J Clin Aesthet Dermatol. 2018;11(10):20–26
 by Monica Janeczek, MD; Lauren Moy, MD; Alexandria Riopelle, BS; Olivia Vetter, BS; Jeave Reserva, MD; Rebecca Tung, MD; and James Swan, MD
by Monica Janeczek, MD; Lauren Moy, MD; Alexandria Riopelle, BS; Olivia Vetter, BS; Jeave Reserva, MD; Rebecca Tung, MD; and James Swan, MD
Drs. Moy, Reserva, Tung, and Swan are with the Department of Dermatology at the Stritch School of Medicine at Loyola University Chicago in Maywood, Illinois. Mses. Janeczek, Riopelle, and Vetter are with the Stritch School of Medicine at Loyola University Chicago in Maywood, Illinois.
FUNDING: No funding was received for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. In recent studies, N-acetylcysteine has been shown to be efficacious in several dermatologic conditions.
Objective.The aim was to review clinical trials that assess the efficacy of N-acetylcysteine in cutaneous disorders.
Design.The PubMed database was searched and a manual search of clinical trials in the references was performed. Studies included randomized, controlled studies, uncontrolled studies, meta-analyses, and systemic reviews published between years 1966 and 2017.
Results.Efficacy of N-acetylcysteine was shown in excoriation disorder, onychophagia disorder, trichotillomania, acne vulgaris, Type I lamellar ichthyosis, bullous morphea, systemic sclerosis, toxic epidermal necrolysis, atopic dermatitis, xeroderma pigmentosum, and pseudoporphyria. Studies also show benefits in wound healing and photoprotection.
Conclusion. The review of available literature suggests that N-acetylcysteine could potentially serve as a safe, tolerable, and effective therapeutic option for a variety of dermatologic conditions.
KEYWORDS: N-acetylcysteine, pharmacology, dermatological disorder, topical therapy, oral therapy, intravenous therapy
N-acetylcysteine (NAC) is a mucolytic and nephroprotective agent and is also the antidote to acetaminophen overdose.1–3 However, NAC is now showing potential for use in other disorders. Its uses have been studied in the fields of neurology, pulmonology, cancer, vascular disorders, and psychiatry. NAC’s potentially diverse clinical applications are attributed to its effects on nitric oxide and its role as an antioxidant. As an antioxidant, NAC affects pathways involved in stress, infection, and inflammatory conditions.4 More recently, NAC has been used in various dermatologic conditions. In this review article, we explore the available literature for publications related to the treatment of skin disorders using NAC therapy, seeking to better understand its methods of action and potential applications in the field of clinical dermatology.
Methods
Data search. A search in the PubMed literature database was conducted to collect relevant articles. The key search terms used included N-acetylcysteine, NAC, N-acetyl cysteine, and combinations thereof, with skin, dermatology, wounds, and TEN. The use of the Boolean operators and or were employed to identify relevant empirical studies and reports.
Inclusion and exclusion criteria. In this review, we focused on randomized, controlled studies, uncontrolled studies, meta-analyses, and systematic reviews published between the years 1966 and 2017. The search was limited to literature published in the English language. Studies that did not show clinical improvement with the administration of N-acetylcysteine, based either on objective or subjective measures, were excluded. Studies were not excluded based on route of administration.
Results
Results are summarized in Table 1. Detailed discussion of results are provided below.
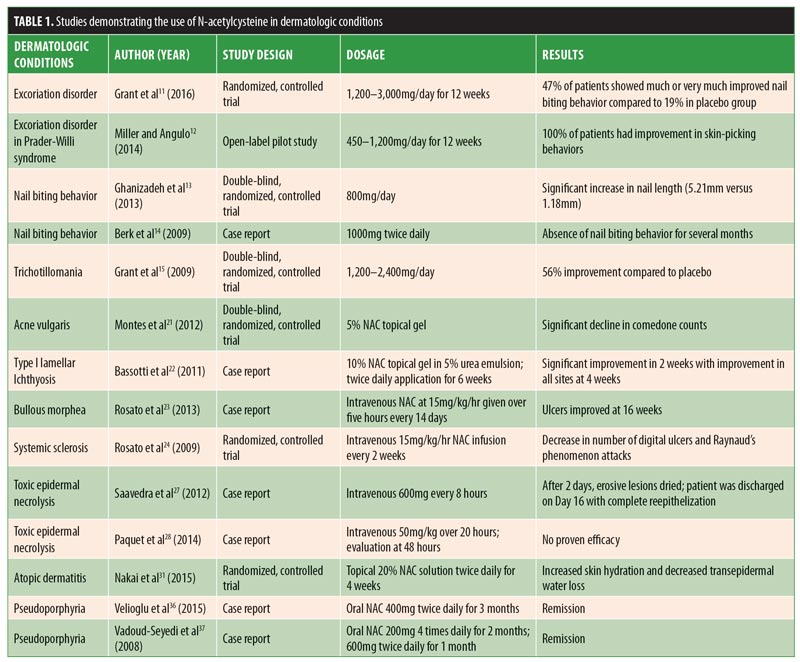
Dermatologic conditions related to psychiatric disorders. Excoriation disorder. Excoriation disorder is characterized by the repetitive and compulsive picking of the skin, leading to tissue damage.5 Excoriation can be triggered by emotional stress, anxiety, changes in routine, and/or the sensation or appearance of a bump, unevenness, blemish, or discoloration of the skin.5 It affects four percent of the general population and can significantly impact a patient’s quality of life.5 It can even pose potentially life-threatening complications. For example, tissue damage caused by excoriation can lead to localized infections and septicemia.6 Currently, there is no United States Food and Drug Administration (FDA)-approved psychoactive medication for excoriation disorder, with studies showing either mixed results or lack of efficacy.7–10 However, in their randomized, double-blind trial conducted at the University of Minnesota, Grant et al11 found that, compared with placebo, NAC treatment yielded significant improvement in skin picking severity. Fifty-three patients were studied, and 15 of 32 patients (47%) who received NAC treatment