The editors of JCAD are pleased to present this biannual column as a means to recognize select medical students, PhD candidates, and other young investigators in the field of dermatology for their efforts in scientific writing. We hope that the publication of their work encourages these and other emerging authors to continue their efforts in seeking new and better methods of diagnosis and treatments for patients in dermatology.
J Clin Aesthet Dermatol. 2019;12(6):46–54
 by Daniela Mikhaylov, BA; Peter W. Hashim, MD, MHS; Tatyana Nektalova, MD; and Gary Goldenberg, MD
by Daniela Mikhaylov, BA; Peter W. Hashim, MD, MHS; Tatyana Nektalova, MD; and Gary Goldenberg, MD
Ms. Mikhaylov and Drs. Hashim, Nektalova, and Goldenberg are with the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Goldenberg is also with Goldenberg Dermatology, PC, in New York, New York.
FUNDING: No funding was received for this study.
DISCLOSURES: Dr. Goldenberg is a consultant and speaker for Abbvie, Celgene, Eli Lilly & Co., Novartis, and SUN and a consultant for Amgen and UCB. The other authors have no conflicts of interest relevant to the content of this article.
ABSTRACTS: Psoriasis is an inflammatory skin disease that is associated with many comorbidities. Several psoriasis treatments approved by the United States Food and Drug Administration have been shown to have beneficial effects on these comorbidities, while others might lead to an exacerbation of these conditions. In this article, we review studies of psoriasis treatments and their level of evidence for use in co-occurring diseases. An awareness of the multifaceted effects of certain psoriasis medications can enable physicians to provide more personalized treatment to their most complicated patients.
KEYWORDS: Psoriasis, comorbidities, cardiovascular disease, metabolic syndrome, depression, psoriatic arthritis, ulcerative colitis, Crohn’s disease, nephrotoxicity, liver disease, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab
Psoriasis is a chronic, immune-mediated, inflammatory, multisystem disease that affects about two percent of the adult population.1 In addition to its effects on the skin, psoriasis is associated with several comorbidities, including cardiovascular disease (CVD), metabolic syndrome (defined as the combination of obesity, hypertriglyceridemia, reduced high-density lipoprotein [HDL], hypertension, and high fasting glucose), psoriatic arthritis (PsA), depression, Crohn’s disease, ulcerative colitis, drug-induced nephrotoxicity, chronic kidney disease, and nonalcoholic fatty liver disease (NAFLD).1–5 Therefore, patients with psoriasis often have higher mortality and hospitalization rates than those of the general population.2
Many psoriatic comorbidities have been linked with the chronic inflammatory nature of psoriasis as well as side effects from psoriasis medications.6 For example, patients with psoriasis often have increased inflammatory mediators in the blood, such as high-sensitivity C-reactive protein, which are predictive of cardiovascular risk. Furthermore, patients with psoriasis often have dysregulation of inflammatory and lipid metabolism genes that have been shown to be related to atherosclerotic CVD.1 In addition, certain psoriasis medications can increase the risk of CVD.7
PsA also shares specific immunopathologic and genetic pathways with psoriasis, providing a possible explanation for the frequent co-occurrence of these diseases. Specifically, patients with psoriasis often demonstrate the upregulation of tumor necrosis factor-alpha (TNF-alpha), a proinflammatory marker that is central to psoriasis pathogenesis. The upregulation of TNF-alpha results in the infiltration of T-cells and proliferation of keratinocytes in psoriatic plaques. TNF-alpha is often upregulated in the synovium of PsA patients. Polymorphisms in the TNF-alpha promoter have been shown to increase susceptibility of both psoriasis and PsA.8
Psoriasis-associated metabolic syndrome is also believed to be caused by increased levels of psoriasis proinflammatory factors, including alpha.6 There is evidence suggesting that the psoriasis proinflammatory mediators TNF-alpha and interleukin (IL)-6 are linked with depression.6 In addition, inflammatory bowel disease (IBD) most likely co-occurs in patients with psoriasis because it shares many genetic risks and inflammatory pathways with psoriasis.2 As an example, patients with psoriasis and IBD often have genetic polymorphisms in IL-23R, which lead to changes in the IL-12/23 pathway.4
In addition, kidney disease is directly related to risk factors that are common in patients with psoriasis, such as hypertension, diabetes, obesity, dyslipidemia, and metabolic syndrome.7 Since these risk factors put patients with psoriasis at higher risk for atherosclerosis, these individuals can become predisposed to developing chronic kidney disease and even end-stage renal disease.5 Several psoriasis medications, such as methotrexate and cyclosporine, are nephrotoxic, thus increasing the likelihood of kidney disease or kidney function exacerbation.7
NAFLD is another disease associated with psoriasis and ranges from simple steatosis to cirrhosis.9 It is thought that the chronic inflammation in psoriasis, caused by proinflammatory adipokines or skin-derived cytokines, can trigger NAFLD by increasing insulin resistance and leading to hepatic lipid accumulation.10
Systemic therapies that target psoriasis can reduce the risk of systemic comorbidities. These effects are possible because psoriasis shares many of the mechanisms of disease and inflammation with those of its comorbid conditions.1 In this article, we review United States Food and Drug Administration (FDA)-approved systemic treatments available for psoriasis, with a focus on their multisystem benefits as well as possible exacerbating characteristics. Knowledge about these treatment options for patients with psoriatic comorbidities can help physicians better individualize care for their most complex patients.
