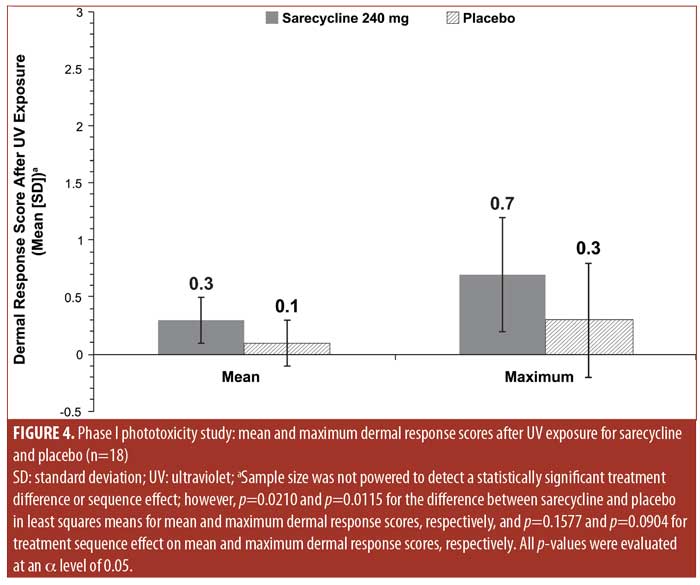
Mean and maximum numerical UV-induced dermal response scores were low for both sarecycline and placebo (0.3 and 0.7 points for sarecycline and 0.1 and 0.3 points for placebo, respectively) (Figure 4).
No TEAEs or serious AEs were reported in the phototoxicity study.
Discussion
A Phase III, long-term, open-label study was conducted in 483 patients with moderate-to-severe acne who completed one of two identically designed, pivotal, Phase III, 12-week, double-blind, placebo-controlled clinical trials involving treatment with sarecycline. Patients received once-daily oral sarecycline for up to 40 weeks. The results demonstrated the long-term safety and tolerability of sarecycline. In addition, a phase I, single-dose, randomized, double-blind, placebo-controlled crossover study examined the dermal response to UV exposure with 240mg of sarecycline or placebo in 18 healthy adult male subjects. The results supported the expectation that sarecycline has a low potential to cause clinically significant phototoxicity.
Existing oral tetracycline-class antibiotics have been associated with side effects such as nausea, vomiting, diarrhea, esophagitis, candidiasis, and photosensitivity, which limit their tolerability in the treatment of acne. Additionally, minocycline crosses the blood–brain barrier and was associated with vestibular events, such as dizziness, tinnitus, and vertigo in nine percent, two percent, and one percent of patients, respectively, in clinical trials described in the prescribing information.6,15,25 Serious AEs associated with minocycline include tissue discoloration, liver dysfunction, autoimmune hepatitis, lupus erythematosus, and other immune disorders.6,25 Doxycycline has a greater potential to cause photosensitivity than minocycline.6 Prescribing information for both doxycycline and minocycline warns of the potential development of fungal infections, which may include vaginal candidiasis.15,16 Headache is another AE commonly associated with doxycycline and minocycline usage.15,26,27
In contrast, the Phase III, long-term, open-label safety study of the novel tetracycline-class antibiotic sarecycline revealed that the three TEAEs with the highest frequencies overall were nasopharyngitis (3.7%), upper-respiratory-tract infection (3.3%), and headache (2.9%). Total rates of TEAEs commonly associated with other tetracycline-class antibiotics, including GI (6.6%), phototoxic (0.4%), fungal infection (1.4%), and vestibular (4.3%), were low.
Patients receiving doxycycline are cautioned to limit their sun exposure due to the risk of phototoxicity with the drug.16 In contrast, photosensitivity reactions in patients treated with sarecycline in the present Phase III long-term safety study were uncommon, with sunburn reported in only one patient. This is consistent with the Phase I phototoxicity study, which found that dermal response to UV exposure did not exceed mild erythema with either sarecycline or placebo at any time point and that the mean and maximum UV-induced dermal response scores for both sarecycline and placebo were low.
Neither the Phase III long-term safety study of sarecycline nor the Phase I phototoxicity study reported any clinically significant laboratory findings. It should be noted that minocycline prescribing information recommends reduced or intermittent doses of the antibiotic in cases of renal impairment to minimize the potential for excessive systemic drug levels.15 Sarecycline pharmacokinetics are not affected by mild-to-moderate hepatic impairment or by renal impairment.21 Unlike minocycline, dose adjustments are not required in conjunction with sarecycline for patients with renal impairment.15,21 Similarly, patients with mildly to moderately impaired hepatic function do not require dose adjustments of sarecycline.
Sarecycline is a new oral antibiotic therapy for the treatment of moderate-to-severe non-nodular inflammatory lesions of acne vulgaris. The clinical study results described here demonstrate that sarecycline is a well-tolerated tetracycline-class antibiotic, with a favorable safety and tolerability profile. Based on nonclinical studies, sarecycline demonstrates activity against C. acnes but has limited activity against enteric Gram-negative bacteria. Broad-spectrum tetracycline-class antibiotics for acne have been associated with disruption of the gut microbiome.28–30 The limited activity of sarecycline against enteric Gram-negative bacteria, if confirmed by clinical studies, has the potential to confer less disruption of the GI microbiome at doses recommended for acne treatment.