were “much” or “very much” improved, compared with four of the 21 patients (19%) who received placebo treatment. Miller and Angulo12 showed that NAC significantly reduced symptoms of excoriation and skin-picking lesions in patients with Prader-Willi syndrome (PWS). PWS is a neurodevelopmental disorder caused by an abnormality on the long arm of chromosome 15 that results in behavioral dysfunctions, such as excessive food interest, skin picking, and obsessive-compulsive behaviors. The authors studied 35 patients with confirmed PWS and excoriation disorder for more than one year. Patients were treated with NAC, 450 to 1,200mg per day, for 12 weeks. All 35 patients showed improvement in skin-picking behaviors. Ten patients did not have complete resolution of symptoms but still showed a significant reduction in active skin lesions.
Onychophagia. Onychophagia is another common behavioral disorder that currently does not have an effective pharmacological treatment. A randomized, double-blind, placebo-controlled clinical pilot study performed by Ghanizadeh et al13 showed that NAC treatment (800mg per day) in 42 children and adolescents yielded a statistically significant difference between the two groups in terms of increased nail length after the first month of treatment. The mean increased nail lengths in the experimental and control groups were 5.21 and 1.18 millimeters, respectively. However, there was no statistical significance after two months of observation, suggesting that NAC decreases nail biting behavior only in the short-term. Another study by Berk et al14 demonstrated, in three case reports, that NAC decreased the impulsivity and anxiety associated with onychophagia. In all three cases, 1,000mg of NAC was given twice daily, which resulted in the absence of nail biting behavior for several months.
Trichotillomania. Trichotillomania is characterized by repetitive hair pulling that causes noticeable hair loss. Studies on serotonergic medications have resulted in conflicting data regarding effective treatment for this condition. A double-blind, placebo-controlled study conducted in 2009 by Grant et al15 demonstrated that, in 50 patients with trichotillomania, those given NAC 1,200 to 2,400mg per day had significantly greater reductions in hair-pulling symptoms, with a 56-percent improvement using the Massachusetts General Hospital Hair Pulling Scale and the Psychiatric Institute Trichotillomania Scale, compared with placebo.
Wound healing. Many recent experiments have studied potential topical uses of NAC, including its application as a wound healing agent. Oguz et al16 compared dexpanthenol, an alcohol analog of vitamin B5 used as a moisturizer to improve wound healing, with 3% NAC on wound healing in rats. Thirty rats received a linear 2cm incision in the skin and were divided into three groups: control (no treatment), dexpanthenol treatment, and NAC treatment. On Day 21, excision and histological evaluation showed that the two treatment groups had similar wound healing rates and fibrosis versus the control group, but a higher angiogenesis rate was observed in the NAC group, compared with the other groups.
Similarly, Aktunc et al17 studied NAC’s effect on wound healing in diabetic and nondiabetic mice. They measured vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS) expression, and wound-breaking strength (WBS) in 48 mice. The mice were divided into two groups of alloxan-induced diabetic mice (a control group and an NAC treatment group) and two groups of healthy mice (a control group and an NAC treatment group). Each mouse received incisional wounds on its back. NAC treatment groups received intraperitoneal NAC 150mg/kg for five days. Of note, the investigators found that both diabetic and nondiabetic mice groups who received NAC treatment had significantly decreased levels of oxidative stress markers and higher VEGF expression, which resulted in higher WBS measurements, compared with the control groups. Specifically, the WBS measurements in the NAC groups ranged from 87 to 136 versus 69 to 106 in the control groups.
Further research has also shown promising results using NAC on burn wounds in mice. Tsai et al18 reported that NAC improved burn wounds with regard to reepithelization in vitro as well as induced cellular wound healing pathways in vivo. The researchers observed GSH levels, cell proliferation and migration, scratch wound healing activities, epithelization-related proteins, matrixmetalloproteinase-1 (MMP-1), and proteins involved in regulating MMP-1 expression in the skin cell line CCD-966SK were increased in a dose-dependent manner with NAC concentrations of 0.1mM to 1.0mM. In vivo, the authors reported that, histopathologically, topical NAC showed more significant characteristics of reepithelization.
