J Clin Aesthet Dermatol. 2019;12(10):39–44
 by Rachel Lubart, Phd; Inbar Yariv MSc; Dror Fixler, PhD; and Anat Lipovsky, Phd
by Rachel Lubart, Phd; Inbar Yariv MSc; Dror Fixler, PhD; and Anat Lipovsky, Phd
Prof. Lubart and Dr. Lipovsky are with the Chemistry Department at Bar-Ilan University in Ramat-Gan, Israel. Prof. Lubart is also with the Physics Department at Bar-Ilan University in Ramat-Gan, Israel. Ms. Yariv and Prof.. Fixler are with the Faculty of Engineering and the Institute of Nanotechnology and Advanced Materials, Bar-Ilan University in Ramat-Gan, Israel.
ABSTRACT: Background. Topical hyaluronic acid (HA) has shown effectiveness in maintaining skin hydration. Topical creams containing HA are widely available, but their efficacy is limited by their lack of penetration into the skin due to the large molecule size of HA, the result of being formulated into a cream base.
Objective. In this three-part study (in vitro, ex vivo, and in vivo), molecule sizes, penetration levels, and antiaging qualities of a topical HA facial cream that was formulated using a new technology that micronizes HA molecules (m-HA) were assessed.
Methods and Results. Particle sizes of m-HA were evaluated using electron microscopy, which showed varying sizes, the smallest of which was 100nm in diameter. The antioxidation capabilities of m-HA were measured using electron spin resonance and were found to be higher than original HA. Skin penetration of the m-HA formulation was evaluated via immunohistochemical staining of porcine skin samples, which demonstrated penetration of the formulation into the stratum corneum and the deep epidermal layers toward the dermis. Antiaging qualities of the m-HA formulation were assessed in an open-label clinical study that included 36 healthy adult women. Skin parameters were measured objectively (e.g., Corneometer, Cutometer) and subjectively via patient questionnaire, results of which indicated significant improvements in facial skin hydration, elasticity, and wrinkle depth.
Conclusion.The topical HA facial cream with m-HA technology demonstrated penetration into the epidermal skin layer, and, to our knowledge, our formulation is the first HA facial cream to achieve this. Clinical application of the facial cream demonstrated objective and subjective improvements in facial skin quality of healthy adult female subjects. Our results support the use of this new HA facial cream with m-HA technology as an effective antiaging topical therapy. Larger randomized, controlled studies are needed to confirm our findings.
KEYWORDS: Antiaging, micronized hyaluronic acid, skin penetration, antioxidant, skin hydration
Hyaluronic acid (HA), a macromolecule, plays a vital role in the synthesis of extracellular matrix molecules and epidermal cell interactions with the surrounding environment.1 It is reported to be nontoxic, nonimmunogenic, and noncarcinogenic.2 HA is widely used for multiple medical purposes.3 One of its most valued properties is its ability to hold a large amount of moisture.4 Youthful skin remains hydrated because it contains large amounts of HA. However, as we age, the amount of HA in the skin decreases, and, by the time we become adults, this amount decreases to five percent of the baseline. HA is also known as an excellent free radical scavenger. With its anti-inflammatory properties, HA could potentially be used help to stop inflammation and irritation to the skin.5
HA lacks the ability to permeate the skin, and when topical HA is applied to the skin, it remains on the skin’s surface and functions as a skin-surface moisturizer; thus HA injections must be used for its delivery into deeper skin layers. A technology that enables topically applied HA to deeply penetrate the skin would likely be well received among dermatologists and the patients they treat. Decreasing the size of a substance has additional advantages. For example, when the particle size decreases, the proportion of the surface area increases, which predicts a greater biological activity per given mass when compared with larger particles.6
Many methods have been applied to depolymerize HA into lower-molecular-mass fragments, such as oxidative degradation,7 high-temperature exposure in an autoclave,8 and acid hydrolysis.9 Today, the raw material of micronized HA powder (50KDa) is available on the market. The challenging aspect is keeping the HA molecules “small” after they are formulated into a cream base. Once the dry micronized molecules are embedded in solution before being incorporated into the cream, they immediately react with water and aggregate into bulky HA complexes, which are, once again, too large to permeate the skin.
