J Clin Aesthet Dermatol. 2019;12(10):29–34
 by Nisrine Kawa, MD; Kachiu C. Lee, MD, MPH; R. Rox Anderson, MD; and Lilit Garibyan, MD, PhD
by Nisrine Kawa, MD; Kachiu C. Lee, MD, MPH; R. Rox Anderson, MD; and Lilit Garibyan, MD, PhD
Drs. Kawa, Anderson, and Garibyan are with the Wellman Center for Photomedicine at Massachusetts General Hospital and the Department of Dermatology at Harvard Medical School in Boston, Massachusetts. Dr. Lee is with the Department of Dermatology at Brown University in Providence, Rhode Island.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Onychomycosis is a challenging nail condition to treat. The gold standard treatment relies on long-term systemic therapy, which carries risks of potential side effects and drug interactions. Topical alternatives exist; however, treatment outcomes remain disappointing. In this article, we review newer topical formulations that are approved by the United States Food and Drug Administration, as well as other topical drugs that are still undergoing clinical trials. Lasers and energy-based devices have also been used for the treatment of onychomycosis; however, standardized parameters and clear treatment endpoints have yet to be specified. Currently, device-based therapies are considered as options for improving the cosmetic appearance of nails. The use of lasers to improve the penetration of topical antifungal treatments as possible combination treatments is also reviewed.
KEYWORDS: Antifungal, fungus, laser, nail, onychomycosis
Onychomycosis is estimated to affect 5.5 percent of the world’s population.1 Onychomycosis can be painful and have significant psychosocial effects, negatively impacting quality of life.2 Fungal pathogens disrupt the integrity of the skin by means of scaling and fissure formation. These pathogens typically start by penetrating at the distal end of the nail bed, causing distal subungual onychomycosis, which is the most common form of fungal nail disease. Less frequently, infection at the surface of the nail plate can lead to white superficial onychomycosis; invasion near the cuticle can result in proximal subungual onychomycosis; and candidal infections of the nail can occur in case of human immunodeficiency virus and decreased immunity. This allows for penetration of pathogenic bacterial species, thus predisposing the patient to infections and cellulitis.3 Risk factors for onychomycosis include age, peripheral vascular disease, diabetes, and immunosuppression.4
Various treatment modalities are available for onychomycosis. While mild (<20% nail involvement) and moderate (20%–60% nail involvement) forms of onychomycosis can benefit from topical therapies, severe cases require systemic antifungals. Nevertheless, long-term treatment success rates vary widely and relapse rates remain high.1,4
Currently, a 12-week course of oral terbinafine is considered the gold standard for the treatment of onychomycosis. However, the complete cure rate at 36 weeks after the completion of terbinafine in the original approval study was reported to be only 38 percent, and the great toenail alone was used for outcomes assessments.5 There are studies that report cure rates ranging from 14 to 90 percent with oral terbinafine for onychomycosis.6 This range could be due to different parameters and outcomes measures used to evaluate cure rates.
Treatment failure with oral and topical antifungals is thought to be multifaceted.7 While patient factors such as age, health status, adherence, and polypharmacy play a role in long-term clearance, drug and fungal properties are also responsible. For example, in a study assessing residual drug content of nail clippings after oral treatment cessation, the clippings showed persistence of drug levels for months after the cessation of systemic treatment.1 A large discrepancy exists when comparing ex-vivo drug studies for fungal susceptibility to those with in-vivo results. In ex-vivo experiments, antifungal drugs tend to demonstrate much higher positive outcomes typically not seen when evaluating the efficacy of these drugs in-vivo.8 The variation in outcomes can be due to multiple factors, including drug bioavailability, penetration, local actions by keratin, and fungal behavior at the site of infection. A study showed that terbinafine affinity to keratin reduced the bioavailability and ability to target fungus in the nail bed.9 In the study by Ghannoum et al,9 the drug was found to be sequestered within the nail plate, thus reducing its efficacy in vivo. Despite changes in drug formulation to allow for better nail penetration, the drugs still appear to be less effective than expected. Fungal growth patterns within the nail and spore formation also lead to less favorable outcomes in vivo. The slow growth rate of nails further contributes to suboptimal treatment outcomes, as a high fungal load is harbored within the affected nail.