Methods
To investigate the systemic effects of FDA-approved psoriasis treatments on well-documented psoriasis comorbidities, we conducted a PubMed search of articles using the term psoriasis combined with each of the following: systemic treatment, comorbidities, cardiovascular disease, metabolic syndrome, depression, psoriatic arthritis, ulcerative colitis, Crohn’s disease, nephrotoxicity, liver disease, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, secukinumab, ixekizumab, brodalumab, and guselkumab. For each article, the type of study and endpoints were noted. Phase III clinical trials and meta-analyses of randomized, controlled studies were preferentially chosen, but, if none existed, lower-evidence studies, such as cohort studies, case reports, and case-control studies were used. Some additional sources were found by looking at the references of the articles identified during the initial search.
We used the guidelines by Shekelle et al11 to document the highest level of available evidence for each medication and indication. Level IA indicates evidence for meta-analysis of randomized, controlled trials (RCTs). Level IB represents evidence from at least one RCT. Level IIA represents evidence from at least one controlled study without randomization. Level IIB represents evidence from at least one other type of quasi-experimental study. Level III represents evidence from nonexperimental descriptive studies, including comparative studies, correlation studies, and case-control studies. Lastly, Level IV represents evidence from expert committee reports, opinions, or clinical experience of respected authorities.
Nonbiologic Systemic Medications
Nonbiologic systemic medications that are FDA-approved for psoriasis include methotrexate, acitretin, cyclosporine, and apremilast. A summary of these medications and their level of evidence for psoriatic comorbidities can be found in Table 1.

Methotrexate. Methotrexate is an antimetabolite that inhibits the synthesis of deoxyribonucleic acid (DNA) by blocking dihydrofolate reductase and thymidylate reductase.12 Methotrexate has been shown to have several systemic effects on patients with psoriasis. For example, a large, five-year cohort study showed a decrease in the incidence of cerebrovascular disease and atherosclerosis in patients with psoriasis and rheumatoid arthritis taking a low cumulative dose of methotrexate.13 Another large cohort study showed that patients with severe psoriasis who were treated with methotrexate had a lower risk of cardiovascular death, myocardial infarction (MI), and stroke as compared to patients treated with topicals, phototherapy, and climate therapy.14 In contrast, a retrospective study showed that methotrexate does not significantly improve metabolic syndrome in patients with PsA.15 Another study associated methotrexate treatment with an increase in triglycerides and a decrease in HDL in patients with psoriasis.16 One meta-analysis showed methotrexate’s efficacy in treating PsA,17 while another demonstrated its benefit in maintaining remission from Crohn’s disease.18 However, a different meta-analysis revealed no benefit in inducing remission from ulcerative colitis relative to placebo.19 Methotrexate has also been shown to lead to renal damage and even acute renal failure; therefore, patients should be well hydrated and monitored for drug-drug interactions and creatinine levels while taking methotrexate.20 Methotrexate has also been shown to decrease renal and creatinine clearance, and thus should be used with caution in patients with renal disease.21 Patients with NAFLD or any chronic liver disease are at an increased risk for methotrexate-induced hepatotoxicity and hepatic fibrosis.22 A retrospective case series showed that preexisting liver pathology in patients with psoriasis might be a risk factor for severe hepatotoxicity.23 In this study, 62.5 percent of the patients with preexisting periportal fibrosis progressed to bridging fibrosis or cirrhosis upon methotrexate treatment. Due to the potential for acute and chronic hepatotoxicity, the package insert cautions against use of methotrexate in the presence of preexisting chronic liver disease.20
Acitretin. Acitretin is an oral retinoid that is approved for psoriasis treatment.24 In addition to its effectiveness in psoriasis, several studies in both humans and animals have shown that retinoids such as acitretin slow atherosclerotic disease progression.25,26 However, a cohort study revealed that, in a subset of patients with psoriasis, acitretin was associated with an increased risk of hyperlipidemia.27 Therefore, these patients should have regular lipid screenings and should be treated for hyperlipidemia to avoid increased CVD risk. More studies are needed to determine the effect of acitretin on CVD risk.25 Dermatologists should use caution when prescribing acitretin to patients with obesity and/or high blood lipid levels.28,29 Acitretin is considered ineffective for PsA, and the package insert cautions against use in patients with kidney disease.28 Regarding hepatotoxicity, a study linked acitretin with transaminitis, but there was no evidence of hepatotoxicity in biopsies after two years of treatment.30 Furthermore, acitretin rarely results in severe hepatotoxic reactions (0.26%).31 However, since acitretin can cause hyperlipidemia, it should be avoided in patients with psoriasis and NAFLD.32
