 J Clin Aesthet Dermatol. 2019;12(5):34–38
J Clin Aesthet Dermatol. 2019;12(5):34–38
by SHILPI SHARMA, MD; DEEPAK K. MATHUR, MD; VIJAY PALIWAL, MD; and PUNEET BHARGAVA,MD
All authors are with the Department of Dermatology, Venereology, and Leprology at SMS Medical College and Attached Hospitals in Jaipur, India.
FUNDING: No funding was received for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Introduction.Acne vulgaris is a chronic inflammatory disorder of the pilosebaceous units and has been associated with hyperandrogenism, which, in women, is most commonly caused by polycystic ovary syndrome (PCOS). Metformin treatment can correct ovarian and functional adrenal hyperandrogenism in adolescents with PCOS.
Objective. We evaluated the efficacy of metformin therapy in women with acne and PCOS in terms of acne load.
Methods.This was a hospital-based, interventional, longitudinal study that included 40 female patients with acne and PCOS diagnosed using the Rotterdam criteria. Hormonal assay, including serum levels of testosterone, dehydroepiandrosterone sulphate, luteinizing hormone, follicle-stimulating hormone, prolactin, and blood sugar, was conducted on the third or fourth day of the menstrual cycle in a fasted state. An abdominal ultrasound was performed on the ninth day of menstrual cycle to diagnose PCOS. Baseline acne severity was assessed as per the Definition Severity Index. Metformin 500mg was given to all selected patients three times a day for eight weeks. Patient follow-up and acne severity reassessment was conducted at Weeks 3 and 6. At Week 8, all work-ups were repeated. Intention-to-treat analysis was done. Wilcoxin signed-rank sum test was used to identify significance in acne severity.
Results.Metformin treatment significantly reduced acne severity in patients with PCOS (p<0.001).
Conclusion. Metformin reduces ovarian hyperandrogenism, leading to clinical improvement of acne in women with PCOS.
KEYWORDS: Acne, acne load, hyperandrogenism, PCOS, acne severity
Acne vulgaris is a chronic inflammatory disorder of the pilosebaceous units1 and has been associated with hyperandrogenism. In women, the most common cause of hyperandrogenemia is polycystic ovary syndrome (PCOS), which usually manifests as acne, hirsutism, and menstrual irregularities.2 Insulin resistance and raised plasma levels of insulin are responsible for the high androgen concentration in patients with PCOS.3 Metformin, a biguanide hypoglycemic drug, improves insulin sensitivity, decreases insulin levels, corrects ovarian and functional adrenal hyperandrogenism in PCOS, and leads to clinical improvement of acne.3 However, literature studying the effects metformin has on acne severity in patients with PCOS is sparse. We evaluated the efficacy and safety of metformin therapy in reducing acne severity in women with PCOS and acne who were being treated at a skin outpatient department at SMS Hospital in Jaipur, India.
Methods
This was a hospital-based, interventional, longitudinal study of 40 patients conducted in the Department of Dermatology, Venereology, and Leprosy at SMS Hospital in Jaipur, India following clearance from the Institutional Review Board.Written informed consent to participate in the study, as well as photoconsent from the patient used as the illustrative case example in this article, were obtained from all participants
Inclusion and exclusion criteria. Adult female patients who presented to our clinic between June 2014 and May 2015, had a diagnosis of PCOS, and met the other inclusion criteria of the study were recruited to participate in our investigation. Other inclusion criteria included having a diagnosis of comorbid acne and willingness to provide informed consent. Patients who met these criteria were excluded if they were pregnant; lactating; taking oral contraceptive pills or other hormonal replacement drugs known to cause acne; or were undergoing any therapies for acne that included systemic treatment, topical treatment, or any form of dermatological procedure.
Baseline evaluation. Forty-five women met study requirements and were recruited as participants in the study. Written informed consent and detailed demographic and historical clinical data were obtained and recorded for each patient. Hormonal assay, including testosterone, dehydroepiandrosterone sulphate (DHEA), luteinizing hormone (LH)/follicle-stimulating hormone (FSH) ratio, and prolactin levels, was performed on all patients on the third or fourth day of their menstrual cycle in a fasted state. A pelvic ultrasound was performed on all patients on the ninth day of their menstrual cycle. Fasting blood sugar was recorded in all patients, and total acne load was assessed.
