J Clin Aesthet Dermatol. 2019;12(5)40–45
 by Brandon Kirsch, MD; Paul M. Hoesly, MD; Anokhi Jambusaria, MD, MSCE; Michael G. Heckman, MS; Nancy N. Diehl, MS; and Jason C. Sluzevich, MD
by Brandon Kirsch, MD; Paul M. Hoesly, MD; Anokhi Jambusaria, MD, MSCE; Michael G. Heckman, MS; Nancy N. Diehl, MS; and Jason C. Sluzevich, MD
Drs. Kirsch (at the time of this study), Hoesly (at the time of this study), and Sluzevich are with the Department of Dermatology at the Mayo Clinic in Jacksonville, Florida. Dr. Jambusaria is with the University of Texas Austin Dell Medical School in Austin, Texas. Mr. Heckman and Ms. Diehl are with the Division of Biomedical Statistics and Informatics at the Mayo Clinic in Jacksonville, Florida. Dr. Kirsch is now in private practice with Minars Dermatology in Hollywood, Florida, and is the Medical Director and Vice President of Clinical Development at Brickell Biotech, Inc. in Boulder, Colorado. Dr. Hoesly is now in private practice at the Austin Regional Clinic in Austin, Texas.
FUNDING: This study was supported in part by the Mayo Foundation for Medical Education and Research.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background.Melasma is a common hyperpigmentation disorder of the skin. Combination therapy of topical retinoids, corticosteroids, and hydroquinone has been effective in treating melasma, but long-term use is limited by corticosteroid atrophy and exogenous ochronosis. The aim of this pilot study (NCT02730819) was to determine the efficacy, safety, and tolerability of a novel composition (2013-MCN-333) comprising tazarotene 0.075%, azelaic acid 20%, tacrolimus 0.1%, and (microfine) zinc oxide 10% for the treatment of melasma.
Methods.Sixteen patients with moderate-to-severe melasma were treated daily with sunscreen and 2013-MCN-333 for 20 weeks. Primary outcome measure was change in Melasma Area and Severity Index (MASI) score.
Results.Twenty-five percent of patients met the primary endpoint of a MASI score of less than eight points at Week 20. MASI score also decreased significantly from baseline (median: 18.9 points) through Week 4 (median: 17.3 points; p=0.006), Week 12 (median: 16.0 points; p=0.001), and Week 20 (median: 13.3 points; p=0.001). Treatment-related adverse events were mild, most of which decreased or resolved over the course of the study.
Limitations.The small sample size and nonblinded nature of treatment intervention are potential limitations.
Conclusion. Our results suggest daily 2013-MCN-333 could potentially be an effective, safe, and tolerable treatment for moderate-to-severe melasma.
KEYWORDS: Cosmetic, aesthetic, drugs, melasma, photosensitivity, tacrolimus
Melasma is an acquired, chronic hyperpigmentation disorder that mostly affects women in their reproductive years. Melasma is characterized primarily by the accumulation of epidermal melanin and is known to be associated with several pathophysiologic factors, including augmented production and facilitated transfer of melanosomes to basal and suprabasal keratinocytes.1,2 Triggers of melasma include ultraviolet light, heat, inflammation, genetic factors, vascular influences, pregnancy, hormonal contraception, and abnormal release of estrogen, progesterone, and ?-melanocyte-stimulating hormone.3–5
In 1975, a widely cited paper by Kligman and Willis6 was published on topical treatment of melasma with a synergistic combination of approved pharmaceutical agents, including corticosteroid (dexamethasone 0.1%), melanin synthesis inhibitor (hydroquinone 5.0%), and retinoid (tretinoin 0.1%). The “Kligman formulation” was created to allow for the application of lower concentrations of each active pharmaceutical agent to reduce adverse effects, decrease response time, and improve clinical results. Various similar combination products that contain hydroquinone, tretinoin, and corticosteroids have been studied and successfully used in clinical practice.7–9
The main limitation of compositions based on the Kligman formulation is restricted use to short-term (up to 8 weeks) and intermittent application due to potential adverse effects, such as skin atrophy, telangiectasia, and paradoxical hyperpigmentation, that are associated with long-term use.10 Consequently, relapse is a frequent problem, and no satisfactory, long-term topical treatment is currently available. The purpose of this study was to determine the efficacy, safety, and tolerability of a novel formulation comprising azelaic acid, tazarotene, tacrolimus, and zinc oxide for the treatment of melasma. We hypothesized that combination therapy using these lightening and anti-inflammatory agents would safely and effectively improve hyperpigmentation without the use-limiting adverse effects associated with long-term daily application of current combination therapies containing hydroquinone, tretinoin, and corticosteroids.