Cyclosporine. Cyclosporine is an immunosuppressive medication that is approved for psoriasis and has both positive and negative effects on certain psoriatic comorbidities.33 In a nationwide cohort study, cyclosporine failed to reduce cardiovascular events in patients with psoriasis.14 Another cohort study showed an association with increased triglyceride levels, increased risk of hypercholesterolemia (odds ratio: 1.34), and increased relative risk for developing arterial hypertension and diabetes (odds ratios: 3.31 and 2.88, respectively) in patients with psoriasis.27 A separate study found that 12 percent of patients with psoriasis treated with cyclosporine developed new-onset hypertension.34 It is therefore advised that cyclosporine be used only for a short duration, with alternative medications started once the patient’s skin has improved.25
A meta-analysis demonstrated that cyclosporine was effective in the treatment of PsA.17 One meta-analysis showed that high doses or cyclosporine resulted in clinical improvements in Crohn’s disease, and another showed moderate efficacy of cyclosporine for ulcerative colitis.35,36 Cyclosporine should be avoided in patients with renal abnormalities due to nephrotoxicity.37 A recent study reported that cyclosporine increased the risk of renal dysfunction in patients with psoriasis and preexisting kidney disease.38 According to expert opinion, the length of treatment with cyclosporine correlates with nephrotoxicity. Intermittent treatment with 12-week courses decreases nephrotoxic risk when compared to continuous or long-term therapy.37 Additionally, the package insert notes associations with hepatotoxicity and liver injury in some cases, especially in patients with psoriasis and underlying comorbidities.33 Specifically, since cyclosporine increases lipid levels, it can potentially exacerbate NAFLD.32
Apremilast. Apremilast is an oral phosphodiesterase-4 inhibitor approved for use in patients with psoriasis and PsA.39 It has also been shown to be generally safe in patients with psoriasis and certain comorbidities. A safety analysis that was pooled from two Phase III RCTs showed that patients with psoriasis treated with apremilast for up to 156 weeks did not demonstrate an increased risk of major adverse cardiovascular effects (MACE).40 However, additional long-term studies are needed to evaluate the definitive effects on cardiovascular risk reduction in patients with psoriasis.25 A meta-analysis showed that apremilast is highly effective in treating PsA.41 In regards to kidney disease, one study reported that patients with mild-to-moderate renal impairment did not exhibit changes in renal elimination upon apremilast treatment, while those with severe renal impairment did; therefore, dose reduction is recommended in patients with severe renal dysfunction.42 In a Phase III RCT in patients with psoriasis, serum alanine transaminases (ALTs) were elevated three times the upper limit of normal in 0.2 percent of the apremilast group and 0.4 percent of the placebo, and there were no liver-related serious adverse events. Therefore, apremilast might be suitable for patients with psoriasis and comorbid NAFLD.43
TNF-alpha Inhibitors
Etanercept, adalimumab, infliximab, and certolizumab pegol are TNF-alpha inhibitors that are FDA-approved for psoriasis and PsA. In addition to these indications, adalimumab and infliximab are approved for Crohn’s disease and ulcerative colitis, while certolizumab pegol is approved for Crohn’s disease.4,44 However, with regard to cardiovascular comorbidities, it remains unclear as to whether TNF-alpha inhibitors significantly reduce the risk of CVD. Some studies have associated TNF-alpha inhibitors with a decrease in the risks of MI and CVD in patients with psoriasis.25,45 In comparison, other studies indicate that TNF-alpha inhibitors cause no change in MACE.46 There is conflicting evidence for individual TNF-alpha inhibitors, suggesting that additional studies are needed before firm conclusions can be made regarding effects on the cardiovascular system. A summary of these TNF-alpha inhibitors and their level of evidence for psoriatic comorbidities can be found in Table 2.
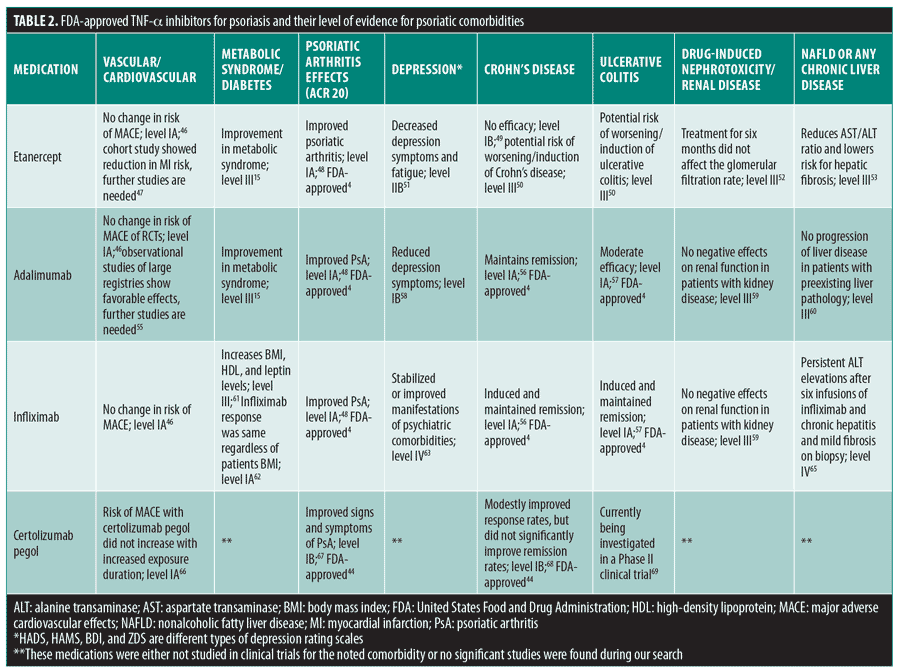