The assessment of treatment efficacy follows specified endpoints (Table 1). While some studies rely on subjective clinical cures, others are more objective, relying on mycological or complete cures. Additionally, while many studies utilize the great toenail as a targeted therapeutic site to evaluate complete cure rate, others include the remaining nails as well. Given the lower responsiveness of the great toenail compared to the other toenails, therapeutic responsiveness might appear higher when all nails are included, and therefore, must be considered when evaluating drug efficacy.6 For example, in the case of oral terbinafine, the complete cure rate using the great toenail alone as the therapeutic response site was reported to be 23 percent, while the cure rates when considering the second, third and fourth toenails in the same study were 65, 51, and 67 percent, respectively.6
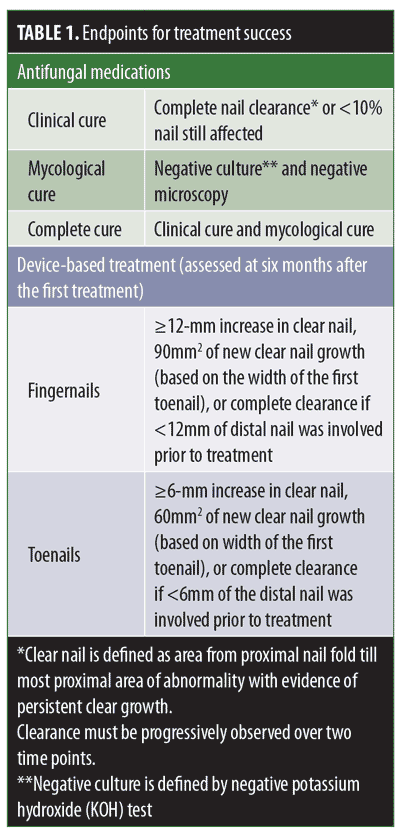
Designated endpoints and selected target nails should be considered judiciously when comparing study results. Here, we provide a review on the uses and outcomes of new and upcoming topical therapies and energy-based devices for the treatment of distal subungual onychomycosis.
Topical Antifungals
Numerous topical treatments are available in the form of nail lacquers or solutions, but the efficacy of these treatments remains low. Up until 2014, ciclopirox, which has been shown to have efficacy against both dermatophytes and yeast, was the only topical antifungal approved by the United States Food and Drug Administration (FDA) for the treatment of onychomycosis.10,11 It was postulated that the inability of the drug to penetrate the hard nail plate hinders clinical efficacy, leading to its poor overall performance.10,11 Therefore, the penetration capacity of ciclopirox with and without added diffusion enhancers was assessed in various studies. In one study using an ex-vivo experimental setup, it was demonstrated that ciclopirox reached sufficiently high concentrations within healthy nails, especially when using oil-based diffusion enhancers.12,13 The limitations of this study were the use of nondiseased nails in an ex-vivo system. In another study, using ex-vivo models of diseased nails to evaluate fungicidal and fungistatic activity of ciclopirox against Trichophyton mentagrophytes (T. mentagrophytes) in the presence of keratin powder, it was shown that the drug was not active.14 Under these conditions, the drug was not able to kill the fungal pathogens in the same way that it did in the absence of keratin, indicating, aside from optimal penetration into the nail plate and strong antifungal activity ex vivo, that the drug also needs to function within the infected nail in the presence of keratin. For that reason, newer antifungal options aim to achieve higher efficacy by making use of a combination of factors, such as good penetration, new low surface tension formulations, lower keratin affinity, and alternative mechanisms of action.14 As a result, the efficacy of these novel drugs is often compared to ciclopirox as a reference. Table 2 summarizes the newer topical antifungals, with a focus on their mechanism of action and efficacy.