Methods
Study patients. This single-arm, open-label, single-center pilot study was conducted at the Mayo Clinic in Jacksonville, Florida, from May 1, 2016, through September 30, 2016. Eligible patients were recruited from the outpatient clinic of the Department of Dermatology. Men and women between the ages of 18 and 65 years, with moderate-to-severe melasma (as defined by a Melasma Area and Severity Index [MASI] score of 16 points or higher [scale range: 0–48 points]), were eligible for study inclusion. Patients who received photosensitizing drugs, immunosuppressive medications, topical hydroquinone, azelaic acid, or a retinoid within the previous 60 days were excluded. Patients were screened by telephone and, if eligible, underwent an additional screening examination by investigators at the dermatology clinic before enrollment in the study. Because this was a pilot study, the sample size was not chosen on the basis of any formal power calculations. This study was approved by the Mayo Clinic Institutional Review Board. Informed consent, including consent for photography, was obtained from all individuals interested in participating in the study before enrollment or any study-related procedures.
Sixty-five patients were screened by telephone. Twenty-one patients met the inclusion criteria, and a total of 19 patients were enrolled. Three out of the 19 enrolled patients did not return for follow-up visits and were not included in the analysis. The final sample size of the study was 16 patients.
Of these 16 patients, 15 returned for follow-up at Week 2; 16 at Week 4; 14 at Week 8; 11 at Week 12; 11 at Week 16; and 10 at Week 20. Six of 16 patients discontinued treatment at some point during the study. Reasons for discontinuation included the following: Two patients reported severe dryness at Week 4 and chose to discontinue further treatment. One patient reported severe scaling and burning at Week 8 and also chose to discontinue treatment. One patient called and reported a self-described allergic reaction after the initial baseline visit and withdrew from the study without any in-person follow-up. Two patients discontinued treatment because of a lack of perceived efficacy, but they acknowledged active sunbathing and inconsistent use of sunscreen. All adverse events were deemed to be treatment-related.
Data collection and outcomes. Baseline information was collected regarding age; sex; Fitzpatrick skin type; use of photosensitizing medications, oral contraceptives, and daily sunscreen; onset of melasma during pregnancy; and prior treatments. Changes in MASI scores were measured from baseline through Weeks 4, 12, and 20. The primary outcome measure was the proportion of patients who achieved a MASI score of less than eight points at Week 20.
As a secondary outcome, Melasma Quality of Life Scale (MELASQOL) score was assessed at baseline and Week 20. A narrow-band reflectance spectrophotometer (Mexameter; Courage-Khazaka Electronic, Köln, Germany) was used to measure melanin index at the forehead, chin, and left and right cheeks of participants at baseline and Weeks 2, 4, 8, 12, 16, and 20; photographs of these areas were also taken at these time points. Patient global assessments, patient and physician assessments of symptoms and complications, and assessments of missed applications of the medication were performed at Weeks 2, 4, 8, 12, 16, and 20. Physician global assessments were performed at Weeks 4, 12, and 20.