Etanercept. There are mixed reports on the effects of etanercept on the cardiovascular system. A retrospective cohort study showed that etanercept decreased the risk of MI, compared to topical agents in patients with psoriasis.47 However, a meta-analysis showed no change in the risk of MACE relative to placebo.46 A retrospective study reported that etanercept improved metabolic syndrome components (waist circumference, triglycerides, HDL, and glucose) in patients with PsA.15 In addition, most studies report that etanercept improves PsA.48 However, etanercept does not appear to be effective in treating Crohn’s disease.49 A large retrospective study found that etanercept can actually induce or worsen IBD in some patients.50 Therefore, physicians should be careful when prescribing etanercept to patients with psoriasis and comorbid IBD.4 Etanercept might also help with depression. A Phase III RCT showed that patients with psoriasis who were treated with etanercept had improvements in both depressive symptoms and fatigue as shown on the Hamilton Depression Rating Scale (HAM-D) and Beck’s depression inventory (BDI).51 Additionally, a study showed that etanercept treatment for six months did not affect the glomerular filtration rate in patients with psoriasis.52 Another study comparing etanercept to phototherapy in patients with psoriasis, NAFLD, and metabolic syndrome found significant reductions (p<0.05) in aspartate transaminase to alanine aminotransferase ratio (AST/ALT) and significant increases in insulin sensitivity after 24 weeks of treatment. As insulin resistance is directly correlated with hepatic fibrosis, this study highlights that etanercept seems to be more effective in reducing the risk of hepatic fibrosis than psoralen and ultraviolet A (PUVA) light therapy in patients with psoriasis and NAFLD.53
Adalimumab. There are conflicting findings on the effects of adalimumab on the cardiovascular system. A meta-analysis reported that adalimumab does not appear to cause a change in the risk of MACE.46 Another study showed no difference in vascular inflammation in the carotids of patients with psoriasis after 52-week treatment with adalimumab.54 However, observational studies have shown reductions in MIs alpha inhibitors such as adalimumab. Therefore, further studies are needed to definitively conclude the effect of adalimumab on heart disease in patients with psoriasis.55
A retrospective study showed that adalimumab improved metabolic syndrome components in PsA patients.15 Meta-analyses have also shown that adalimumab is effective in both PsA and Crohn’s disease.48,56 Furthermore, a meta-analysis demonstrated that adalimumab has a moderate efficacy in ulcerative colitis, but that biologics, such as infliximab, might be more effective.57 With regard to depression, an RCT in patients with psoriasis treated with adalimumab showed a significant reduction in depression symptoms, as measured by the Zung Self-rating Depression Scale (ZDS) (p<0.001).58 Furthermore, another study reported that adalimumab and other TNF-alpha inhibitors did not have a negative effect on renal function in patients with kidney disease.59 A retrospective investigation indicated that, in patients with psoriasis and liver disease who were treated with adalimumab for five years, including three patients with NAFLD, none had progression of liver disease.60
Infliximab. A meta-analysis found no change in the risk of MACE in patients with psoriasis after treatment with infliximab compared to placebo.46 However, a pretest–post-test study revealed that infliximab increased body mass index (BMI), as well as HDL and leptin levels, suggesting that lipid profiles and weight should be monitored in patients receiving this treatment.61 A meta-analysis showed efficacy in patients with obesity, as infliximab response is independent of BMI, unlike response with other biologics.62 Infliximab has been found to benefit patients with PsA, ulcerative colitis, and Crohn’s disease.4 Meta-analyses have demonstrated that infliximab improves PsA, as well as reduces and maintains remission of Crohn’s disease and ulcerative colitis.48,56,57
Infliximab can also have beneficial effects on psychiatric disorders, although the level of evidence is weak. A case series reported three cases of psoriasis and comorbid depression in which infliximab was effective in stabilizing or symptoms of depression.63
A separate study showed that infliximab and other TNF-alpha inhibitors do not have a negative effect on renal function in patients with kidney disease.59 Furthermore, severe liver toxicity is rare in infliximab treatment.64 A case report of a 53-year old patient with PsA revealed persistent ALT elevations after six infusions of infliximab, as well as chronic hepatitis and mild fibrosis on biopsy.65
Certolizumab pegol. A meta-analysis conducted in patients with rheumatoid arthritis showed that the risk of MACE with certolizumab pegol treatment did not increase with increased drug exposure duration.66 A Phase III RCT in patients with PsA showed improvements in symptoms of PsA upon certolizumab pegol treatment.67 Another Phase III RCT conducted in patients with Crohn’s disease showed modest improvement in response rates, but no significant improvement in remission rates upon certolizumab pegol treatment.68 Certolizumab pegol is currently being investigated in a Phase II clinical trial for its potential use in patients with ulcerative colitis.69
Other Biologic Medications
Other biologic medications that are FDA-approved for psoriasis include ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab, and tildrakizumab. A summary of these medications and their level of evidence for psoriatic comorbidities can be found in
Table 3.