NAC has also been studied to assist in the treatment of tissue necrosis, especially in ischemically endangered flap tissue. Bachle et al19 evaluated the effects of NAC on microcirculatory dysfunction that leads to tissue necrosis. Investigators created laterally based skin flaps in mice and, through intravital fluorescence microscopy, analyzed arteriolar perfusion, capillary density, leukocytic inflammation, apoptotic cell death, and nonperfused tissue over 10 days. They observed significantly high arteriolar dilation and increased capillary perfusion in the treatment group with 100mg/kg NAC, which led to reduced ischemia and apoptotic cell death in all areas of the flap. Specifically, arterial dilation increased 100 percent by Day 10 in the NAC-treated mice, with a 37-percent reduction in necrotic flap tissue.
Research has demonstrated potential antimicrobial effects of NAC against biofilms of gram-positive and gram-negative bacteria. Eroshenko et al20 reported significantly reduced biofilm formation and growth of mixed culture Propionibacterium acnes and Staphylococcus epidermis following initiation of 12.5mg/mL NAC. A concentration of 25mg/mL of NAC diminished the growth of biofilm by at least 50 percent in all tested bacteria. These results suggest that NAC could be an effective nonantibiotic alternative for biofilm-associated infections.
Disease-specific uses. NAC has been reported useful in the treatment of a variety of specific skin disorder, including acne vulgaris, lamellar ichthyosis, bullous morphea, ulcers in scleroderma, toxic epidermal necrolysis, and dermatitis.1
Acne vulgaris. NAC has been used topically for acne vulgaris. Montes et al21 conducted a double-blind, randomized, controlled trial using 5% NAC topical gel in 99 patients. The investigators observed significantly superior results among patients treated with NAC, compared with the placebo group, in reduction of comedone counts, suggesting NAC might be an effective treatment option for mild-to-moderate acne vulgaris. This effect might be due to NAC’s ability to suppress sebaceous activity and minimize the growth of Propionibacterium acnes.
Lamellar ichthyosis. Bassotti et al22 reported that topical application of 5 to 10% NAC prepared in a 5% urea emulsion greatly benefited children with Type I lamellar ichthyosis. Application was two times a day for six weeks, with a daily maintenance application. During the first two weeks, a significant improvement in scaling was noted. After four months of maintenance application, a reduction in scaling was observed in all treated areas. After four months of maintenance application, marked improvement was observed in all treated areas. The authors concluded that topical NAC might be beneficial for lamellar ichthyosis in children, as well as for prevention of ectropion after a surgical procedure.
Bullous morphea. NAC has demonstrated effectiveness as a wound healing agent in patients with bullous morphea,23 a form of localized scleroderma. The pathogenesis of bullous morphea is unknown, but it is generally thought to be an autoimmune disorder, and treatment of coexistent ulcers can be challenging. The cause of bullae formation is multifactorial, but reactive oxygen species production is thought to play a key role. The authors reported the case of a 79-year-old woman with bullous morphea and long-standing ulcers who was successfully treated with topical NAC as part of a wound care treatment plan. She had previously failed treatment with nifedipine 30mg/day, intravenous iloprost, systemic antibiotics, and an autologous skin graft. An infusion of NAC at 15mg/kg per hour was given over five consecutive hours every 14 days with daily topical wound care. After 16 weeks, her ulcer had significantly improved in terms of erosions, hemorrhagic vesicles, eroded bullae, and plaques.
Systemic sclerosis and Raynaud’s phenomenon. Rosato et al24 reported that NAC might be useful in both short- and long-term treatment of patients with systemic sclerosis (SSc) and severe comorbid Raynaud’s phenomenon with digital ulcers. The authors observed a reduction in digital ulcers and a decrease in the number of attacks of Raynaud’s phenomenon in 50 patients with SSc who received NAC infusions at 15mg/kg per hour every two weeks. On average, patients treated with NAC had a reduction of 4.5 digital ulcers per year versus 0.81 in the control group. In addition, NAC patients had approximately three attacks per year as compared with seven in the control group.