Assessment methods. PCOS was diagnosed using the Rotterdam criteria,4 which requires the presence of at least two of the following: oligo/anovulation, hyperandrogenism, and/or polycystic ovaries on ultrasound.
Acne was diagnosed clinically, and its severity was measured using a four-point Definition Severity Index (DSI)5 assessment scale, defined as follows: 0.5=no-inflamed comedones, open and closed (no erythema); 1=comedones/papules with surrounding erythema and/or superficial pustules less than 2mm in diameter with no or little erythema; 2=pustules with greater than 2mm in diameter and/or with significant erythema ; 3=deep infiltrates with or without pustules/nodules/isolated cysts. Total acne load was calculated for each patient by multiplying the total number of each type of lesion with its severity index and adding all of these sums together.
We used the Ferriman-Gallwey system6 to assess hirsutism, a 0-to-4-point scale (0=no hair and 4=frankly virile) that assesses hair quantity on nine different body sites: chin, upper lip, chest, upper and lower back, upper and lower abdomen, arm and thighs. A score of 8 or higher is commonly accepted as abnormal.
Statistical analysis. Acne load percent reduction was calculated using the Friedman test,7 a nonparametric alternative to the one-way analysis of variance (ANOVA) with repeated measures. The Friedman test can be used to test for differences between groups when the dependent variable being measured is ordinal. It can also be used for continuous data that has violated the assumptions necessary to run the one-way ANOVA with repeated measures. A P value of <0.05 was taken as significant in percent improvement as well as in percent reduction of acne from baseline to Weeks 3, 6, and 8 of therapy.
Dosage and administration. Metformin 500mg was administered to all patients three times a day for eight weeks. Patient follow-up was conducted at Weeks 3, 6, and 8. Acne severity reassessment was conducted at Weeks 3, 6, and 8, and detailed clinical evaluation and radiological and biochemical investigations were repeated at Week 8.
Results
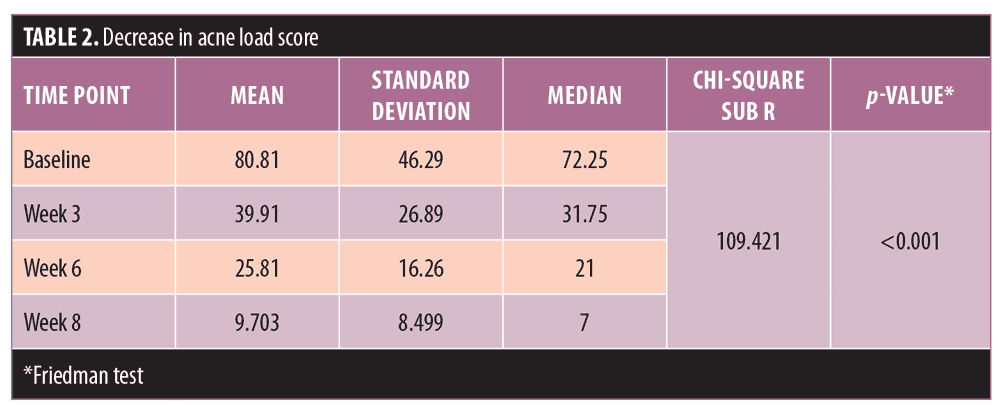
In this hospital-based, interventional, longitudinal study, 45 patients met the study inclusion/exclusion criteria and were recruited as participants. Two women dropped out of the study due to adverse events (AE), and three women were excluded from further analysis due to failure to attend the required follow-up visits. Forty women in all completed the study, and results are based on data collected from these 40 participants. The mean age of study participants was 20.98±3.423 years with a median age of 20 years. Mean age of onset for acne was 18.45±3.7 years, with a median onset age of 18 years (Table 1).