We have developed a promising technique that prepares nanoparticles of organic materials (NOPs) to stay small after they are incorporated into a cream base. The NOPs were found to possess an increased biological activity as well as increased penetration into the tissue.10 In this article, the properties of the micronized HA we prepared are discussed. For the in-vitro portion of our study, micronized HA was characterized by scanning electron microscopy (SEM) and electron spin resonance (ESR) analysis, and the skin-penetration depth of a cream formulation containing the micronized HA (m-HA) was tested on porcine skin samples. For the in-vivo portion of our study, we evaluated the clinical efficacy of the new cream formulation using an open-label study model that measured objective and subjective improvements in facial skin quality parameters among a group of healthy adult women volunteers.
Materials and Methods
In-vitro study. Molecule size. To determine the size of m-HA, SEM was performed using the JSM 840 system (JEOL Ltd., Tokyo, Japan). To determine the antioxidative capability of HA, electron paramagnetic resonance (ESR) spectroscopy was used. ESR is a very sensitive technique for determining reactive oxygen species, such as the presence of hydroxyl radicals. Here, hydroxyl radicals were generated by performing a Fenton reaction (Eq. 1).
Fe2+ + H2O2 Fe3+ + OH* + OH–
Hydroxyl radicals have a short half-life (ns-ms), which makes them difficult to detect directly; thus, it is necessary to add a spin trap that binds the hydroxyl radicals to ensure a long-lived free radical, called a spin adduct, that can be detected by the ESR technique11
We used 5,5 dimethyl-1-pyrroline-N-oxide (DMPO) to trap hydroxyl radicals to yield DMPO-OH, which has a quartet signal in the EPR spectrum. Figure 1 shows a typical ESR spectrum of DMPO-OH adduct that is characterized by its 1:2:2:1 quartet of lines and hyperfine splitting constant aN and aH=14.9. The area under the quartet signal is proportional to the amount of hydroxyl radicals.
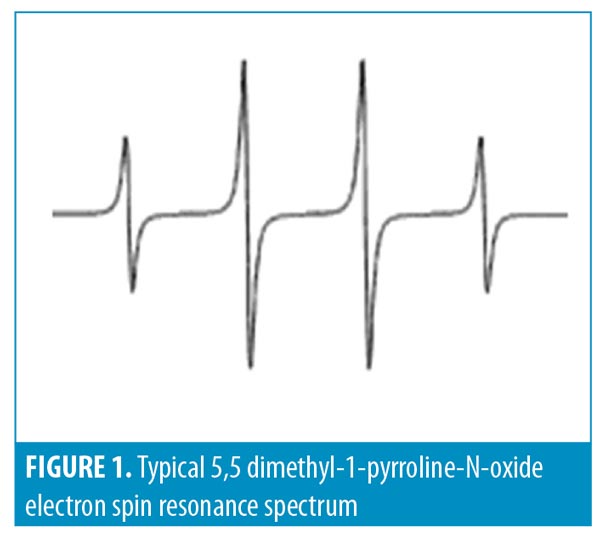
Antioxidative qualities. The antioxidative properties of small HA relative to those of original HA were measured by the reduction of hydroxyl radicals generated in a Fenton reaction in the presence of small or original HA. In the Fenton reaction (H2O2 + Fe2+ HO– + HO– + Fe3+), hydroxyl radicals (HO*) are formed, mimicking the oxidative stress formed in vivo. When the Fenton reaction is performed in the presence of HA, there is a decrease in the quartet signal, which indicates its antioxidative activity.
The effects of HA on the formation of hydroxyl radicals was investigated by adding HA to a Fenton reaction system (H2O2+ FeCl2 + DMPO + ddH2O/HA). In order to detect hydroxyl radical coupled with the spin trap, 5,5-dimethyl-1- pyrroline-N-oxide (DMPO; 0.02 M; Sigma-Aldrich, St. Louis, Missouri), Fenton reagents with HA were drawn by a syringe into a gas-permeable Teflon capillary (Zeus Industries, Raritan, New Jersey) and inserted into a narrow quartz tube that was kept open at both ends. The tube was then placed in the ESR cavity and the spectra were recorded after five minutes of reaction time, on an EPR 100d X-band spectrometer (Bruker, Billerica, Massachusetts). The EPR measurement conditions were as follows: frequency, 9.74 GHz; microwave power, 20 mW; scan width, 65 G; resolution, 1,024; receiver gain, 2×105; conversion time, 82ms; time constant, 655ms; sweep time, 84 seconds; scans, n=2; modulation frequency, 100 kHz.