Toxic epidermal necrolysis. Toxic epidermal necrolysis (TEN) is the most severe form of drug-induced skin reaction, with over 30-percent involvement of the total body surface area. It is a dermatologic emergency with extensive destruction of the epidermis and mucosal epithelia. TEN affects between 0.4 and 1.5 cases per million people every year, with a mortality rate ranging from 15 to 40 percent.25 Most patients die from infections and multiorgan failure, and treatment is centered on supportive care with adjunctive corticosteroids and/or intravenous immunoglobulin (IVIG) therapy. The use of NAC for the treatment of TEN has been reported in several case reports,26 but most recently by Saavedra et al27 in 2012, who reported a case of cefazolin-induced TEN with an excellent response to NAC therapy. In this report, a 38-year-old female patient with no previous reported antibiotic allergies was admitted to the hospital for an acute urinary tract infection. Her admission vitals revealed septic and hypovolemic shock requiring aggressive fluid resuscitation and dopamine; she was started on intravenous cephazolin. Three days later, she developed an erythematous rash that rapidly progressed, affecting nearly 52 percent of her body surface, with bullae appearing on the erythematous areas. The patient also demonstrated bilateral conjunctivitis and erosions of the genital and oral mucosae. TEN was confirmed by skin biopsy. No systemic corticosteroids were given. IVIG was administered on a daily dosage of 1g/kg of body weight for three consecutive days, with rash progression to 75-percent body surface area and epidermal detachment. The authors then administered NAC 600mg every eight hours intravenously. Two days later, the erosive lesions of the skin and mucosae had dried and her eyes were reepithelized. She was discharged after 16 days, with complete reepithelization of the lesions. The authors concluded that NAC might be an effective alternative for patients who do not respond to IVIG therapy, especially in sepsis patients. Recently, Paquet et al28 tested the use of NAC for the treatment of TEN in a proof-of-concept study. Five patients with drug-induced TEN were treated with NAC in combination with anti-tumor necrosis factor alpha (anti-TNF-?) infliximab. In comparison with the control group, who received infliximab only, the combination therapy group did not demonstrate improved efficacy. Due to the small number of documented cases, it is difficult to determine the true clinical utility of NAC for the treatment of TEN; however, the results suggest a lack of benefit.
Atopic dermatitis. Atopic dermatitis (AD) is a chronic, inflammatory, pruritic skin disorder caused, in part, by skin barrier dysfunction. It has been shown that patients with AD have low levels of skin hydration and high levels of TEWL.29,30 In 2015, Kozo et al31 evaluated topical NAC for improving skin hydration and transepidermal water loss (TEWL) in patients with AD. Here, the researchers studied 11 patients with AD who had received previous treatments of topical corticosteroids and tacrolimus ointment, and 10 healthy volunteers. A topical 20% NAC solution or control solution was applied to patient forearm skin twice a day for four weeks. Nine of the 11 patients with AD and nine of the 10 healthy volunteers in the NAC group had increased skin hydration, while decreased TEWL was present in nine of the 10 patients with AD and eight of the 10 healthy volunteers. The researchers concluded that NAC treatment for patients with AD generally results in lower levels of skin hydration and higher levels of TEWL; however, due to conflicting results in a minority of patients, further studies are warranted with a larger sample size.
Neurological disorders. NAC has been shown to improve peripheral neuropathy, a result of damage to peripheral nerves, with symptoms of numbness, tingling, and pain that can result in cutaneous manifestations. Li et al33 found that, in rats, oral NAC inhibited matrix metalloproteinase (MMP)-9 and MMP-2 production, which are key factors in neuropathic pain. Further clinical studies are warranted in human subjects.
Kamboj et al34 demonstrated protective effects of NAC for oxidative stress and apoptosis in diabetic mice with decreased tail-flick latency by thermal hyperalgesia. Between 1.4 and
1.5g/kg of NAC was administered to the mice once daily for seven weeks via the drinking water. The researchers observed improved motor coordination and ameliorated hyperalgesia, while electron microscopy revealed that NAC reversed structural deficits.
Porphyria disorders. NAC has also been reported to be efficacious in the treatment of pseudoporphyria, a bullous disease characterized by blisters and atrophic scarring in the absence of elevated porphyrin. Pseudoporphyria can be induced by ultraviolet (UV) exposure, diuretics, renal failure, and nonsteroidal anti-inflammatory drugs.35 There is no reliable treatment available to date. Velioglu et al36 reported a case of a 34-year-old woman with renal failure who developed pseudoporphyria. The authors observed complete resolution of her lesions following a three-month trial of oral NAC, 400mg twice daily. The pseudoporphyria returned within two weeks of NAC discontinuation. Subsequently, 600mg of NAC twice daily was resumed, which led to complete resolution of lesions within six weeks.36 Two additional cases have been reported with similar efficacy in patients with chronic renal failure, one of whom went into full remission at two months with 200mg of NAC four times daily and the other of whom received 600mg twice daily, with remission occurring in one month.37 Similarly, a 35-year-old man with human immunodeficiency virus (HIV) and porphyria cutanea tarda was treated with oral NAC three times daily at 600mg. After four weeks, lesions were improved, with complete resolution after three months of treatment with NAC.38
Xeroderma pigmentosum. Kunisada et al42 provided evidence supporting the use of NAC to treat xeroderma pigmentosum complementation group A, an autosomal recessive disease that causes early onset of skin cancers, as well as freckle-like pigmented maculae, after relatively minimal sun exposure.41 The authors studied Xpa-deficient mice as an animal model for the UV radiation-induced, skin cancer-prone phenotype seen in xeroderma pigmentosum. Notably, these mice experienced an intense erythema 24 hours after a dose of UVB exposure that produced barely discernible erythema in wild-type (WT) mice. Following microarray studies on the Xpa-deficient mice, levels of CXCL1, a neutrophil attractant chemokine gene, were measured after UVB irradiation in WT and Xpa-deficient mice using enzyme-linked immunosorbent assay (ELISA). The investigators found that CXCL1 levels were significantly higher in Xpa-deficient mice than in WT mice at both 48 hours and 96 hours after irradiation, even when only a small area of skin was exposed to irradiation. However, upon administration of NAC-containing food, investigators observed significantly reduced CXCL1 levels in Xpa-deficient mice. Furthermore, oral administration of NAC exhibited significant tumor reduction and a decrease in the levels of UV-induced reactive oxygen species.