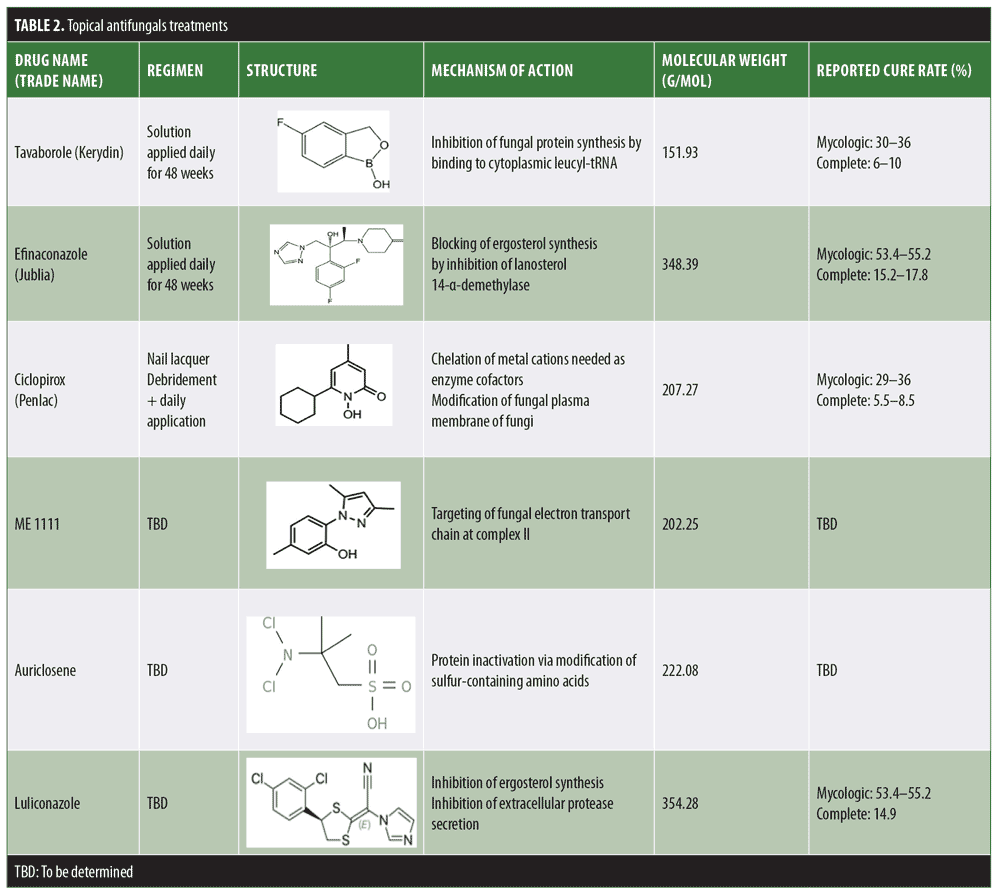
Efinaconazole. Efinaconazole (Jublia 10% solution; Ortho-Dermatologics, Bridgewater, New Jersey) is a topical imidazole active against dermatophytes and yeasts. It received FDA approval in 2014.15 Having a low affinity for keratin, this drug is able to penetrate the nail plate easily and selectively target pathogenic fungi.15 Despite having a higher molecular weight than ciclopirox, its low surface tension is thought to allow better penetration.11 Once within the nail, efinaconazole is observed to have a better efficacy than what was noted for ciclopirox. In fact, the same ex-vivo infection model using keratin powder demonstrated complete eradication of T. mentagrophytes at concentrations of 5 and 20mcg/mL.13 Studies using a fluorescein vehicle showed that a single application of efinaconazole to the hyponechium was able to reach the site of infection at the nail bed and underside of the nail plate.17 Further studies were also conducted in cadaveric nails following the application of nail polish. In this case, the drug was able to penetrate the nail without disrupting the polish, resulting in a similar concentration as seen in control specimens free of polish.18,19
Tavaborole. Tavaborole (Kerydin, 5% solution; PharmaDerm, Princeton, New Jersey) is a boron-based antifungal containing oxaborole molecules approved in 2014. The increased affinity for fungal enzymes allows the drug to be selective against the pathogen without interfering with host protein synthesis. It is indicated for the treatment of onychomycosis caused by T. rubrum and T. mentagrophytes; however, it is also effective against an array of less commonly encountered pathogens, such as yeast.20 The low molecular weight of tavaborole allows for easy nail penetration.21 Drug levels in the nail remain high even at three months after treatment cessation. Using liquid chromatography and mass spectrometry, ex-vivo cadaveric nail studies showed significantly higher drug penetration of tavaborole compared to ciclopirox.22 Moreover, studies evaluating the effects of nail polish on drug penetration determined that the presence of nail polish did not hinder the transungual delivery of tavaborole and might even enhance it.23 Despite the concern of boron-related toxicity, tavaborole appears to have an excellent safety profile, showing no cytochrome enzyme inhibition and negligible plasma levels after topical use.22 Rodent studies assessing potential carcinogenic effects also determined that the product was suitable for long-term use in humans.22
Other Topical Onychomycosis Treatments Under Investigation
Auriclosene. NVC 422 (auriclosene) is a broad-spectrum antibacterial and antifungal agent belonging to a family of compounds known as aganocides.24 Current clinical studies are focusing mainly on the treatment of impetigo, conjunctivitis, and urinary catheter infections (clinicaltrials.gov; NCT00781339, NCT01532336, NCT01367314, NCT01243125, NCT01877694). However, preclinical studies have assessed its utility in the treatment of onychomycosis; an in-vitro evaluation on a cadaveric model utilized nanoemulsion lacquers and gel formulations to assess the growth inhibition of dermatophytes, which showed significant drug penetration and fungal eradication.25