Ex-vivo study—permeation. The newly prepared m-HA was inserted (0.15%) into a base cream containing water, cetyl alcohol, isopropyl myristate, C12-15 alkyl benzoate, steareth-2, hydroxyethyl urea, cetearyl ethylhexanoate, castor isostereate succinate, theobroma cacao (cocoa) seed butter, steareth-21, phenoxyethanol, dimethicone, caprylyl glycol chlorphenesin, disodium ethylenediaminetetraacetic acid, sodium pyroglutamic acid, fructose, glycine inositol, niacinamide, and sodium lactate. Fresh porcine skin samples were used for the analysis. Three different formulations of HA in the same base cream were applied on the porcine skin samples and were left for five hours. The samples were then fixed and prepared on histological slides. Immunohistochemistry (IHC) analysis was performed using, first, an “anti-HA” antibody and then a second layer of fluorescent antibody against the first “anti-HA” antibody.
In-vivo study: skin quality improvement measures. The clinical study was conducted in accordance with the Guideline for Good Clinical Practice-ICH Harmonised Tripartite Guideline, applicable United States Food and Drug Administration (FDA) regulations/guidelines set forth in the 21 Code of Federal Regulations parts 11 and 50, and the standard practices of BioScreen Testing Services. Prior to initiating the study, an informed consent form, which outlined reasons for the study, possible adverse effects, associated risks and potential benefits of the treatment, and limits of liability for BioScreen Testing Services, Inc. was obtained from each volunteer, along with a photography release form.
The study was performed by an independent clinical research organization (BioScreen Testing Services) in Torrance, California. Thirty-six healthy female participants, 35 to 65 years of age, were enrolled in the study. Eligible participants were healthy women (American Society of Anesthesiologists [ASA] Physical Classification System I–II) who were free of any chronic or acute dermatological disorder. Potential participants were excluded if they had damaged skin at or close to test sites (e.g., sunburn, tattoos, scars, excessive hair, nonremovable piercings, other disfigurations); were pregnant, nursing, or planned to become pregnant during the study period; reported usage of injectable insulin; had undergone medical procedures (e.g., laser resurfacing, plastic surgery, or other injectable fillers) at the test sites within the previous 12 months; were currently using or who had used retin A, other prescription or over-the-counter retinyl A products, other astringent-derived products, or alpha hydroxyl acid treatments for photoaging and fine lines/wrinkles in the last three months; and/or had a known history of hypersensitivity to any cosmetics, personal care products, and/or fragrances. Study participants were instructed to refrain from using personal care products with antiaging properties for the duration of the study (12 weeks). Participants were also instructed not to wear any form of cosmetics on the study visit days.
For test product use, participants were instructed to apply the cream on five designated areas, including the forehead, left and right periorbital skin areas, upper part of the mouth, and lower part of the mouth. The cream was applied twice daily, morning and evening, on clean and completely dry skin. Participants were instructed to apply small amounts of the cream first into wrinkles within the designated areas (e.g., forehead, around/on the lips, around the eyes), and, after two to three minutes, massage the cream around and into the whole face. The test product was weighed at each visit to monitor adherence.
Skin hydration measurements. Moisture levels in the skin were measured by testing changes in skin electrical properties. The Corneometer CM 825 (Courage and Khazaka, Cologne, Germany) or the Dermalab Series SkinLab Combo Moisture probe (Cortex Technology, Denmark) were used to measure the electrical capacitance/hydration or conductance/hydration, respectively, of the skin as recommended by the manufacturer. Three replicate measurements were taken on designated test sites at each interval.
Skin firmness (R0) and elasticity (R7). The firmness and the elasticity of the skin were measured using the Cutometer MPA 580 Courage and Khazaka, Cologne, Germany). This device measures the elasticity of the upper skin layer using negative pressure, which deforms the skin mechanically. The measuring principle is based on the suction method. Negative pressure is created in the device and the skin is drawn into the aperture of the probe before being released again after a defined time. Inside the probe, the penetration depth is determined by a noncontact optical measuring system. The resistance of the skin to the negative pressure (firmness) and its ability to return to its original position (elasticity) are displayed as curves (penetration depth in mm/time) in real time during the measurement. This measurement allows one to obtain information about the elastic and mechanical properties of the skin surface and objectively quantify skin aging.