In an in-vivo study on human skin by Kang et al,43 researchers evaluated whether the antioxidant activity of NAC could inhibit responses to UV light exposure, a primary cause of photoaging in the skin of humans. Photoaging is characterized by premature skin aging, wrinkling, blotchy dyspigmentation, and rough skin textures.44 UV signaling pathways that lead to matrix destruction in the dermis are responsible for this premature skin aging and provide potential targets for photoaging prevention strategies, such as NAC, which increases tripeptide GSH, an important endogenous antioxidant.45 Among healthy adults exposed to UV radiation, topical NAC treatment appeared to be effective in interrupting the UV signaling cascade that leads to photoaging.43 NAC did not provide UV transmission inhibition (i.e., a sunscreen effect), but it did significantly increase the total amount of GSH in the viable epidermis of the treated skin. This allowed for an increase in the antioxidant capacity of the human skin by stockpiling the reduced form of GSH, which is often times the limiting factor in a cell’s ability to heal after UV damage occurs.
Photoprotective effects. Cotter et al39 studied whether NAC could protect melanocytes from UV-induced oxidative stress/damage in vitro and from UV-induced melanoma in vivo. In vitro, the authors used the mouse melanocyte cell line melan-A. NAC (1–10mmol/L) protected melan-A cells from several UV-induced oxidative sequelae. For in-vivo experiments, mice transgenic for hepatocyte growth factor and survivin, who were shown previously to develop melanoma following a single neonatal dose of UV irradiation, were given NAC (7mg/mL) transplacentally and through nursing until two weeks after birth. NAC was shown to significantly delay the mean onset of UV-induced melanocytic tumors, compared with the control mice. Overall, the authors concluded that NAC might be useful as a chemopreventive agent. Goodson et al40 recruited patients from a screening clinic who were at an increased risk for melanoma. Nevi were removed from the patients following UV-irradiation (4,000J/m2) before and after oral ingestion of a single (1,200mg) dose of NAC. In nevi removed three hours after NAC ingestion, levels of reduced GSH and the NAC metabolite cysteine were significantly increased compared with nevi removed prior to drug ingestion. The authors concluded that NAC might be safely used in patients to modulate UV-induced oxidative stress in nevi. The study further suggests that patients could potentially take NAC prophylactically before acute UV exposure to prevent pro-oncogenic oxidative stress in nevi, ultimately reducing long-term melanoma risk. NAC has demonstrated beneficial effects on radiation-induced dermatitis in rats.
In addition, NAC has shown to have chemoprotective effects, according to Dermirel et al.32 The investigators reported that the rats in the NAC treatment group had significantly lower severity of radiodermatitis than the control group, both clinically and histopathologically.
Discussion
Mechanisms of action. NAC is a thiol and mucolytic agent. When free NAC enters a cell, it is rapidly hydrolyzed to release cysteine, a precursor of glutathione (GSH). GSH, which maintains the protective redox state of the cell, is one of the most well-studied antioxidants. It is endogenously synthesized in all cells and has been found to bind to the glutamate recognition site in N-methyl-D-aspartate (NMDA) receptors, which allows it to function as an endogenous neuromodulator.5 There are three amino acids in GSH: glutamate, glycine, and cysteine. Cysteine has the lowest intracellular concentration, and the availability can limit the rate of GSH synthesis during oxidative stress.6
NAC is an acetylated cysteine residue that is able to increase cell protection against oxidative stress. This is accomplished through the release of cysteine, which is the rate-limiting precursor in the synthesis of GSH. Cysteine also acts independently as an effective scavenger of free radicals, minimizing reactive oxygen species (e.g., OH, H2O2, and O2-) that damage deoxyribonucleic acid (DNA), proteins, and lipids in cells.11 NAC is, therefore, considered to be an antioxidant and free-radical scavenger that increases GSH.6
NAC affects nuclear factor kappa B (NF-kB), which regulates the genetic stress response during inflammatory and oxidative stressors, both in vitro and in vivo.12 Specifically, NAC is thought to affect thioredoxin expression, which regulates DNA binding of NF-kB and glutaredoxin expression that is responsible for detecting changes in the redox state of GSH. Overall, this can modulate the inflammatory responses.13,14
By decreasing free radicals and replenishing intracellular cysteine levels, NAC is thought to increase GSH concentrations, inhibit inflammatory factors, and assist in epidermal proliferation.6,12–14 In this way, NAC might be useful as a treatment modality for dermatological conditions.