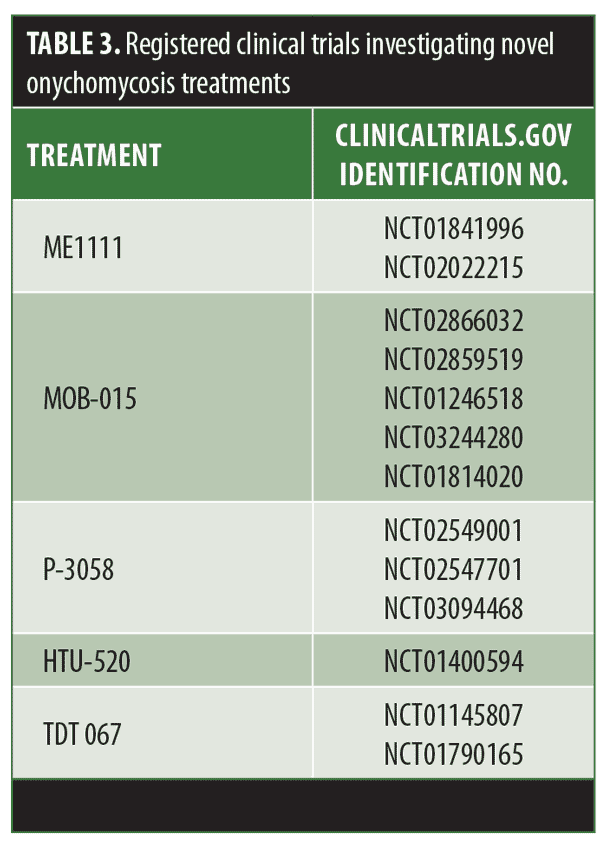
ME1111. ME1111 is a fungicidal compound effective against dermatophytes, reaching sufficiently high concentrations in the nail to overcome the minimum inhibitory concentration (MIC) of these organisms.26 The ability of this drug to exert antifungal activity in the presence of keratin has not been assessed. However, the effect of ME1111 showed selective toxicity to the fungal organisms.27 Phases I and II clinical trials assessing the pharmacokinetics and safety of ME1111 were recently completed, but results have not been published yet (Table 3).
Luliconazole. Luliconazole is an imidazole antifungal with an added ketone dithioacetate component. In spite of its higher molecular weight, a modified molecular structure endows this novel antifungal with lower keratin affinity and, in turn, potentially improved potency.28 This drug is FDA-approved in a cream formulation for the treatment of fungal infections of the skin. Studies pertaining to onychomycosis used solutions of 5% applied daily for 48 weeks and assessed outcomes at the end of the treatment course;29 at this concentration, randomized control studies showed similar efficacy for Luliconazole and other approved topical treatments, reporting around a 14.9 percent complete cure rate. A 10% solution was tested for safety and tolerability; however, it has not yet been assessed for treatment efficacy.30
Non-oral terbinafine alternatives. Given the well-known efficacy of terbinafine, novel formulations of the drug are also being evaluated. This includes topical, transungual routes, and patches (MOB-015, P-3058, and HTU-520, respectively; Table 3). Additionally, compounding terbinafine with new vehicles such as transferosome (TDT 067) is also being investigated as these lipid vesicles are thought to allow improved transport through the nail barrier.11,31
Summary. Although the aforementioned drugs provide alternatives to systemic treatments, optimal efficacy has not yet been achieved. Due to the thick keratin structure of the onychomycotic nail, drugs must be able to effectively penetrate the nail plate in order to reach the deeper site of infection.14 Nevertheless, reaching mycological MIC is insufficient for efficacy given the potential interaction between drugs and the keratinized structure.14 Higher concentrations might be needed before the drug is able to act in vivo as it does ex vivo. Furthermore, penetration studies focusing on healthy nails fail to consider morphological changes accompanying the diseased nail, such as increased porosity and hyperkeratosis.13,18 These factors warrant potential changes in how drugs are evaluated. Additionally, the propensity of fungal organisms to move to a spore stage makes them resistant to treatment. A better method of targeting spores within the nail plate is needed to eradicate the disease.