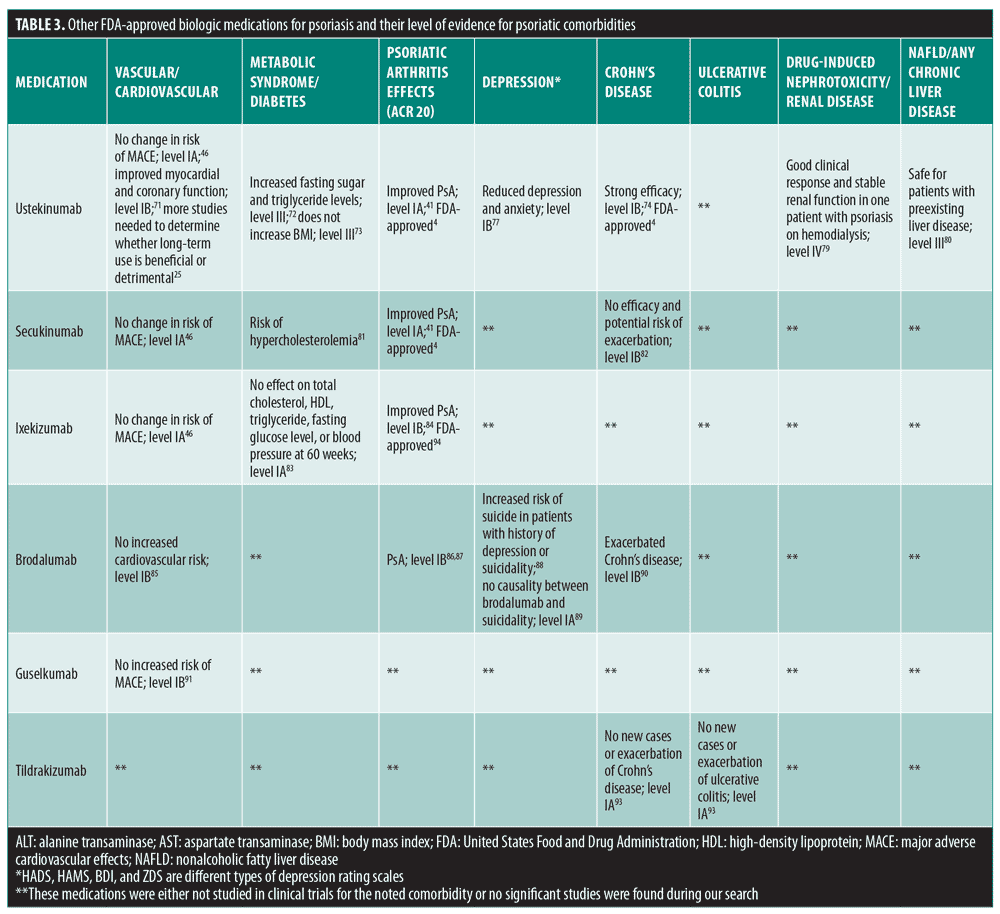
Ustekinumab. Ustekinumab is an IL-12/23 inhibitor that offers systemic effects that can be beneficial to patients with psoriasis and certain comorbidities.70 The drug has also been FDA-approved for use in patients with PsA, Crohn’s disease, and ulcerative colitis.4 However, ustekinumab’s effect on the vascular system is unclear. A meta-analysis showed that patients with psoriasis treated with ustekinumab had no change in the risk of MACE relative to placebo (Table 3).46 Conversely, a recent RCT indicated that ustekinumab might improve myocardial and coronary function in patients with psoriasis,71 while a cohort study reported that its use increased fasting sugar and triglyceride levels.72 However, a separate study reported that ustekinumab did not increase BMI in patients with chronic plaque psoriasis.73 These conflicting findings demonstrate the need for further research investigating ustekinumab’s effects on the cardiovascular system.25
A meta-analysis of RCTs showed that ustekinumab is effective in PsA.41 Other RCTs have also reported efficacy of ustekinumab use in Crohn’s disease.74–76 Ustekinumab has also been demonstrated to help with depression and anxiety in patients with psoriasis. A Phase III RCT that investigated ustekinumab treatment in patients with psoriasis reported significant reductions in the Hospital Anxiety and Depression Scale scores (p<0.001 for both depression and anxiety).77 According to the package insert, nephrotoxicity has not been reported or formally studied for ustekinumab.78 A recent case report described a patient with psoriasis and end-stage renal disease who was on hemodialysis that showed a good clinical response and renal stabilization after ustekinumab treatment.79 Furthermore, a retrospective study reported that ustekinumab-induced liver injury was rare and mild in patients with psoriasis, and that ustekinumab is safe for patients with psoriasis and preexisting liver disease.80
Secukinumab. Secukinumab is an IL-17A inhibitor that is approved for psoriasis and PsA,4 and has been shown to have variable effects on different comorbidities in patients with psoriasis. For example, a meta-analysis showed no difference in the risk of MACE in patients with psoriasis treated with secukinumab compared to placebo.46 On the other hand, according to the package insert, clinical trials have reported that a higher percent of patients on secukinumab developed hypercholesterolemia compared to those on placebo.81 Another meta-analysis suggested that secukinumab is effective in treating PsA.41 Importantly, a Phase II clinical trial found that secukinumab exacerbated Crohn’s disease.82 Therefore, secukinumab should be prescribed with caution in patients with psoriasis and comorbid IBD.