NAC is thought to influence dermatologic disorders through several mechanisms. In compulsive psychiatric disorders associated with cutaneous involvement, its method of action is predominately related to its ability to regulate the level of glutamate in the nucleus accumbens, which influences impulse control. Specifically, NAC is hydrolyzed to release cysteine, which is able to cross the blood-brain barrier. Cysteine is then converted into cystine, and high levels of the latter compound stimulate the exchange of glutamate for cystine through the cystine-glutamate antiporter. This results in an increase in nonsynaptic glutamate, which then acts on the glutamate receptors (mGluR2/3) of presynaptic neurons. Binding of this receptor leads to the inhibition of glutamate release in the nucleus accumbens. The decline in the level of glutamate leads to improved impulse control.46
Likewise, NAC is effective in accelerating wound healing through various mechanisms. As a precursor of GSH, NAC increases the synthesis of this antioxidant, which reduces free radical damage. It has been shown that free radicals inhibit the healing process by damaging proteins, lipids, and DNA. Moreover, NAC has also been shown to increase PI3K, the regulator of the PKC family of molecules, which is responsible for growth factor and cytokine production necessary for wound healing. Angiogenesis is also increased with NAC topical administration. This is accomplished through the upregulation of Stat3, a protein essential for migration of dermal microvascular endothelial cells. MMP-1, a protein responsible for extracellular matrix formation, is also increased with NAC administration.18
It has been postulated that the beneficial effect of NAC on ischemic ulcers and Raynaud’s phenomenon in scleroderma occurs through two mechanisms. First, NAC leads to a decline in reactive oxygen species, which is thought to be the mechanism for healing ischemic ulcers by minimizing free radical damage. Second, Raynaud’s phenomenon is alleviated with NAC use through its vasodilatory role in microvasculature, which counteracts the vasospasms seen in this disease. Specifically, the decline in free radicals leads to an increase in the production of nitric oxide, which causes vasodilation and relief of symptoms.24
The beneficial role of NAC in atopic dermatitis is also due to its ability to decrease free radical species. In the setting of reduced oxidative stress, it has been observed that cell adhesion molecules are able to restore the skin barrier, suggesting that topical administration of NAC will decrease TEWL and increase skin hydration. Atopic dermatitis has been partially attributed to a decline in TEWL and skin hydration; therefore, it would appear that NAC helps to counteract this phenomenon.31
Similarly, NAC’s ability to reduce free radicals makes it a viable treatment for TEN. There is evidence to suggest that drugs that induce TEN lead to proinflammatory cytokine release through the activation of T-lymphocytes. Reactive oxygen species contribute to keratinocyte damage, which is normally scavenged by GSH. As a precursor to GSH, NAC provides a therapeutic role in TEN.28
In cases of pseudoporphyria and porphyria cutanea tarda, sun exposure and ongoing inflammation leads to a decline of GSH in the skin. NAC is able to increase the synthesis of GSH, counteracting the inflammatory process in the skin.36
Lastly, through its ability to regulate gene expression, NAC has a beneficial effect on xeroderma pigmentosa through its inhibition of the activation of c-Jun N-terminal kinase, p38 mitogen-activated protein kinase, redox-sensitive activating protein 1, and NF-kB transcription factor. NAC also appears to decrease UV-induced free radical damage.47
Dosage and method of administration. NAC can be given orally, intravenously, topically, or by inhalation. NAC is rapidly absorbed after oral administration in both animals and humans, reaching maximum plasma concentration 2 to 3 hours after administration.15 The usual dose of oral NAC is 1,200 to 2,400mg per day; however, the precise therapeutic dosages of NAC have not been definitively established in the literature, and dosage studies are needed to determine optimal therapeutic dosing.
Adverse events. When NAC is given intravenously, the most common adverse effects (>10%) include autoimmune disease (14–18%) and nonimmunological anaphylactic reaction (1–18%).48 Other less common adverse side effects (<10%) include flushing, tachycardia, edema, rash, nausea, and vomiting. Rare side effects (?1%) include pharyngitis, rhinorrhea, rhonchi, and throat tightness. NAC is contraindicated in patients who have demonstrated a hypersensitivity to acetylcysteine.49
No significant adverse effects have been reported in association with oral administration of NAC. The most commonly reported adverse effects are mild gastrointestinal symptoms, including nausea, vomiting, diarrhea, and abdominal gas.51–53 Other side effects include a metallic taste, sleepiness, light-headedness, and wheezing.53
Limitations. There are several limitations to our review. First, for many of the dermatologic disorders discussed in this review, data are preliminary regarding the safety, tolerability, and efficacy of NAC as a method of treatment. Second, there were significant variations in study methodology among the research included in our review. Human studies differed in terms of dosage, route of administration, disease severity, and patient demographics, making it difficult to determine if the results could apply to the population at large. The included investigations also varied in terms of study design, ranging from case reports to double-blind, randomized, controlled trials, and study subjects, with some using animals and others using humans as the test subjects. Additional long-term, randomized, controlled research in humans is warranted for each dermatologic condition to firmly establish safety, efficacy, appropriate dosage, and generalizability of NAC therapy.