Dosing and application. The study formulation cream (2013-MCN-333) contained tazarotene 0.075%, azelaic acid 20%, tacrolimus 0.1%, and (microfine) zinc oxide 10%, and was applied once daily to affected areas of the face at bedtime for 20 weeks. The Mayo Clinic Research Pharmacy prepared, stored, and distributed the study formulation. Each month, patients received 15g of 2013-MCN-333 in an ointment jar. Patients returned the used jars, which were weighed by the Mayo Clinic Research Pharmacy to determine treatment adherence. Patients were instructed to avoid sun exposure between 10 AM and 4 PM and were advised to apply sunscreen often.
Statistical analysis. Continuous variables are summarized as medians and ranges, and categorical variables are summarized as numbers and percentages of patients. The paired Wilcoxon signed-rank test was used to compare MASI scores, MELASQOL scores, and melanin index between the baseline and follow-up time points. For MASI score, melanin index, and patient and physician assessments of symptoms and complications, all of which were assessed at multiple follow-up time points, we imputed a small-to-moderate amount of missing data, when possible, by using the last-observation-carried-forward (LOCF) method. This was performed to account for any potential bias in estimates that could be introduced by excluding patients with missing data (e.g., patients who dropped out of the study because of adverse events or a lack of efficacy). Specifically, using the LOCF method, missing values are assigned to be equal to the most recent value. The only instances when LOCF imputation was not possible were the Week 2 measurements for melanin index, as well as the Week 2 patient and physician global assessments of efficacy, symptoms, and complications, due to missing data from one patient for which no post-baseline data could be used for imputation. Similarly, missing MELASQOL information at Week 20 for six missing patients was not imputed due to lack of postbaseline measurements. Of note, sensitivity analyses were performed without imputation of missing MASI, melanin index, and patient and physician global assessment data regarding efficacy, symptoms, and complications, and results were similar (data not shown). A p value of less than 0.05 was considered significant, and all tests were two-sided. Statistical analyses were performed using the R Statistical Software version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline patient characteristics are summarized in Table 1. Median (range) age was 