Ixekizumab. Ixekizumab is another IL-17A inhibitor approved for psoriasis and PsA treatment.4 A meta-analysis showed no difference in the risk of MACE in patients with psoriasis treated with ixekizumab compared to placebo.46 Similarly, a meta-analysis showed no significant changes between patients with psoriasis treated with ixekizumab or placebo for total cholesterol, HDL, triglyceride, fasting glucose levels or for blood pressure at 60 weeks.83 However, a Phase III clinical trial suggests that ixekizumab is effective for treating PsA.84
Brodalumab. Brodalumab, a human immunoglobulin (Ig) G2 monoclonal antibody, inhibits the human IL-17 receptor A and is approved for use in patients psoriasis.4 Regarding psoriatic comorbidities, brodalumab was studied in Phase II and III trials and did not appear to increase cardiovascular risk.85 In addition, multiple Phase II trials have found that brodalumab is effective in patients with PsA.86,87 On the other hand, according to the package insert, brodalumab has a suicide and depression warning and should be carefully considered before prescribing to patients with a history of suicidality or depression.88 However, a recent study that analyzed several psoriasis clinical trials did not find causality between suicide and brodalumab treatment.89 Thus, more research is needed regarding the safety of brodalumab use in patients with psoriasis and comorbid depression. In addition, a Phase II trial that was conducted for treatment of Crohn’s disease with brodalumab was terminated early because it exacerbated the disease.90 Therefore, brodalumab is contraindicated in patients with Crohn’s disease.
Guselkumab. Guselkumab is a human IgG1 monoclonal antibody that inhibits the p19 subunit of IL-23, and is approved for use in patients with psoriasis.4 Phase III trials have shown that guselkumab does not appear to be associated with an increased risk of MACE as compared with placebo.91
Tildrakizumab. Tildrakizumab is a humanized IgG1 kappa monoclonal antibody that inhibits IL-23 p19 and has been recently gained FDA approval for use in psoriasis.92 A recent analysis of three large clinical trials reported that no new cases of IBD or exacerbation of IBD occurred in patients with psoriasis who were taking tildrakizumab.93
Conclusion
Psoriasis is associated with numerous comorbidities, and selecting the proper treatment can be challenging. Some medications can help with one comorbidity and exacerbate another. Studies of psoriasis medications should continue to explore the effects of study drugs on comorbid disease among patients with psoriasis to help minimize the number of medications required for these patients and to help ensure the application of more personalized therapies that avoid adverse events.
References
- Churton S, Brown L, Shin TM, Korman NJ. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74(2): 169–182.
- de Oliveira M de FSP, Rocha B de O, Duarte GV. Psoriasis: Classical and emerging comorbidities. An Bras Dermatol. 2015;90(1):9–20.
- Amir Y, Lebwohl MG. Review of available and investigational biologics and non-biologic small molecules for the treatment of plaque psoriasis. J Psoriasis Psoriatic Arthritis. 2016;2(1):11–21.
- Whitlock SM, Enos CW, Armstrong AW, et al. Management of psoriasis in patients with inflammatory bowel disease: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2018;78(2):383–394.
- Ungprasert P, Raksasuk S. Psoriasis and risk of incident chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2018;50(7):1277–1283.
- Gottlieb AB, Chao C, Dann F. Psoriasis comorbidities. J Dermatolog Treat. 2008;19(1): 5–21.
- Gonzalez-Parra E, Dauden E, Carrascosa JM, et al. Kidney disease and psoriasis. a new comorbidity? Actas Dermosifiliogr. 2016;107(10):823–829.
- Veale DJ, Ritchlin C, FitzGerald O. Immunopathology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2): ii26–ii29.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231.
- Wenk KS, Arrington KC, Ehrlich A. Psoriasis and non-alcoholic fatty liver disease. J Eur Acad Dermatol Venereol. 2011;25(4):383–391.
- Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999;318(27):593–596.
- Strober BE. Methotrexate and cyclosporine in psoriasis revisited. Semin Cutan Med Surg. 2014;33(2 Suppl 2):S27–S30.
- Prodanowich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262–267.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatology Venereol. 2015;29(6):1128–1134.
- Costa L, Caso F, Atteno M, et al. Impact of 24-month treatment with etanercept, adalimumab, or methotrexate on metabolic syndrome components in a cohort of 210 psoriatic arthritis patients. Clin Rheumatol. 2014;33(6):833–839.
- Owczarczyk-Saczonek A, Drozdowski M, Maciejewska-Radomska A, et al. The effect of subcutaneous methotrexate on markers of metabolic syndrome in psoriatic patients–preliminary report. Adv Dermatology Allergol Dermatologii i Alergol. 2018;35(1):53–59.
- Ash Z, Gaujoux-Viala C, Gossec L, et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: Current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2012;71(3):319–326.
- Patel V, Wang Y, Macdonald JK, et al. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014;2014(8):CD006884.
- Chande N, Wang Y, Macdonald JK, Mcdonald JWD. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014;2017(12):CD006618.
- Methotrexate [package insert]. Hospira Inc. Lake Forest, IL. 2011.
- Kremer JM, Petrillo GF, Hamilton RA. Pharmacokinetics and renal function in patients with rheumatoid arthritis receiving a standard dose of oral weekly methotrexate: association with significant decreases in creatinine clearance and renal clearance of the drug after 6 months of therapy. J Rheumatol. 1995;22(1):38–40.