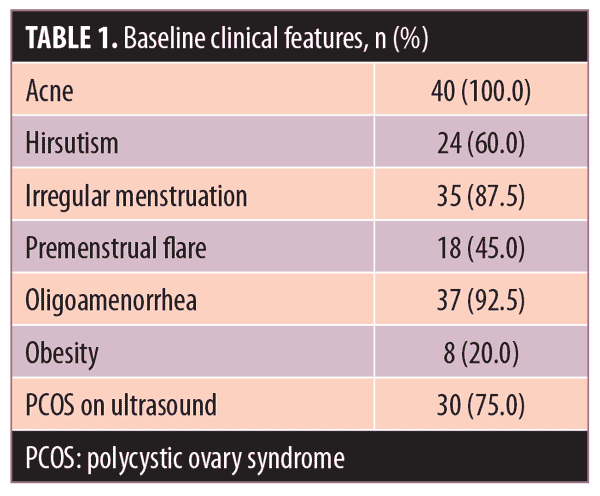
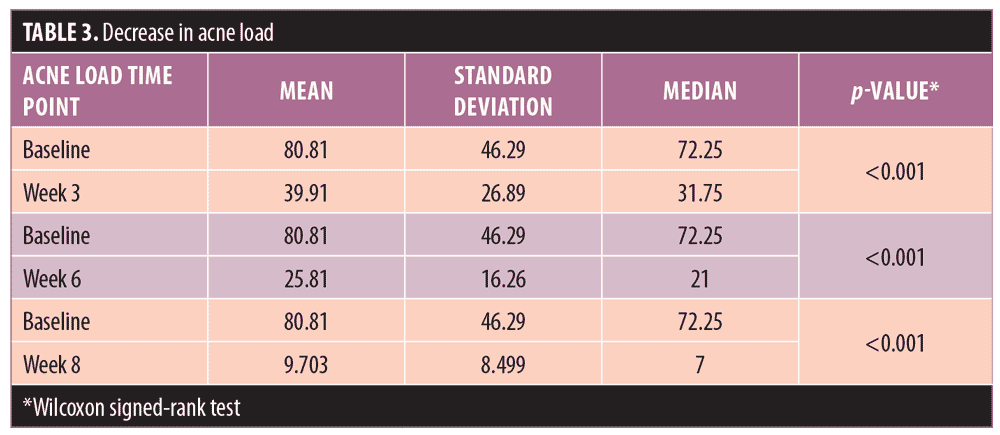
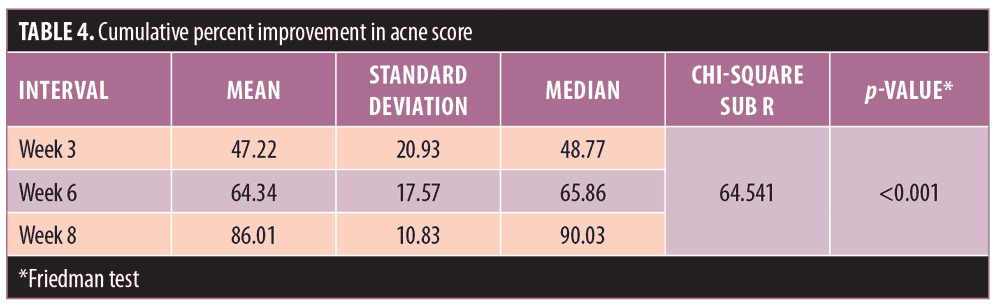
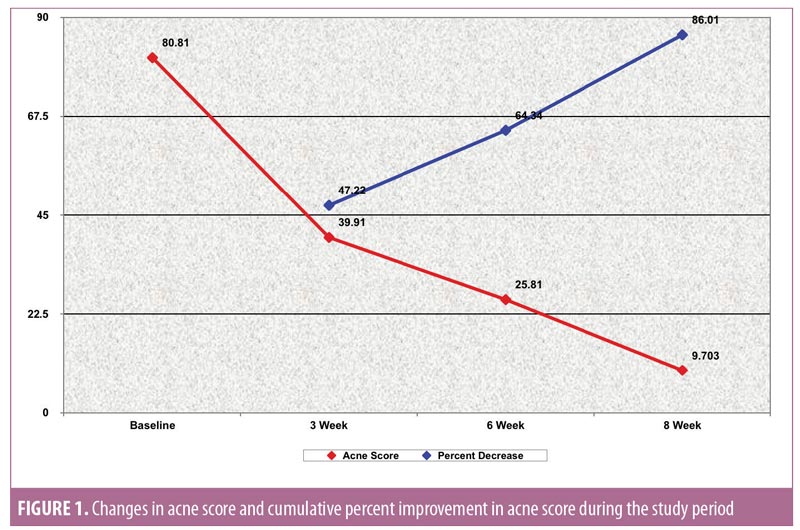
Acne severity. Using the DSI, the mean acne severity at baseline was calculated to be 80.81±46.29. Acne load decreased to 39.91±26.89 at Week 3 of treatment with metformin 500mg. At Week 6, acne load decreased to 25.81±16.26. At the end of treatment (8 weeks), acne load was reduced to 9.703±8.5 (Figure 2). Upon application of the Friedman test, we found the reduction in acne load to be highly statistically significant (Table 2) (p<0.001). Comparison of acne severity at Weeks 3, 6, and 8 to acne severity at baseline showed a significant decrease in acne severity at Week 3 that was sustained through Weeks 6 