Patient questionnaire. Each participant was instructed to complete a self-assessment questionnaire.
Statistical analysis. Statistical analyses were performed using a z-test. Statistical significance was set at 0.05.
Results and Discussion
In-vitro study. Molecule size. The main purpose for reducing the size of HA is to facilitate its delivery to human tissues and to enhance its penetration across the skin barrier. In addition, decreasing HA size results in an intensifying of its characteristics, including its anti-oxidation capability and hygroscopy, which are of great importance in medical cosmetics.
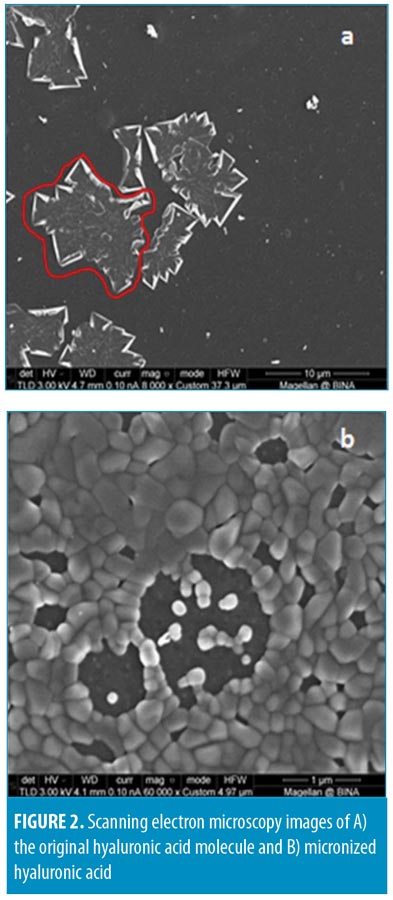
In the present research work, we used a new technique to produce a significant reduction in the HA size. The reduced sizes were evaluated using electron microscopy techniques. As shown in Figure 2, the original, irregularly shaped HA molecule of ~15 × 20 microns (circled, Figure 2A) went through a micronization procedure, resulting in the formation of m-HA. Figure 2 depicts the change in the size of HA/m-HA particles. In Figure 2A, the original HA molecule is seen as a large irregular structure (marked by a red line). In Figure 2B, the m-HA particles can be observed as spherical and monodispersed.
Antioxidative activity. Significant attention has been paid to antioxidants that prevent reactive oxygen species-induced damage. HA is known for its antioxidative properties both in vitro and in vivo.12,13 This makes HA a good antiaging agent. By reducing the size of HA, it is expected that its antioxidative capability will be increased. Therefore, we examined the antioxidative capability of m-HA prepared using our technique relative to original HA. As is demonstrated in Figure 3, m-HA (Figure 3C) scavenged hydroxyl radicals better than original HA (Figure 3B).
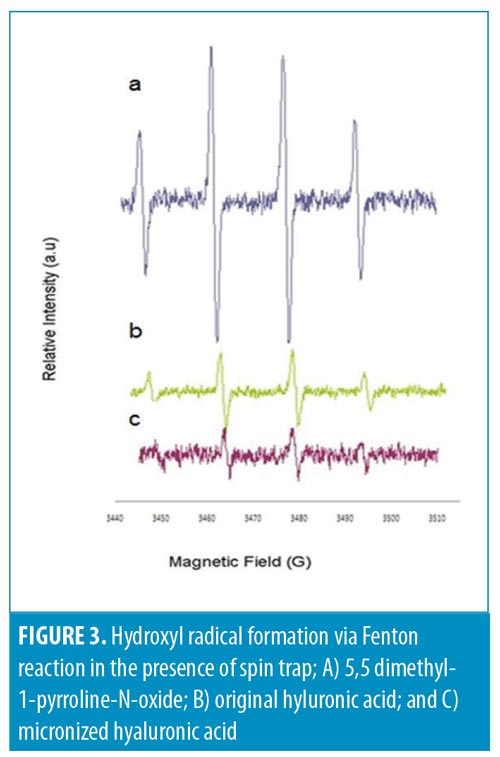
Preventing oxidative damage in living organisms by scavenging free radicals is important. m-HA appears to have a high radical scavenging effect relative to that of original HA, which suggests better oxidative skin damage management.