Limitations. The two clinical studies presented here are limited in that they do not offer direct comparisons of sarecycline and other tetracycline-class antibiotics, nor do they include clinical data supporting the possibility that sarecycline has the potential for minimized impact on the intestinal microbiome. The open-label design and lack of a placebo control group in the long-term safety study are further limitations. Additionally, it is possible that the allowance of concomitant topical acne medications and treatment discontinuations/reinitiations were confounding variables in the Phase III open-label study, although these allowances might represent conditions likely to occur in real-life use. A pooled and integrated analysis of these data with those of the two Phase III, pivotal, double-blind, placebo-controlled trials might offer a more comprehensive dataset for interpretation. The Phase III open-label study also lacked a formal efficacy analysis, with no lesion counts taken, and the sample size of the phase I study was insufficient to detect statistical significance for treatment difference or sequence effect. Finally, in the Phase III long-term safety study, sarecycline was taken one hour before or two hours after eating; however, sarecycline may be taken with or without food.21 It is possible that rates of GI AEs would have been reduced if dosing with food was allowed in this study. However, the evidence from these studies successfully conveys the safety and tolerability of sarecycline.
Conclusion
Results of this Phase III open-label study demonstrate that sarecycline, a novel, narrow-spectrum, tetracycline-class antibiotic, has demonstrated no clinically meaningful safety findings and low rates of TEAEs, with nasopharyngitis, upper-respiratory-tract infection, headache, and nausea being the only TEAEs reported by two percent or more of patients with moderate-to-severe acne vulgaris aged nine years or older treated with sarecycline once daily for up to 40 weeks.
References
- Del Rosso JQ. A status report on the use of subantimicrobial-dose doxycycline: a review of the biologic and antimicrobial effects of the tetracyclines. Cutis. 2004;74(2):118–122.
- Roberts MC. Tetracycline therapy: update. Clin Infect Dis. 2003;36(4):462–467.
- Smith JG, Jr., Chalker DK, Wehr RF. The effectiveness of topical and oral tetracycline for acne. South Med J. 1976;69(6):695–697.
- Chopra I, Hawkey PM, Hinton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992;29(3):245–277.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: Current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8(5):19–26.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33.
- Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260.
- Schnappinger D, Hillen W. Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch Microbiol. 1996;165(6):359–369.
- Scholz CF, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016;66(11):4422–4432.
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54(2): 258–265.
- Krout C, Lio PA. Tetracyclines: history and current formulation review from a dermatology perspective. Pract Dermatol. 2015;(2):51–54.
- Simonart T, Dramaix M, De Maertelaer V. Efficacy of tetracyclines in the treatment of acne vulgaris: a review. Br J Dermatol. 2008;158(2):208–216.
- Tanghetti EA. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 2013;6(9):27–35.
- Farrah G, Tan E. The use of oral antibiotics in treating acne vulgaris: a new approach. Dermatol Ther. 2016;29(5):377–384.
- Solodyn [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America; 2017.
- Acticlate [package insert]. Exton, PA: Aqua Pharmaceuticals; 2017.
- Nakase K, Nakaminami H, Takenaka Y, et al. Propionibacterium acnes is developing gradual increase in resistance to oral tetracyclines. J Med Microbiol. 2017;66(1):8–12.
- Ozuguz P, Callioglu EE, Tulaci KG, et al. Evaluation of nasal and oropharyngeal flora in patients with acne vulgaris according to treatment options. Int J Dermatol. 2014;53(11):1404–1408.
- Bienenfeld A, Nagler AR, Orlow SJ. Oral antibacterial therapy for acne vulgaris: an evidence-based review. Am J Clin Dermatol. 2017;18(4):469–490.
- Del Rosso JQ, Gallo RL, Thiboutot D, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 2: perspectives on antibiotic use and the microbiome and review of microbiologic effects of selected specific therapeutic agents commonly used by dermatologists. J Clin Aesthet Dermatol. 2016;9(5):11–17.
- Seysara [package insert]. Barcelona, Spain: Almirall; 2018.
- Zhanel G, Critchley I, Lin LY, Alvandi N. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63(1):e01297-18.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind, clinical trials. J Drugs Dermatol. 2018;17(9):987–996.
- Kircik LH, Bhatia ND, Lain E, et al. Once-daily oral sarecycline 1.5 mg/kg/day for moderate to severe acne vulgaris: pooled data from two phase 3 pivotal studies. Presented at: Annual Meeting of the American Academy of Dermatology; February 16–20, 2018; San Diego, CA.
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012(8):CD002086.
- Leyden JJ, Bruce S, Lee CS, et al. A randomized, phase 2, dose-ranging study in the treatment of moderate to severe inflammatory facial acne vulgaris with doxycycline calcium. J Drugs Dermatol. 2013;12(6):658–663.
- Moore A, Ling M, Bucko A, et al. Efficacy and safety of subantimicrobial dose, modified-release doxycycline 40 mg versus doxycycline 100 mg versus placebo for the treatment of inflammatory lesions in moderate and severe acne: a randomized, double-blinded, controlled study. J Drugs Dermatol. 2015;14(6): 581–586.
- Becker E, Schmidt TSB, Bengs S, et al. Effects of oral antibiotics and isotretinoin on the murine gut microbiota. Int J Antimicrob Agents. 2017;50(3): 342–351.
- Ferrer M, Mendez-Garcia C, Rojo D, et al. Antibiotic use and microbiome function. Biochem Pharmacol. 2017;134:114–126.
- Zaura E, Brandt BW, Teixeira de Mattos MJ, et al. Same exposure but two radically different responses to antibiotics: resilience of the salivary microbiome versus long-term microbial shifts in feces. mBio. 2015;6(6):e01693-15.