and 8 (p<0.001) (Table 3). There was a 47.22-percent improvement in acne load in the first three weeks of therapy, which increased to 64.34 percent at six weeks and 86.01 percent at eight weeks. This change in rate of improvement was found to be significantly increased (p<0.001) during the study period (Table 4 and Figure 1).
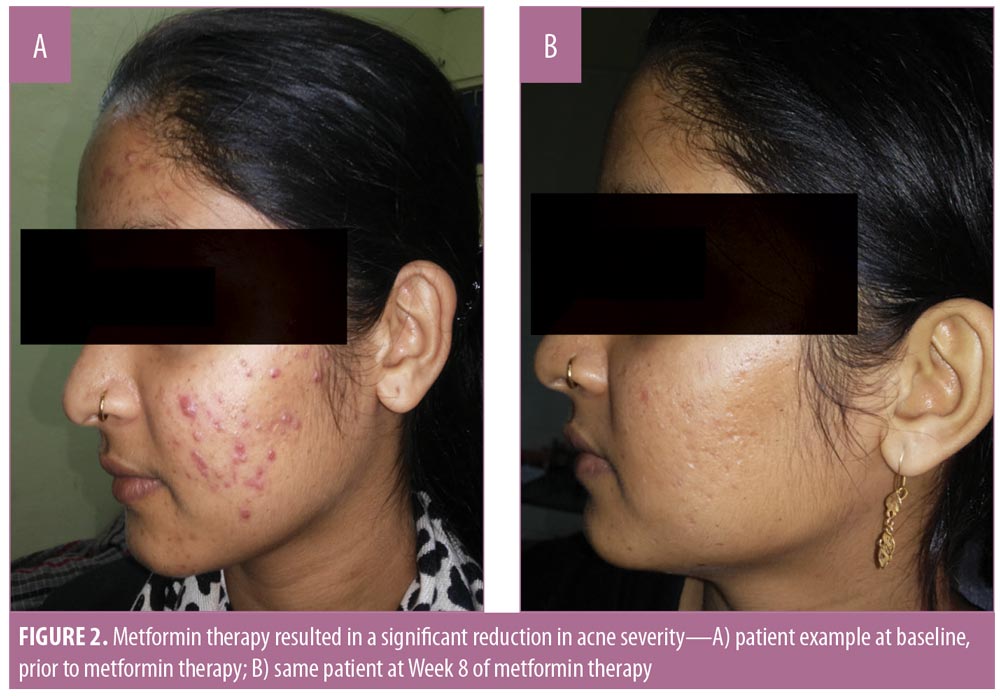
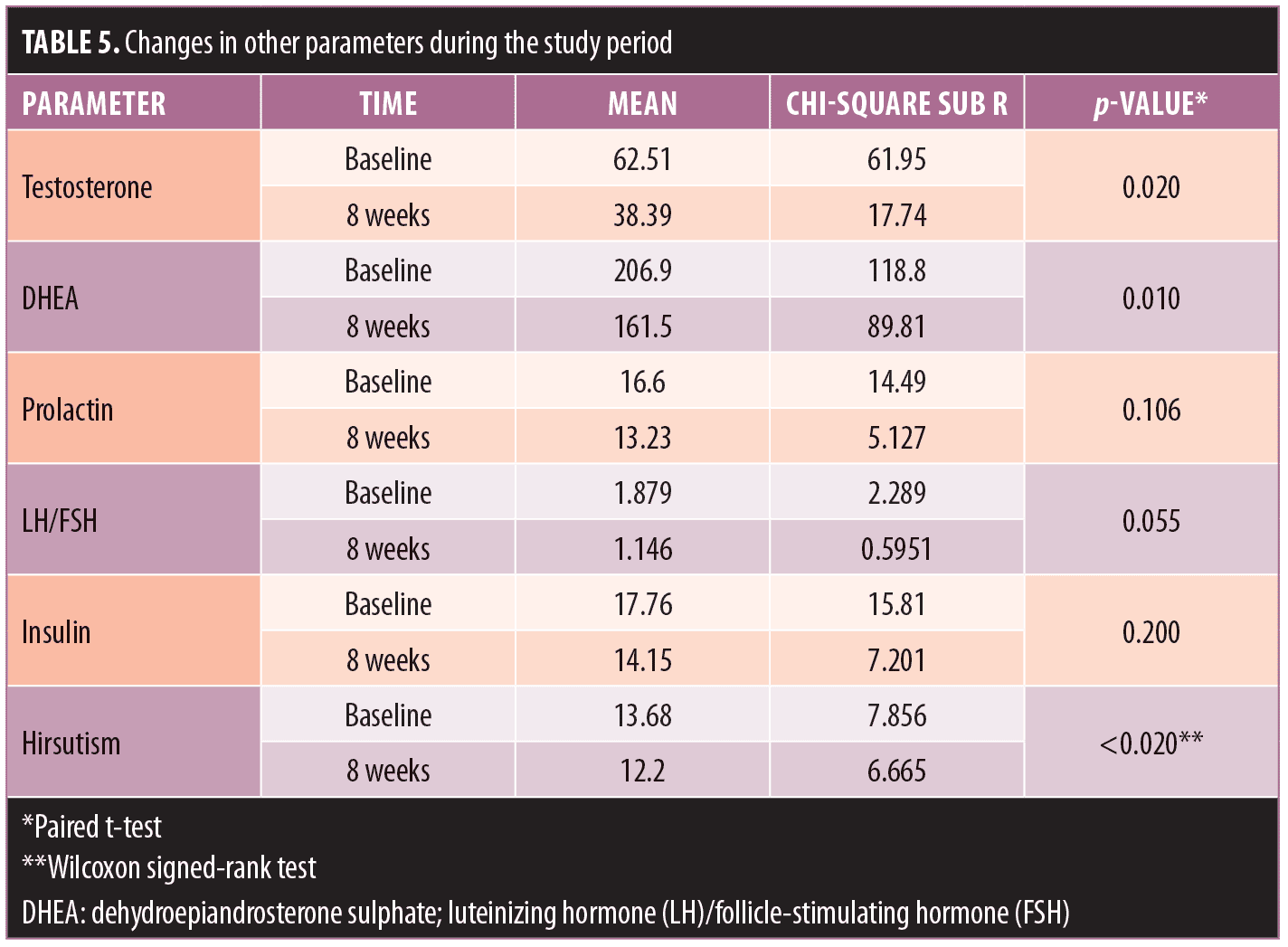
Hirsutism. Using the Ferriman-Gallwey assessment system, we also observed a significant reduction in the hirsutism score (p<0.020), as well as in testosterone (p<0.05) and DHEA (p<0.05) levels at Week 8 of metformin therapy. Serum prolactin, serum insulin, and luteinizing hormone (LH)/follicle-stimulating hormone (FSH) ratio also decreased; however, these reductions were not statistically significant (p>0.05) (Table 5).
Adverse events. Reversible gastrointestinal side effects were seen in about 20 percent of the participants taking metformin, including diarrhea, nausea, abdominal discomfort, anorexia, and a metallic taste in the mouth. These effects were not severe in most cases, and could be amended by taking metformin with food, as well as taking H2 blockers for the first 1 to 2 weeks of metformin therapy. However, in two women, symptoms of gastrointestinal distress (diarrhea), considered by the investigators to be treatment related, were severe enough to cause them to discontinue treatment with metformin; thus, these two patients, after being switched to alternate treatment regimen, were not included in the study analysis.