Conclusion
Our review of the available literature suggests that NAC could potentially serve as a safe, tolerable, and effective therapeutic option for a variety of dermatologic disorders, some of which currently have limited treatment options available. NAC’s modulation of the inflammatory pathways might contribute to the benefits seen with treatment of certain dermatologic disorders.
References
- Hutter D, Greene JJ. Influence of the cellular redox state on NF-kappaB-regulated gene expression. J Cell Physiol. 2000;183(1):45–52.
- Sheffner AL, Medler EM, Bailey KR, et al. Metabolic studies with acetylcysteine. Biochem Pharmacol 1966;15(10):1523–1535.
- Wong JW, Koo JY. Delusions of parasitosis. Indian J Dermatol. 2013;58(1):49–52.
- Middelveen MJ, Stricker RB. Morgellons disease: a filamentous vorrelial dermatitis. Int J Gen Med. 2016;9:349–354.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation: clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351–359.
- Odlaug BL, Grant JE. Pathologic skin picking. In: Grant JE, Stein DJ, Woods DW, Keuthen NJ. Trichotillomania, Skin Picking, and Other Body-Focused Repetitive Behaviors. Washington DC: American Psychiatric Publishing, Inc.; 2012:21–41.
- Simeon , Stein DJ, Gross S, et al. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry. 1997;58(8):341-347.
- Bloch MR, Elliott M, Thompson H, et al. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics. 2001;42(4):314-319.
- Arbabi M, Farnia V, Balighi K, et al. Efficacy of citalopram in treatment of pathological skin picking, a randomized double blind placebo controlled trial. Acta Med Iran. 2008;46(5):367-372.
- Grant JE, Odlaug BL, Chamberlain SR, et al. A double-blind, placebo-controlled trial of lamotrigine for pathological skin picking: treatment efficacy and neurocognitive predictors of response. J Clin Psychopharmacol. 2010;30(4):396-403
- Grant JE, Chamberlain SR, Redden SA, et al. N-acetylcysteine in the treatment of excoriation disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):490–496.
- Miller JL, Angulo M. An open-label pilot study of N-acetylcysteine for skin-picking in Prader-Willi syndrome. Am J Med Genet A. 2014;164A(2): 421–424.
- Ghanizadeh A, Derakhshan N, Berk M. N-acetylcysteine versus placebo for treating nail biting, a double blind randomized placebo controlled clinical trial. Antiinflamm Antiallergy Agents Med Chem. 2013;12(3):223–228.
- Berk M, Jeavons S, Dean OM, et al. Nail-biting stuff? The effect of N-acetyl cysteine on nail-biting. CNS Spectr. 2009;14(7):357–360.
- Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66(7):756–763.
- Oguz A, Uslukaya O, Alabal?k U, et al. Topical N-acetylcysteine improves wound healing comparable to dexpanthenol: an experimental study. Int Surg. 2015;100(4):656–661.
- Aktunc E, Ozacmak VH, Ozacmak HS, et al. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin Exp Dermatol. 2010;35(8):902–909.
- Tsai ML, Huang HP, Hsu JD, et al. Topical N-acetylcysteine accelerates wound healing in vitro and in vivo via the PKC/Stat3 pathway. Int J Mol Sci. 2014;15(5):7563–7578.
- Bächle AC, Mörsdorf P, Rezaeian F, et al. N-acetylcysteine attenuates leukocytic inflammation and microvascular perfusion failure in critically ischemic random pattern flaps. Microvasc Res. 2011;82(1):28–34.
- Eroshenko D, Polyudova T, Korobov V. N-acetylcysteine inhibits growth, adhesion and biofilm formation of Gram-positive skin pathogens. Microb Pathog. 2017;105:145–152.
- Montes L, Wilborn W, Montes CM. Topical acne treatment with acetylcysteine: clinical and experimental effects. Skinmed. 2012;10(6) 348–351.
- Bassotti A, Moreno S, Criado E. Successful treatment with topical N-acetylcysteine in urea in five children with congenital lamellar ichthyosis. Pediatr Dermatol. 2011;28(4): 451–455.
- Rosato E, Veneziano ML, Di Mario A, et al. Ulcers caused by bullous morphea: successful therapy with N-acetylcysteine and topical wound care. Int J Immunopathol Pharmacol. 2013;26(1):259–262.
- Rosato E, Borghese F, Pisarri S, Salsano F. The treatment with N-acetylcysteine of Raynaud’s phenomenon and ischemic ulcers therapy in sclerodermic patients: a prospective observational study of 50 patients. Clin Rheumatol. 2009;28(12):1379–1384.