- Shetty A, Cho W, Alazawi W, Syn WK. Methotrexate hepatotoxicity and the impact of nonalcoholic fatty liver disease. Am J Med Sci. 2017;354(2):172–181.
- Malatjalian DA, Ross JB, Williams CN, et al. Methotrexate hepatotoxicity in psoriatics: report of 104 patients from Nova Scotia, with analysis of risks from obesity, diabetes and alcohol consumption during long term follow-up. Can J Gastroenterol. 1996;10(6):369–375.
- Lee CS, Li K. A review of acitretin for the treatment of psoriasis. Expert Opin Drug Saf. 2009;8(6): 769–779.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: Focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18(10). pii: E2211.
- Mottaghi A, Salehi E, Keshvarz A, et al. The influence of vitamin A supplementation on Foxp3 and TGF-beta gene expression in atherosclerotic patients. J Nutrigenet Nutrigenomics. 2013;5(6):314–326.
- Gisondi P, Cazzaniga S, Chimenti S, et al. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the Italian Psocare Registry. J Eur Acad Dermatol Venereol. 2013;27(1):e30–e41.
- SORIATANE® (acitretin) [package insert]. Stiefel Laboratories, Inc. Research Triangle Park, NC; 2014.
- Gisondi P, Del Giglio M, Girolomoni G. Considerations for systemic treatment of psoriasis in obese patients. Am J Clin Dermatol. 2016;17(6):609–615.
- Roenigk HHJ, Callen JP, Guzzo CA, et al. Effects of acitretin on the liver. J Am Acad Dermatol. 1999;41(4):584–588.
- Vahlquist C, Selinus I, Vessby B. Serum lipid changes during acitretin (etretin) treatment of psoriasis and palmo-plantar pustulosis. Acta Derm Venereol. 1988;68(4):300–305.
- Prussick R, Prussick L, Nussbaum D. Nonalcoholic fatty liver disease and psoriasis: what a dermatologist needs to know. J Clin Aesthet Dermatol. 2015;8(3):43–45.
- Sandimmune® [package insert]. Novartis Pharmaceuticals Corporation: East Hanover, NJ; 2015.
- Ho VC, Griffiths CE, Albrecht G, et al. Intermittent short courses of cyclosporin (Neoral®) for psoriasis unresponsive to topical therapy: a 1-year multicentre, randomized study. Br J Dermatol. 2001;141(2):283–291.
- McDonald JWD, Feagan BG, Jewell D, et al. Cyclosporine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2005;(2):CD000297.
- Feuerstein JD, Akbari M, Tapper EB, Cheifetz AS. Systematic review and meta-analysis of third-line salvage therapy with infliximab or cyclosporine in severe ulcerative colitis. Ann Gastroenterol. 2016;29(3):341–347.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850.
- Kim BR, Yang S, Doh EJ, et al. Risk factors affecting adverse effects of cyclosporine A in a real-world psoriasis treatment. Ann Dermatol. 2018;30(2):143–149.
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75(12):1393–1403.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: Pooled safety analysis for 156 or more weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77(2):310–317.e1.
- Ramiro S, Smolen JS, Landewé R, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature review for the 2015 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2016;75(3):490–498.
- Liu Y, Zhou S, Assaf M, et al. Impact of renal impairment on the pharmacokinetics of apremilast and metabolite M12. Clin Pharmacol Drug Dev. 2016;5(6):469–479.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49.
- Lee EB, Amin M, Bhutani T, Wu JJ. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5–9.
- Wu JJ, Poon KYT, Channual JC, Shen AYJ. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148(11):1244.
- Rungapiromnan W, Yiu ZZN, Warren RB, et al. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2017;176(4):890–901.
- Wu JJ, Poon K-YT, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12(8):899–903.
- Cawson MR, Mitchell SA, Knight C, et al. Systematic review, network meta-analysis and economic evaluation of biological therapy for the management of active psoriatic arthritis. BMC Musculoskelet Disord. 2014;15(1):26.
- Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn’s disease: A randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121(5):1088–1094.
- O’Toole A, Lucci M, Korzenik J. Inflammatory bowel disease provoked by etanercept: report of 443 possible cases combined from an IBD referral center and the FDA. Dig Dis Sci. 2016;61(6): 1772–1774.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35.
- Gisondi P, Girolomoni G. Glomerular filtration rate in patients with psoriasis treated with etanercept. J Int Med Res. 2016;44(1 Suppl):106–108.
- Campanati A, Ganzetti G, Di Sario A, et al. The effect of etanercept on hepatic fibrosis risk in patients with non-alcoholic fatty liver disease, metabolic syndrome, and psoriasis. J Gastroenterol. 2013;48(7):839–846.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-alpha antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137(8):1638–1645.
- Lebwohl M. Does treatment of psoriasis reduce cardiovascular comorbidities?. J Invest Dermatol. 2017;137(8):1612–1613.
- Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology. 2015;148(2):344–354.
- Mei WQ, Hu HZ, Liu Y, et al. Infliximab is superior to other biological agents for treatment of active ulcerative colitis: a meta-analysis. World J Gastroenterol. 2015;21(19):6044–6051.
- Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62(5):812–818.