38 (31–63) years, and all 16 patients were women. Eight patients (50.0%) had Fitzpatrick Skin Type II, five (31.3%) had Type III, one (6.3%) had Type IV, and two (12.5%) had Type V. Melasma onset during pregnancy was reported in five patients (31.3%), and 12 patients (75.0%) had received prior treatment for melasma.
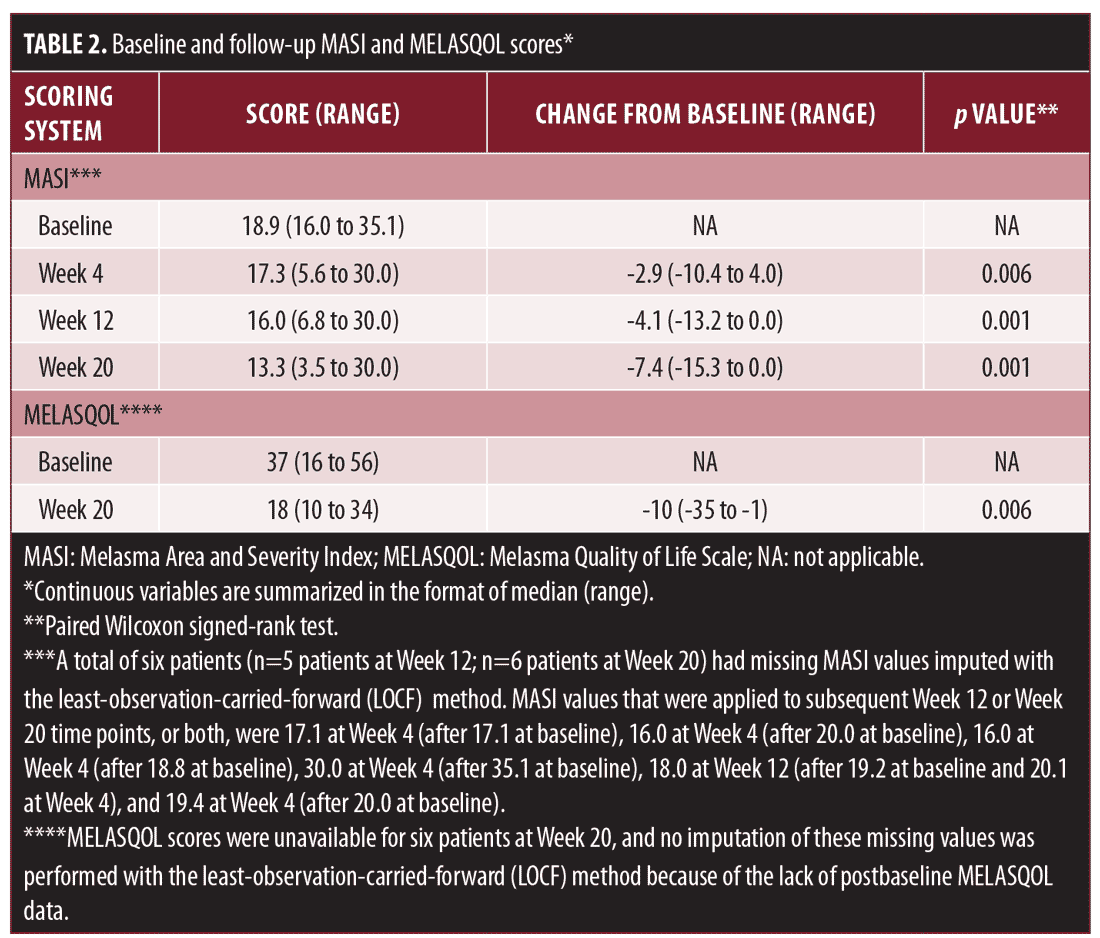
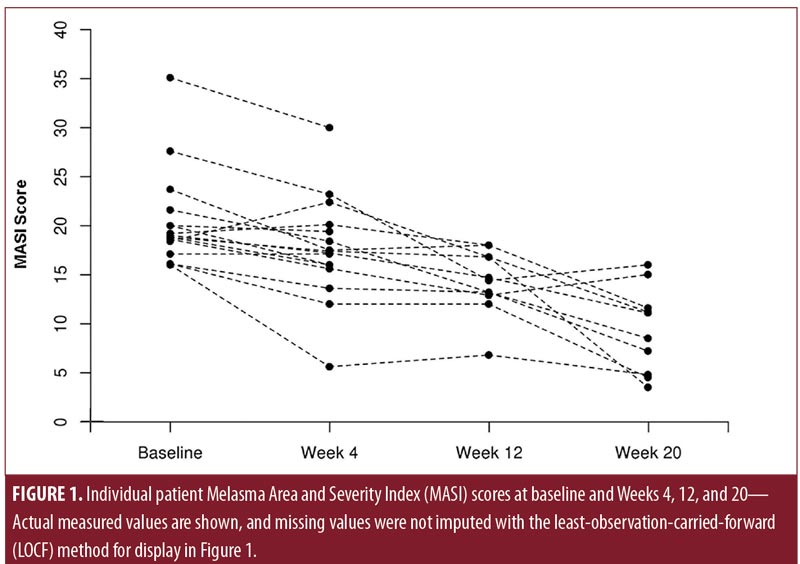
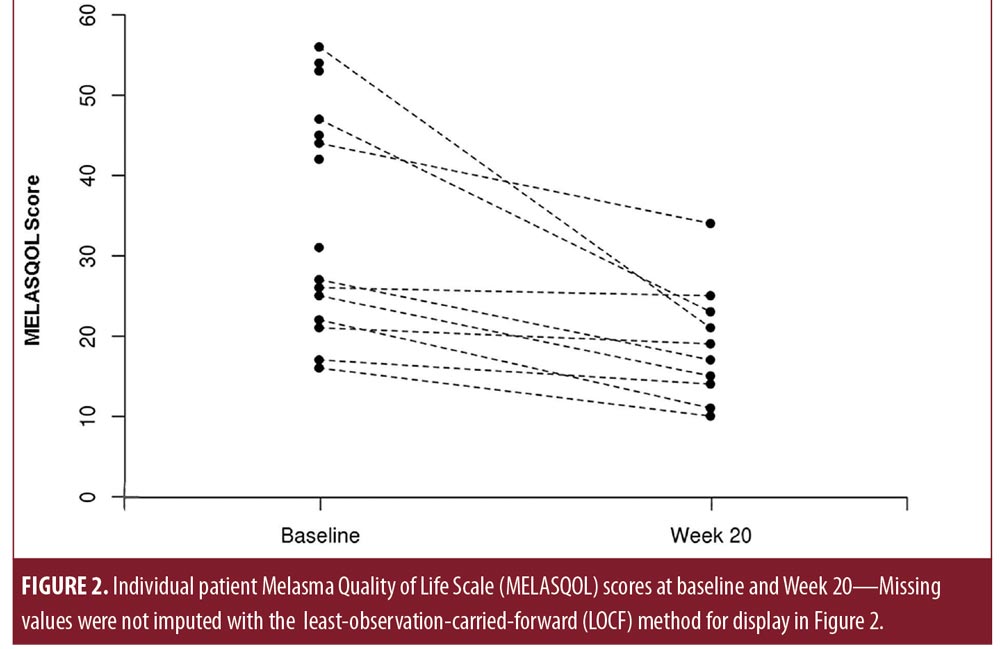
MASI score and MELASQOL score at baseline and follow-up time points are displayed in Table 2. Median (range) baseline MASI score was 18.9 (16.0–35.1) points. Compared with baseline, MASI scores were significantly lower at Week 4 (median: 17.3 [range: 5.6–30.0]; p=0.006), Week 12 (16.0 [6.8–30.0]; p=0.001), and Week 20 (13.3 [3.5–30.0]; p=0.001) (Figure 1; nonimputed values shown). Four patients (25.0%) reported MASI scores of less than eight points at Week 20. Similarly, MELASQOL scores decreased significantly from baseline (median: 37 [range: 16–56]) to Week 20 (18 [10–34]; p=0.006) (Figure 2). The spectrophotometric assessments showed no significantly different findings between baseline and any follow-up time point (p?0.19).
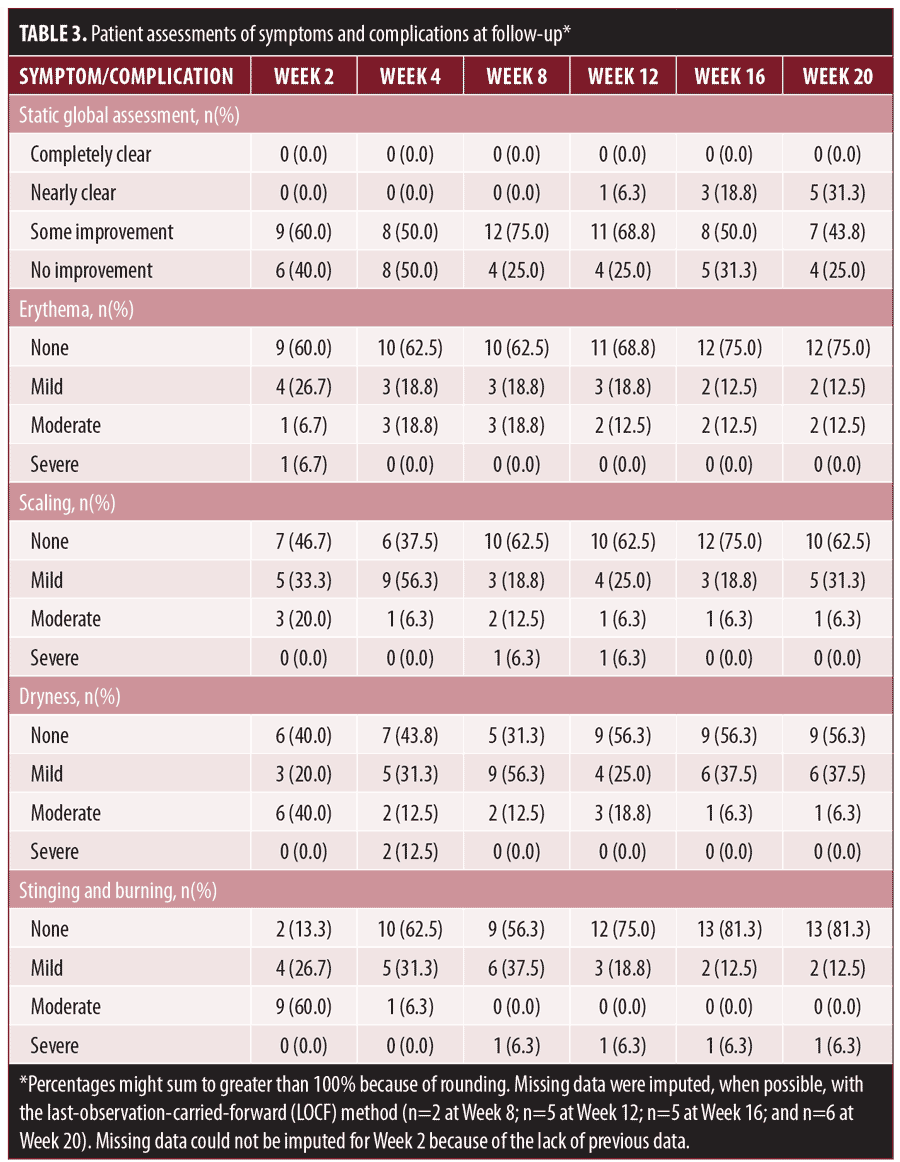
Static global assessments revealed that 60 percent (n=15) of patients showed some improvement in melasma severity as early as Week 2, with 75 percent (n=16) reporting at least some improvement by Week 12 (Table 3), including one patient who reported nearly clear melasma at Week 12. At Week 20, the proportion of patients who reported near clearance increased to 31 percent. No patients reported complete clearance at any time point. In addition, five patients reported improvements in acne severity, and 10 patients noted an improvement in overall skin texture.
The number of missed applications of 2013-MCN-333 per evaluable period (baseline–Week 2, Week 2–Week 4, etc.) was five or fewer for every patient at all follow-up time points except for in Week 8 when three of the assessed 14 patients (21%) missed 6 to 10 applications. The most common reason (61%) for missed application was forgetting to use the treatment.
Patient assessments of erythema, scaling, dryness, and stinging and burning were mostly categorized as mild or moderate and improved over the course of the study. The most common complaint was stinging and burning at Week 2 (87%), which was likely due to tacrolimus. This decreased to affecting 38 percent of patients at Week 4 and 19 percent of patients at Week 20, suggesting increased tolerability with use.