Laser Treatment
Lasers are widely used in the field of dermatology for the treatment of various skin conditions. Difficulties associated with topical treatments and high side effect profile linked to oral therapies have led researchers to test laser treatments as a novel therapy for onychomycosis. These are only FDA-approved for cosmetic endpoints and are judged based on the ability to “temporarily increase the amount of clear nail.”32 Various lasers have been used for the treatment of onychomycosis. We have summarized some of the major laser modalities, treatment regimens, and outcomes in Table 4. Given the lack of standardized parameters, expected clinical outcomes vary from one study to the next, and therefore, cannot be generalized.
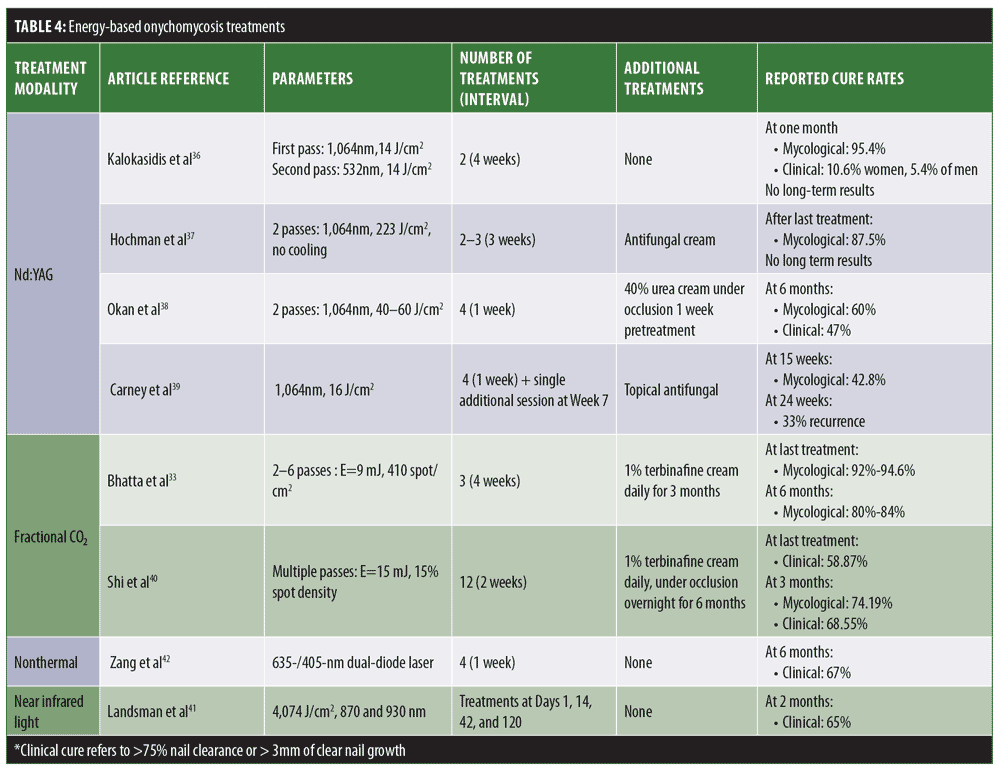
Neodymium-doped yttrium aluminum garnet (Nd:YAG) lasers. The utility of laser treatments is thought to rely on heat transfer to fungal mycelium, reaching fungicidal temperatures of 43°C to 51°C for 2 to 3 minutes; lower temperatures can lead to stimulation of fungal growth.33 Such high temperatures can promote increased local circulation and ultimately strengthen the body’s natural immunological response.33 The heat-induced mitochondrial production of reactive oxygen species within the fungal pathogens also pushes these cells to undergo apoptosis.32 Within the fungal cell, target chromophores are thought to be melanin for the 1,064-nm Q-switched Nd:YAG laser, and xanthomegnin in the case of the 532-nm Q-switched Nd:YAG laser. Currently, long-pulsed Nd:YAG is one of the most commonly used laser modalities for onychomycosis.33 Nevertheless, it does not have a clearly specified optimized treatment regimen. While some studies suggest weekly treatments, others recommend one-month intervals.34–39 The number of total sessions required ranges from 3 to 8, with 2 to 3 passes performed each time depending on operator preference and experience.34–39 Treatment parameters, such as wavelengths, spot sizes, fluence, and pulse duration differ also in various studies, suggesting that the laser effect is not chromophore-dependent.34–36 Some studies evaluating the utility of Nd:YAG relied solely on the laser treatment, while others called for concomitant antifungal use or pretreatment with keratolytics in cases of dystrophic nails.37–39 In spite of the FDA’s preset definition of treatment success, this is defined differently from one study to the next, making adequate comparisons difficult. Most patients undergoing laser treatments for onychomycosis noted that multiple treatments were needed before any visible improvements could be observed.36–39 With regard to safety and tolerability, patients noted mild burning or pain during the sessions; however, such did not prevent them from continuing treatment.36,37