Ex-vivo study. Permeation. The evaluation of HA skin permeation was done ex vivo by applying three different HA formulations on porcine skin tissue for five hours, as follows:
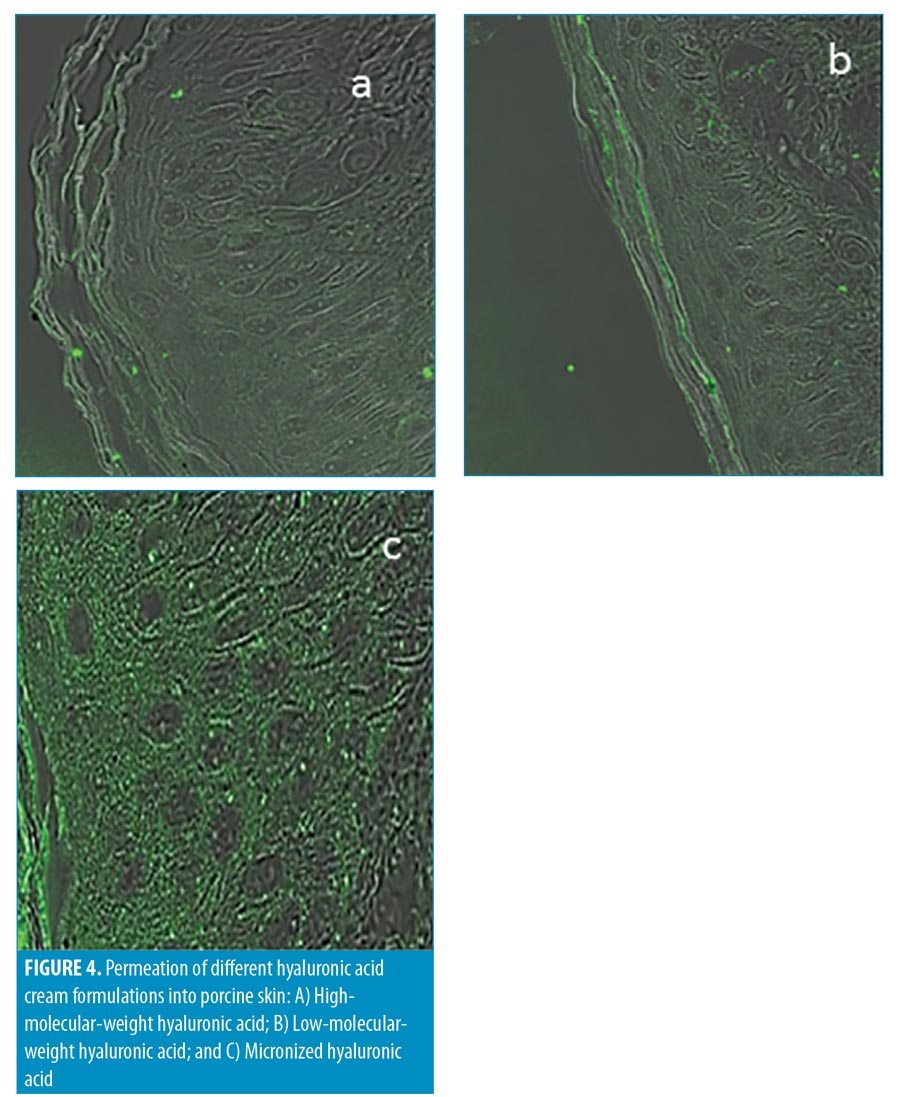
Formulation 1: high-molecular-weight (HMW) HA, inserted in the base cream (Figure 4A).
Formulation 2: low-molecular-weight (LMW) (50 kDa) HA inserted in the base cream (Figure 4B).
Formulation 3: m-HA inserted in the base cream (Figure 4C).
The presence of HA in the skin layers was monitored by marking the HA molecules with fluorescent markers. In Figure 4A, where the base cream formulation containing HMW HA was used, very few glowing bodies (indicating HA) in the stratum corneum were observed. Application of the base cream with LMW HA resulted in more glowing bodies in the stratum corneum, indicating the presence of HA in the layer (Figure 4B). Application of the base cream containing m-HA (Figure 4C) resulted in a very high concentration of glowing bodies in the stratum corneum and the epidermis. The bright image of the stratum corneum and the epidermis demonstrated m-HA penetration beyond the stratum corneum and into the deep epidermal layer.