Discussion
Hyperinsulinism and resultant hyperandrogenism in PCOS chronically alter gonadotropin secretion, leading to increased LH8,9 and disruptions in the normal pituitary-ovarian axis, which can manifest as oligoamenorrhea, infertility, obesity, hirsutism, acne, frequent hypertension, and hyperlipidemia.9,10 The central role of hyperinsulinemia in PCOS has provided an impetus toward the development of treatments for PCOS that lower insulin levels. Metformin, a biguanide hypoglycemic drug, improves insulin levels and insulin sensitivity and has been shown to be a safe and well-tolerated treatment for adult women with PCOS, with minimal side effects and without reported lactic acidosis.8 Metformin’s mechanism of action is not fully understood; however, its major effects appear to involve decreasing hepatic glucose output, which lowers the insulin requirement.8,11
In the present study, the mean (20.98±3.423 years) and median (20 years ) age of participants was similar to participants in a study by Begum et al.13 Out of the 40 patients with acne and PCOS, the most commonly observed symptoms included hirsutism (60%) and irregular menses (87%), similar to a study conducted by Israni et al13 in which researchers observed hirsutism and menstrual irregularities in 69.2 and 82.35 percent of participants, respectively. In our study, obesity was present in 20 percent of patients, similar to the study by Zandi et al,14 in which they observed obesity in 20.8 percent of participants.
Our results suggest that metformin treatment can lead to an improvement in acne severity in patients with PCOS. Mean acne severity in our patients at baseline was 80.81±46.29, and this was reduced to 39.91±26.89 at Week 3 of treatment. By Week 6, it was reduced further to 25.81±16.26, and at Week 8, it was 9.703±8.5. This reduction in acne severity was found to be highly statistically significant (p<0.001). Comparison of acne severity at Weeks 3, 6, and 8 to acne severity at baseline showed a significant decrease in acne load (p<0.001). In our study, there was a 47.22-percent improvement in acne load at Week 3 of therapy, a 64.34-percent improvement at Week 6, and a 86.01-percent improvement at Week 8. The change in percent improvement between each comparative follow-up visit (Weeks 3, 6, and 8) was found to be significant as well, (p<0.001), suggesting that insulin-sensitizing therapy, such as metformin, can have a mitigating effect on acne.
Similar to our study, other studies have demonstrated significant decreases in acne severity in women with PCOS using metformin therapy. One uncontrolled study by Kolodziejczyk et al15 evaluated the effects of a 12-week course of metformin 500mg three times a day on hormonal and clinical indices in 39 women with PCOS. Acne severity was rated as 0=no acne; 1=minor acne, only present on the face; 2=moderate acne, only present on the face; 3=severe acne on the face and chest or back. The investigators observed a significant decrease, from 1.45 to 1.14 (p<0.001), in the acne severity score among the patients taking metformin.
A study by Israni et al13 also studied the effects of metformin on acne vulgaris and other hyperandrogenism conditions associated with PCOS; here, 17 women who met the Rotterdam criteria for PCOS completed three months of metformin therapy (1–1.5g per day). The investigators observed a significant decrease (71%) in the acne severity scores after three months of treatment.
Kazerooni et al3 prospectively studied the hormonal levels and clinical indices of patients with PCOS. They administered metformin 500mg three times a day for eight weeks and found a significant decrease (49%) in the acne severity scores (p<0.0001) after eight weeks of therapy.
Nazari et al16 conducted a self-controlled clinical trial involving 36 adolescent female subjects with PCOS. The patients were treated with 500mg of metformin three times a day for six months. In the patients with acne, Nazari et al reported significant clinical improvement (p=0.003) in acne severity following six months of metformin therapy.
De Leo et al17 evaluated the effects of metformin treatment on ovulatory function, hirsutism, acne, hormonal patterns, and body weight in adolescent girls with PCOS. Eighteen girls, 15 to 18 years of age, were enrolled in the study. All subjects were administered 1,700mg of metformin tablets daily for six months. The investigators reported that all patients showed improvement in menstrual cycle regularity, with a significant reduction in testosterone, androstenedione, and free testosterone levels. Body mass index (BMI) was also restored within normal limits in all girls, and acne and/or seborrhea showed improvement in severity after six months of treatment with metformin.