Fractional CO2 lasers. The mechanism of action of this laser modality is thought to be twofold: it can lead to fungal cell injury and death by photothermal effect and can also create pores in the nail plate, allowing for better penetration of combined topical treatments.33 Again, the optimal number and frequency of treatment sessions and selected parameters have not been established, and are thus selected based on operator experience. For the most part, terbinafine is the combined topical treatment of choice, with few studies evaluating the utility of other agents.35,40 Although early results are promising, studies that included longer follow-up posttreatment periods reported significant relapse rates.33
Other laser treatments under consideration. Near-infrared diode laser application is thought to achieve a cure via its thermal effect on the fungal pathogen, increasing the amount of reactive oxygen species and ultimately leading to apoptosis.33 A dual-wavelength 870-/930-nm laser at 4,074 J/cm2 was tried for four sessions and showed complete irradiation of both bacteria and yeasts.41
Nonthermal low-level laser devices have also been tested for the treatment of onychomycosis in toenails. This method employs 635-/405-nm dual-diode laser application. Although the mechanism of action is not clearly understood, it is thought to involve photobiomodulation leading to increased cellular respiration. For the time being, the only published data are from a retrospective study sponsored by the laser developer and used for FDA approval procedures.42
Conclusion
Ideally, treatments for onychomycosis should boast effective nail penetration and broad-spectrum antifungal activity within the nail, with convenient dosing and limited side effects. This should translate into high overall cure rates and longer effectiveness.
Novel topical and device-based treatments for onychomycosis have brought forth possible alternatives to systemic therapy. The efficacy of these options remains questionable, as cure rates are often still low compared to current standards of care. It is important to note that, currently, very few studies offer a head-to-head, in-vivo comparison of available topical treatments. As a result, reviews of the literature are suboptimal due to the differing treatment endpoints between the various studies. Even when comparing clinical cure, for instance, while some trials consider complete clearance of the nail as the desired goal, others consider clearance of a given percentage of the affected nail (<5%–10%) to be acceptable. Additionally, nonadherence to long treatment courses and daily applications can also hinder the clearance process, so adherence to treatment must be considered.
Laser-based treatments are only considered for cosmetic improvements and not for treatment; thus, their addition to the market offers new challenges in the assessment of improvements. For this reason, treatment success in the setting of laser application is not comparable to that achieved with standard therapies. Variations in laser parameters, treatment frequency, and causative pathogens lead to differences in perceived outcomes. Further standardized treatment regimens are required before the utility of these lasers can truly be evaluated.