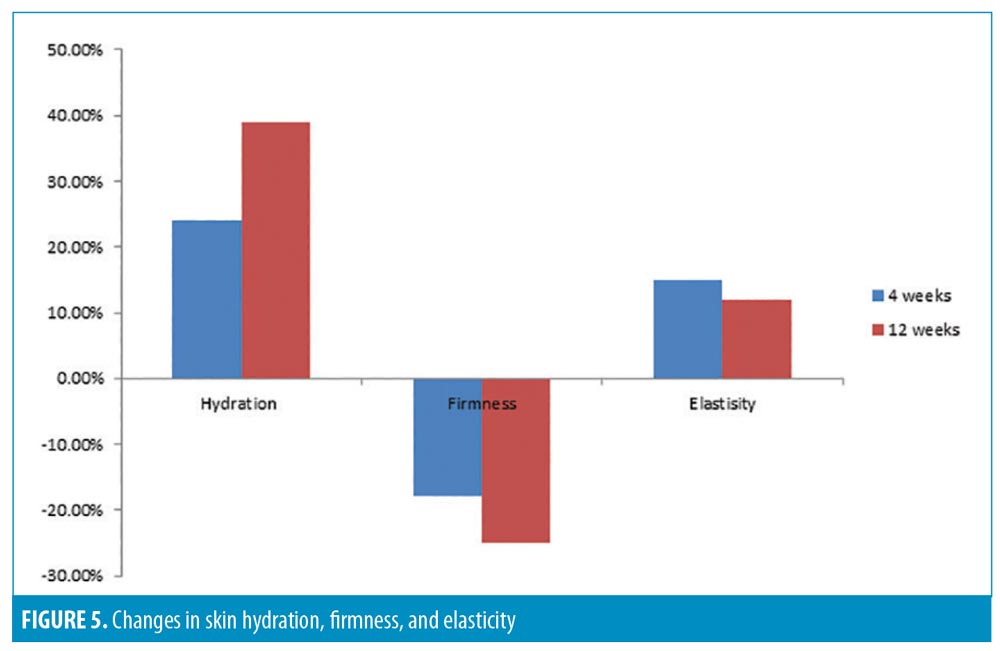
In-vivo clinical study. Thirty-six participants participated in this study, including 13 African-American, 12 Caucasian, six Hispanic, and five Asian participants. Skin hydration (measured by the Corneometer), firmness, and elasticity (both measured by the Cutometer) were evaluated after four and 12 weeks of treatment. Photographic images of fine lines and wrinkles (Vaestro; Canfield Scientific, Parsippany, New Jersey) were also evaluated.
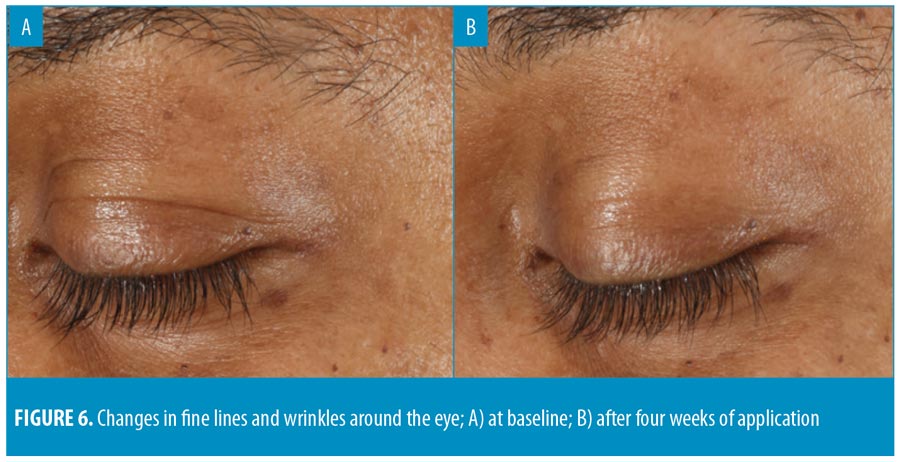
The results demonstrated a statistically significant improvement in skin hydration from baseline to four (24.%) and 12 (38.9%) weeks posttreatment. All participants (100%, p less than or equal to 0.001) showed improvements in skin hydration. Skin hydration, skin firmness, and skin elasticity showed a significant improvement (p less than or equal to 0.005) from baseline in 100 percent, 88.8 percent, and 67.5 percent of participants, respectively (Figure 5). There was also a statistically significant improvement in the appearance of fine lines and wrinkles in the eye area, evaluated using Vaestro, after only four weeks of treatment (Figure 6).