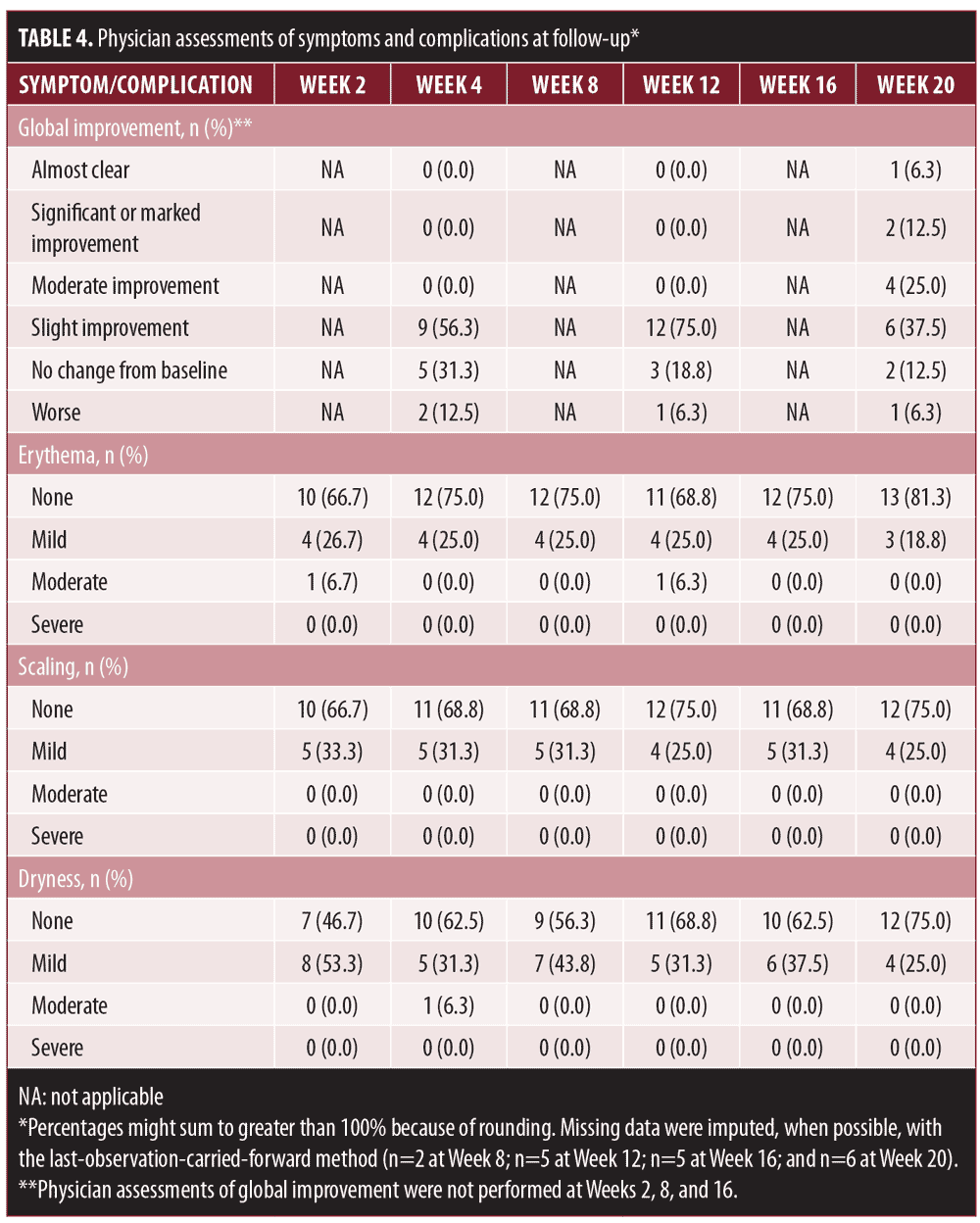
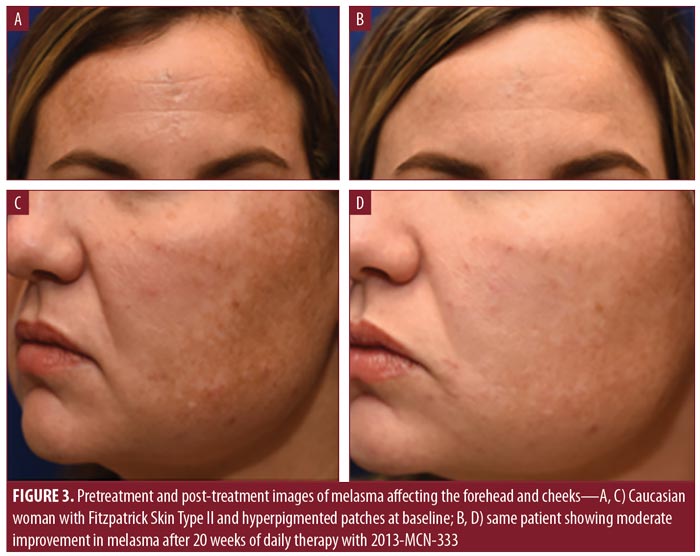
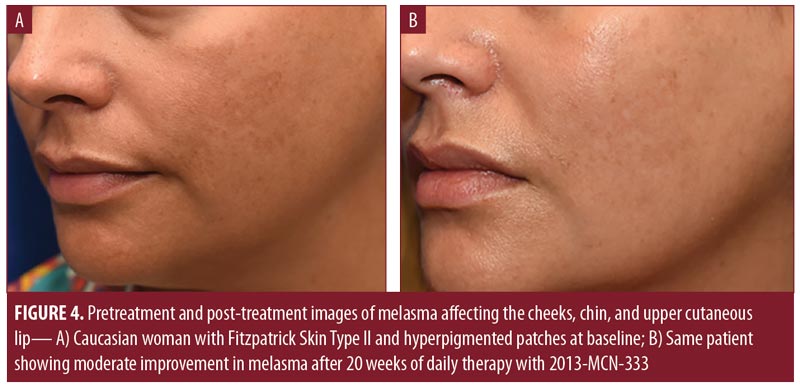
The results of physician assessments of global improvement showed a slight improvement in melasma severity in 56 percent of patients at Week 4 (earliest recorded time point) (Table 4). This increased to 75 percent of patients at Week 12. At Week 20, 44 percent of patients had physician-reported assessments of at least moderate improvement (Figures 3 and 4), with 81 percent of patients showing at least slight improvement. Almost all physician assessments of erythema, scaling, and dryness were recorded as mild or none. One patient had moderate dryness at Week 4, and one patient had moderate erythema at Weeks 2 and 16. No serious adverse events were observed during the study period.
Discussion
Melasma is recognized as one of the most psychologically distressing and difficult-to-treat forms of skin hyperpigmentation and can be associated with substantial quality-of-life impairments.11 Treatments for melasma typically consist of a single depigmenting agent, but some formulations consist of two or more additive or synergistic compounds. Although many treatments are available, melasma is a chronic condition, and long-term therapeutic options are needed to minimize flares and maintain clearance.12