Although some studies have evaluated the combination of topical treatments and laser therapy, no such data exist for the novel antifungals. Despite the ability of efinaconazole and tavaborole to better penetrate the nail plate, it is not clear whether thermal injury or microhole creation would act synergistically with this process. Additionally, the effects of thermal injury on fungal spores should also be evaluated, as eradiation of these might improve outcomes if combined with effective fungicidal topical therapies.
References
- Elewsli B. Onychomycosis: Pathogenesis, Diagnosis, and Management. Clin Microbiol Rev. 1998;11(3):415–429.
- Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5 Pt 1):702–704.
- Bjornsdottir S, Gottfredsson M, Thorisdottir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case–control study. Clin Infect Dis. 2005;41(10):1416–1422.
- Gupta A, Versteeg S, Shear N. Onychomycosis in the 21st century: an update on diagnosis, epidemiology, and treatment. J Cutan Med Surg. 2017;21(6):525–539.
- de Backer M, et al. Twelve weeks of continuous oral therapy for toenail onychomycosis caused by dermatophytes: a double-blind comparative trial of terbinafine 250 mg/day versus itraconazole 200 mg/day. J Am Acad Dermatol. 1998 May;38(5 Pt 3):S57–S63.
- Shemer A, Sakka N, Baran R, et al. Clinical comparison and complete cure rates of terbinafine efficacy in affected onychomycotic toenails. J Eur Acad Dermatol Venereol. 2015 29(3):521–526.
- Finch JJ, Jinna S. Spotlight on tavaborole for the treatment of onychomycosis. Drug Des Devel Ther. 2015;9:6185–6190.
- Zalacain A, Obrador C, Martinez JP, et al. Characterization of the antimicrobial susceptibility of fungi responsible for onychomycosis in Spain. Med Mycol J. 2010;49(5):495–499.
- Ghannoum M, Isham N. Fungal nail infections (onychomycosis): a never-ending story?. PLoS Pathog. 2014;10(6):e1004105.
- Queller JN, Bhatia N. The dermatologist’s approach to onychomycosis. J Fungi. 2015;1(2):173–184.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7(7):10–18.
- Bohn M, Kraemer KT. Dermatopharmacology of ciclopirox nail lacquer topical solution 8% in the treatment of onychomycosis. J Am Acad Dermatol. 2000;43(4 Suppl):57–69.
- Hafeez F, Hui X, Selner M, et al. Ciclopirox delivery into the human nail plate using novel lipid diffusion enhancers. Drug Dev Ind Pharm. 2013;40(6):838–844.
- Tachibana H, Kumagai N, Tatsumi Y. Fungicidal activity in the presence of keratin as an important factor contributing to in vivo efficacy: a comparison of efinaconazole, tavaborole, and ciclopirox. J Fungi (Basel). 2017;3(4). pii: E58.
- Lipner S, Scher R. Efinaconazole in the treatment of onychomycosis. Infect Drug Resist. 2015;8:163–172.
- Tupaki-Sreepurna A, Jishnu B, Thanneru V, et al. An assessment of in vitro antifungal activities of efinaconazole and itraconazole against common non-dermatophyte fungi causing onychomycosis. J Fungi. 2017;3(2). pii: E20.
- Elewski BE, Pollak RA, Pillai R, et al. Access of efinaconazole topical solution, 10%, to the infection site by spreading through the subungual space. J Drugs Dermatol. 2014;13(11):1394–1398.
- Zeichner JA, Stein-Gold L, Korotzer A. Penetration of (14C)-efinaconazole topical solution, 10%, does not appear to be influenced by nail polish. J Clin Aesthet Dermatol. 2014;7(9):34–36.
- Del Rosso JQ. Application of nail polish during topical management of onychomycosis: are data available to guide the clinician about what to tell their patients? J Clin Aesthet Dermatol. 2016;9(8):29–36.
- Gupta G, Foley KA, Gupta AK. Tavaborole 5% solution: a novel topical treatment for toenail onychomycosis. Skin Therapy Lett. 2015;20(6):6–9.
- Sharma N, Sharma D. An upcoming drug for onychomycosis: Tavaborole. J Pharmacol Pharmacother. 2015;6(4):236–239.
- Finch JJ, Jinna S. Spotlight on tavaborole for the treatment of onychomycosis. Drug Des Devel Ther. 2015;20:6185–6190.