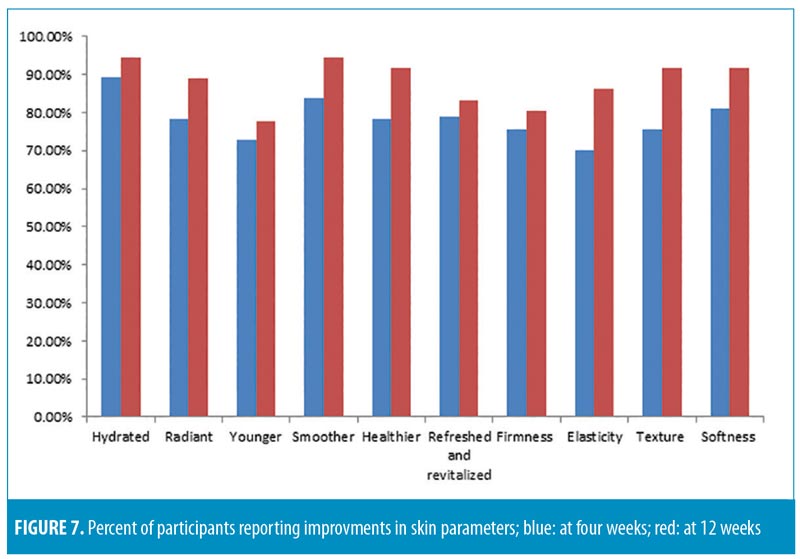
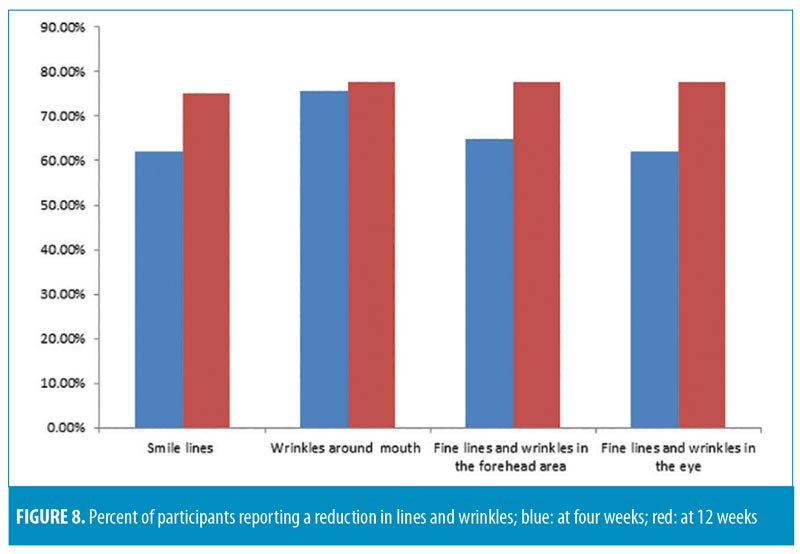
All participants were asked to fill out product efficacy surveys. According to the surveys, an overall improvement in the appearance of the skin was reported by 72.9 percent of participants after four weeks and 80.5 percent after 12 weeks (p=0.05); an overall decrease in fine lines and wrinkles was reported by 70.2 percent and 75 percent of participants (p=0.05) after four and 12 weeks, respectively.
Each tested parameter with participant responses (percentage of subjects in agreement) from the efficacy questionnaire are presented in Figures 7 and 8. All participants (100%) reported ease of application, while 94.4 percent reported the product left the skin feeling pleasant. The majority of subjects (88.8%) were satisfied with the overall results of the product, and no participants reported any unpleasant or uncomfortable sensations. No adverse effects or serious adverse events were reported.
Discussion
Aging is a multifactorial and complex process, and skin aging results in loss of hydration and elasticity.14 Aging is also observed as a consequence of continuous exposure to raised levels of reactive oxygen species.15 One of the most important factors that contributes to skin aging is the reduction in HA and collagen in the dermal matrix.16 In our study, we used in-vivo objective measurements to measure antiaging effects in the newly prepared HA cream. Our results suggest the new technology used to micronize HA leads to better penetration of HA into the epidermal layers. To our knowledge, this study is the first to examine the penetration depth of HA creams in the stratum corneum and epidermal layers. A literature survey revealed few publications have examined the clinical effectiveness of HA-containing creams.17–20 Only Pavicic et al21 considered the efficacy of topical application of creams containing varying sizes of HA molecules, but their results regarding improvement in important skin parameters, such as hydration and elasticity, indicated less effectiveness than our results.