- Hueber AJ, Tunc A, Schett G, Manger B. Anti-tumour necrosis factor alpha therapy in patients with impaired renal function. Ann Rheum Dis. 2007;66(7):981–982.
- Vilarrasa E, Puig L, Alomar A. Biologic treatments for psoriasis in patients with hepatitis C virus infection and other liver diseases: experience in 29 patients.2010;24.
- Ehsani AH, Mortazavi H, Balighi K, et al. Changes in body mass index and lipid profile in psoriatic patients after treatment with standard protocol of infliximab. Acta Med Iran. 2016;54(9):570–575.
- Reich K, Menter A, Plotnick M, Et al. Consistency of infliximab response across subgroups of patients with psoriasis: integrated results from randomized clinical trials. Psoriasis Forum. 2007;13a(1):21–27.
- Bassukas ID, Hyphantis T, Gamvroulia C, et al. Infliximab for patients with plaque psoriasis and severe psychiatric comorbidity. J Eur Acad Dermatology Venereol. 2008;22(2):257–258.
- Remicade (infliximab) [package insert]. Janssen Biotech, Inc. Horsham, PA; 2013.
- Germano V, Picchianti Diamanti A, et al. Autoimmune hepatitis associated with infliximab in a patient with psoriatic arthritis. Ann Rheum Dis. 2005;64(10):1519–1520.
- Bykerk VP, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis. 2015;74(1):96–103.
- Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73(1):48–55.
- Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s Disease. N Engl J Med. 2007;357(3):228–238.
- ClinicalTrials.gov. Study of Cimzia for the Treatment of Ulcerative Colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT01090154. Accessed July 31, 2018.
- Koutruba N, Emer J, Lebwohl M. Review of ustekinumab, an interleukin-12 and interleukin-23 inhibitor used for the treatment of plaque psoriasis. Ther Clin Risk Manag. 2010;6:123–141.
- Ikonomidis I, Papadavid E, Makavos G, et al. Lowering interleukin-12 activity improves myocardial and vascular function compared with tumor necrosis factor-alpha antagonism or cyclosporine in psoriasis. Circ Cardiovasc Imaging. 2017;10(9). pii: e006283.
- Ng CY, Tzeng IS, Liu SH, et al. Metabolic parameters in psoriatic patients treated with interleukin-12/23 blockade (ustekinumab). J Dermatol. 2017;45(3):309–313.
- Gisondi P, Conti A, Galdo G, et al. Ustekinumab does not increase body mass index in patients with chronic plaque psoriasis: a prospective cohort study. Br J Dermatol. 2013;168(5):1124–1127.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960.
- Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367(16): 1519–1528.
- Sands BE, Han C, Gasink C, et al. Tu2006 ustekinumab improves general health status and disease-specific health related quality of life of patients with moderate to severe Crohn’s disease: results from the UNITI and IMUNITI phase 3 clinical trials. Gastroenterology. 2016;150(4):S1004.
- Langley RG, Feldman SR, Han C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63(3):457–465.
- STELARA® (ustekinumab) [package insert]. Janssen Biotech, Inc. Horsham, PA; 2012.
- Nimmannitya K, Tateishi C, Mizukami Y, et al. Successful treatment with ustekinumab of psoriasis vulgaris in a patient undergoing hemodialysis. J Dermatol. 2016;43(1):92–94.
- Llamas-Velasco M, Concha-Garzon MJ, Garcia-Diez A, Dauden E. Liver injury in psoriasis patients receiving ustekinumab: a retrospective study of 44 patients treated in the clinical practice setting. Actas Dermosifiliogr. 2015;106(6):470–476.
- COSENTYX® (secukinumab) [package insert]. Novartis Pharmaceuticals Corporation. East Hanover, NJ; 2018.
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12): 1693–1700.
- Egeberg A, Wu JJ, Korman N, et al. Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J Am Acad Dermatol. 2018;79(1):104–109.e8.
- Mease PJ, Heijde D Van Der, Ritchlin CT, et al. Ixekizumab , an interleukin-17A specific monoclonal antibody , for the treatment of biologic-naive patients with active psoriatic arthritis: results from controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2016;76(1):1–9.
- Strober B, Eichenfield LF, Armstrong A, et al. Overview of adverse cardiovascular events in the brodalumab psoriasis studies. J Am Acad Dermatol. 2017;76(6):AB186.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306.
- Nakagawa H, Niiro H, Ootaki K. Brodalumab , a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis?: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1): 44–52.
- SILIQTM (brodalumab) [package insert]. Valeant Pharmaceuticals International, Inc. Bridgewater, NJ; 2017.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78(1):81–89.e5.
- Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2016;111(11):1599–1607.
- Nakamura M, Lee K, Jeon C, et al. Guselkumab for the treatment of psoriasis: a review of phase III trials. Dermatol Ther (Heidelb). 2017;7(3): 281–292.
- Markham A. Tildrakizumab: first global approval. Drugs. 2018; 78(8):845–849.
- Gooderham M, Elewski BE, Pariser DM, et al. THU0291 Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate to severe plaque psoriasis: data from 3 large randomised clinical trials. Ann Rheum Dis. 2018;77(Suppl 2):364.
- TALTZTM (ixekizumab) [package insert]. Eli Lilly and Company. Indianapolis, IN; 2016.