- Vlahovic T, Coronado D, Chanda S, et al. In vitro study demonstrating nail penetration of tavaborole topical solution, 5% through multiple layers of nail polish. J Am Acad Dermatol. 2015;14:72.
- Ghannoum MA, et al. Efficacy of NVC-422 in the treatment of dermatophytosis caused by trichophyton mentagrophytes using a guinea pig model. Int J Dermatol. 2013 May;52(5):567–571
- Ibrahim S, Debabov D, Ghannoum M, et al. In vitro evaluation of the antifungal activity of NVC-422 (N,N-dichloro-2,2-dimethyltaurine) using a novel cadaver nail model. Abstract presented at: Infectious Diseases Society of America 48th Annual Meeting; October 21–24, 2010; Vancouver, BC, Canada. Available at: https://idsa.confex.com/idsa/2010/webprogram/Paper2675.html.
- Ghannoum M, Isham N, Long L. In vitro antifungal activity of ME1111, a new topical agent for onychomycosis, against clinical isolates of dermatophytes. Antimicrob Agents Chemother. 2015;59(9):5154–5158.
- Tabata Y, Takei-Masuda N, Kubota N, et al. Characterization of antifungal activity and nail penetration of ME1111, a new antifungal agent for topical treatment of onychomycosis. Antimicrob Agents Chemother. 2015;60(2):1035–1039.
- Scher RK, Nakamura N, Tavakkol A. Luliconazole: a review of a new antifungal agent for the topical treatment of onychomycosis. Mycoses. 2014;57(7):389–393.
- Watanabe S, Kishida H, Okubo A. Efficacy and safety of luliconazole 5% nail solution for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2017;44(7):753–759.
- Jones T, Tavakkol A. Safety and tolerability of luliconazole solution 10-percent in patients with moderate to severe distal subungual onychomycosis. Antimicrob Agents Chemother. 2013;57(6):2684–26789.
- Ghannoum M, et al. Activity of TDT 067 (terbinafine in transfersome) against agents of onychomycosis, as determined by minimum inhibitory and fungicidal concentrations. J Clin Microbiol. 2011 May;49(5):1716–1720.
- United States Food and Drug Administration. Medical devices and clinical trial design for the treatment or improvement in the appearance of fungally-infected nails. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/medical-devices-and-clinical-trial-design-treatment-or-improvement-appearance-fungally-infected.
- Bhatta AK, Keyal U, Wang X, et al. A review of the mechanism of action of lasers and photodynamic therapy for onychomycosis. Lasers Med Sci. 2016;32(2):469–474.
- Noguchi H, Miyata K, Sugita T, et al. Treatment of Onychomycosis using a 1064nm Nd:YAG laser. Med Mycol J. 2013;54(4):333–339.
- Bristow IR. The effectiveness of lasers in the treatment of onychomycosis: a systematic review. J Foot Ankle Res. 2014;7:34.
- Kalokasidis K, Onder M, Trakatelli M, et al. The effect of q-switched Nd:YAG 1064-nm/532-nm laser in the treatment of onychomycosis in vivo. Dermatol Res Pract. 2013;2013:379725.
- Hochman LG. Laser treatment of onychomycosis using a novel 0.65-millisecond pulsed Nd:YAG 1064-nm laser. J Cosmet Laser Ther. 2011;13(1): 2–5.
- Carney C, Cantrell W, Warner J, et al. Treatment of onychomycosis using a submillisecond 1064-nm neodymium:yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69(4):578–582.
- Okan G, Tarikci N, Gokdemir G. The effect of long-pulsed Nd:YAG laser for the treatment of onychomycosis. J Am Pod Med Assoc. 2017;107(1):54–59.
- Shi J, Li J, Huang H, et al. The efficacy of fractional carbon dioxide (CO2) laser combined with terbinafine hydrochloride 1% cream for the treatment of onychomycosis. J Cosmet Laser Ther. 2017;19(6):353–359.
- Landsman AS, Robbins AH. Treatment of mild, moderate, and severe onychomycosis using 870- and 930-nm light exposure. J Am Pod Med Assoc. 2012;102:169–171.
- Zang K, Sullivan R, Shanks S. A retrospective study of nonthermal laser therapy for the treatment of toenail onychomycosis. J Clin Aesthet Dermatol. 2017;10:24–30.