Loverro et al18 studied the effects of metformin therapy on clinical and endocrine indices in patients with PCOS. At baseline and at the end of the six-month study, the investigators assessed gonadotropins, androgens (testosterone and androstenedione), insulin, sex hormone binding globulin (SHBG), lipid profile, and clinical indices (e.g., menstrual length, BMI, Ferriman-Gallwey score, waist/hip ratio [WHR]). All women showed improvement in menstrual cycle regularity and experienced a reduction in length of menstruation (reduction rate, 36.9%); a significant decrease in LH, insulin, and androgen levels; and an increase in SHBG plasma concentrations, with a concomitant decrease in WHR and improvement of clinical manifestations of PCOS—in particular, hyperandrogenism and hirsutism.
Limitations. Limitations to this study include small sample size, short duration of treatment and follow-up, nonblinded study design, and lack of control arm. Additionally, patient adherence to taking the study medication as prescribed was not monitored. Larger studies that are randomized, double-blinded, and controlled, with longer treatment duration/follow-up time, are required to support our findings.
Conclusion
Our results suggest that metformin might serve as an effective therapy for ovarian hyperandrogenism and acne in women with PCOS. Few studies have focused on the effects of metformin on acne severity in patients with PCOS to date; thus, larger, prospective, randomized, controlled trials are warranted to confirm these results.
References
- Gollnick H, Zouboulis ChC, Akamatsu H, et al. Pathogenesis and pathogenesis related treatment of acne. J Dermatol. 1991;18(9):489–499.
- Canadian Task Force on Preventive Health Care. New grades for recommendations from the Canadian Task Force on Preventive Health Care. CMAJ. 2003;169(3):207–208.
- Kazerooni T, Dehghan-Kooshkghazi M. Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2003;17(1):51–56.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
- Liden S, Goransson K, Odsell L. Clinical evaluation in acne. Acta Dermato Venereol Suppl (Stockh). 1980;89:47–52.
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447.
- Sawilowsky, S., &Fahoome, G. (2014). Friedman’s Test. Wiley StatsRef: Statistics Reference
- Moghetti P, Castallo R, Negri C. Metformin effects on clinical features, endocrine and metabolic profiles and insulin sensitivity in polycystic ovary syndrome; a randomized, double-blind, placebocontrolled 6 month trial, followed by open long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85(1):139–146.
- Dunaif A, Scott D, Finegood D, et al. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 1996;81(9):3299–3306.
- Cibula D, Cífková R, Fanta M, et al. Increased risk of noninsulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Hum Reprod. 2000;15(4):785–789.
- Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338(13):867–872.
- Begum S, Hossain MZ, Rahman F, Banu LA. Polycystic ovarian syndrome in women with acne. J Pak Assoc Derma. 2012;22:24–29.
- Israni DA, Mehta TY, Shah SR, Goyal RK. Effect of metformin therapy in female visiting dermatologist for acne vulgaris having endocrine and sonographic characteristics of polycystic ovary syndrome (PCOS). Asian J Pharm Clin Res. 2013;6(2).
- Zandi S, Farajzadeh S, Safari H. Prevalence of polycystic ovary syndrome in women with acne: hormone profiles and clinical findings. J Pak Assoc Derma. 2010;20:194–198.
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ, Pawelczyk L. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73(6):1149–1154.
- Nazari T, Bayat R, Hamedi M. Metformin therapy in girls with polycystic ovary syndrome: a self-controlled clinical trial. Arch Iranian Med. 2007;10 2):176–181.
- De Leo V, Musacchio MC, Morgante G, et al.. Metformin treatment is effective in obese teenage girls with PCOS. Hum Reprod. 2006;21(9): 2252–2256.
- Loverro G, Lorusso F, De Pergola G, et al. Clinical and endocrinological effects of 6 months of metformin treatment in young hyperinsulinemic patients affected by polycystic ovary syndrome. Gynecol Endocrinol. 2002;16(3):217–211.