- Revuz J. Alan Lyell and Lyell’s syndrome. J Eur Acad Dermatol Venereol. 2008;22(8):1001–1002.
- Vélez A, Moreno JC. Toxic epidermal necrolysis treated with N-acetylcysteine. J Am Acad Dermatol. 2002;46(3):469–470.
- Saavedra C, Cárdenas P, Castellanos H, et al. Cephazolin-induced toxic epidermal necrolysis treated with intravenous immunoglobulin and N-acetylcysteine. Case Reports Immunol. 2012;2012:931528.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. A proof-of-concept study. Burns. 2014;40(8):1707–1712.
- Werner Y. The water content of the stratum corneum in patients with atopic dermatitis. Measurement with the Corneometer CM 420. Acta Derm Venereol. 1986;66:281–284.
- Werner Y, Lindberg M. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm Venereol. 1985;65:102–105
- Nakai K, Yoneda K, Murakami Y, et al. Effects of topical N-acetylcysteine on skin hydration/transepidermal water loss in healthy volunteers and atopic dermatitis patients. Ann Dermatol. 2015;27(4):450–451.
- Demirel C, Kilciksiz S, Evirgen-Ayhan S, et al. The preventive effect of N-acetylcysteine on radiation-induced dermatitis in a rat model. J BUON. 2010;15(3):577–582.
- Li J, Xu L, Deng X, et al. N-acetylcysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain. 2016;157(8): 1711–1723.
- Kamboj SS, Vasishta RK, Sandhir R. N-acetylcysteine inhibits hyperglycemia-induced oxidative stress and apoptosis markers in diabetic neuropathy. J Neurochem. 2010;112(1):77–91.
- Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44(1):100–108.
- Velioglu A, Ergun T, Ozener C. Pseudoporphyria in a peritoneal dialysis patient. Perit Dial Int. 2015;35(2):234–235.
- Vadoud-Seyedi J, de Dobbeleer G, Simonart T. Treatment of haemodialysis-associated pseudoporphyria with N-acetylcysteine: report of two cases Br J Dermatol. 2008;142(3): 580–581.
- Binet H, Simonart T, Van Vooren JP, et al. Porphyria cutanea tarda in a human immunodeficiency virus-infected patient: treatment with N-acetylcysteine. Int J Dermatol. 1998;37(9):718–719.
- Cotter MA, Thomas J, Cassidy P, et al. N-acetylcysteine protects melanocytes against oxidative stress/damage and delays onset of ultraviolet-induced melanoma in mice. Clin Cancer Res. 2007;13(19):5952–5958.
- Goodson AG, Cotter MA, Cassidy P, et al. Use of oral N-acetylcysteine for protection of melanocytic nevi against UV-induced oxidative stress: towards a novel paradigm for melanoma chemoprevention. Clin Cancer Res. 2009;15(23):7434–7440.
- Kraemer KH. Sunlight and skin cancer: another link revealed. Proc Natl Acad Sci USA. 1997;94(1):11–14.
- Kunisada M, Hosaka C, Takemori C, et al. CXCL1 inhibition regulates UVB-induced skin inflammation and tumorigenesis in Xpa-deficient mice. J Invest Dermatol. 2017;137(9):1975–1983.
- Kang S, Chung J, and Lee J. Topical N-Acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J Invest Dermatol. 2003; 120(5):835-841.
- Weiss JS, Ellis CN, Headington JT, et al. Topical tretinoin improves photoaged skin: a double-blind vehicle-controlled study. JAMA. 1988;259(4): 527–532.
- De Vries N, De Flora S. n-Acetyl L-cysteine. J Cell Biochem Suppl. 1993;17F:270–278.
- Oliver G, Dean O, Camfield D, et al. N-acetyl cysteine in the treatment of obsessive compulsive and related disorders: a systematic review. Clin Psychopharmacol Neurosci. 2015;13(1):12–24.
- Reliene R, Fischer E, Schiestl RH. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res. 2004;64(15):5148–153.
- Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol (Phila). 2009;47(2):81–88.
- Waring WS, Stephen AF, Robinson OD. Lower incidence of anaphylactoid reactions to N-acetylcysteine in patients with high acetaminophen concentrations after overdose. Clin Toxicol (Phila). 2008;46(6):496–500.
- Flanagan RJ, Meredith TJ. Use of N-acetylcysteine in clinical toxicology. Am J Med. 1991;91(3C):131S–139S.
- Mant TG, Tempowski JH, Volans GN, Talbot JC. Adverse reactions to acetylcysteine and effects of overdose. Br Med J (Clin Res Ed). 1984;289(6439):217–219.
- Zyoud SH, Awang R, Syed Sulaiman SA, et al. Incidence of adverse drug reactions induced by N-acetylcysteine in patients with acetaminophen overdose. Hum Exp Toxicol. 2010;29(3):153–160.
- Matuszczak Y, Farid M, Jones J, et al. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve. 2005;32(5):633–638.