Limitations. Limitations of our study include its unblinded methodology, the lack of controls, and the small sample size. Blinded, randomized, controlled clinical trials that include larger participant samples are needed to confirm our results.
Conclusion
The results indicate that our new topical HA facial cream with micronized HA is capable of penetrating the epidermis of facial skin. Our results also suggest effectiveness in improving facial skin quality in a sample of healthy adult women, and suggest its superiority over original topical HA molecule formulations. We believe the study formulation is the first topical solution to provide effective, needle-free administration of fully functional HA molecules into the deeper layers of the skin.
References
- West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228(4705): 1324–1326.
- Becker LC, Bergfeld WF, Belsito DV, et al. Final report of the safety assessment of hyaluronic acid, potassium hyaluronate, and sodium hyaluronate. Int J Toxicol. 2009;28(4 Suppl):5–67.
- Saranraj P, Naidu MA. Hyaluronic acid production and its applications—a review. Int J Pharm Biol Arch. 2013;4(5):853–859.
- Sirikudta W. Moisturizers for patients with atopic dermatitis: an overview. J Allergy Ther. 2013;4(4).
- Schlesinger T, Rowland Powell C. Efficacy and safety of a low-molecular weight hyaluronic Acid topical gel in the treatment of facial seborrheic dermatitis. J Clin Aesthet Dermatol. 2012;5(10):20–23.
- Ansari AA, Khan MN, Alhoshan M, et al. Nanostructured materials: Classification, properties and fabrication. Nanostructured Materials: Classification, Properties and Fabrication. Hauppauge, NY: Nova Science Publishers; 2010: 1–126.
- Hokputsa S, Jumel K, Alexander C, Harding SE. A comparison of molecular mass determination of hyaluronic acid using SEC/MALLS and sedimentation equilibrium. Eur Biophys J. 2003;32(5):450–456.
- Bothner H, Waaler T, Wik O. Limiting viscosity number and weight average molecular weight of hyaluronate samples produced by heat degradation. Int J Biol Macromol. 1988;10(5): 287–291.
- Tokita Y, Okamoto A. Hydrolytic degradation of hyaluronic acid. Polym Degrad Stab. 1995;48(2):269–273.
- Yariv I, Rahamim G, Shliselberg E, et al. Detecting nanoparticles in tissue using an optical iterative technique. Biomed Opt Express. 2014;5(11): 3871–3881.
- Buettner GR. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3(4): 259–303.
- Campo GM, Avenoso A, Campo S, et al. The antioxidant and antifibrogenic effects of the glycosaminoglycans hyaluronic acid and chondroitin-4-sulphate in a subchronic rat model of carbon tetrachloride-induced liver fibrogenesis. Chem Biol Interact. 2004 ;148(3):125–138.
- Balogh GT, Illés J, Székely Z, et al. Effect of different metal ions on the oxidative damage and antioxidant capacity of hyaluronic acid. Arch Biochem Biophys. 2003;410(1):76–82.
- Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2):241–251.
- Wilkinson HN, Hardman MJ. The role of estrogen in cutaneous ageing and repair. Maturitas. 2017;103:60–64.
- Janiš R, Pata V, Egner P, et al. Comparison of metrological techniques for evaluation of the impact of a cosmetic product containing hyaluronic acid on the properties of skin surface. Biointerphases. 2017;12(2):021006.
- Ferrillo M, Vastarella M, Cantelli M, et al. Instrumental, clinical and subjective evaluation of the efficacy of a cosmetic treatment for home use. J Cosmet Laser Ther. 2019;21(4):190–195.
- Garre A, Martinez-Masana G, Piquero-Casals J, Granger C. Redefining face contour with a novel anti-aging cosmetic product: An open-label, prospective clinical study. Clin Cosmet Investig Dermatol. 2017;10:473–482.
- Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7(3):27–29.
- Haeusler H. Efficacy of a hyaluronic acid gel to improve the skin properties. SOFW Journal. 2015;141:16–18.
- Pavicic T, Gauglitz GG, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10(9): 990–1000.