The active pharmaceutical ingredients in 2013-MCN-333 are among the most common topical medications recommended by dermatologists and have long-established records of safety and tolerability. Unlike most other skin-lightening agents that are limited to short-term, episodic application, 2013-MCN-333 does not contain a corticosteroid or hydroquinone and can potentially be used for long-term treatment of epidermal hyperpigmentation without the use-limiting adverse effects associated with corticosteroids and hydroquinone.
The efficacy of 2013-MCN-333 is believed to be due to the synergistic effects of tazarotene, azelaic acid, tacrolimus, and zinc oxide acting at different steps of the pigmentation pathway. Azelaic acid disrupts the synthesis of melanin by interfering with the tyrosine–tyrosinase pathway.13,14 At a concentration of 20%, azelaic acid has a skin-lightening effect equivalent to hydroquinone 4%.15 Tazarotene, a selective retinoic acid receptor agonist, increases the penetration of lightening agents, such as azelaic acid, through accelerated epidermal turnover and also exhibits other inhibitory effects on melanogenesis.16 The nonsteroidal anti-inflammatory agent tacrolimus was selected to prevent and treat possible irritant dermatitis. In addition, because some mediators of melanogenesis are produced after exposure to proinflammatory stimuli, the anti-inflammatory properties of tacrolimus were also postulated to indirectly prevent epidermal hyperpigmentation. Combination treatments comprising broad-spectrum sunscreens, such as those made with zinc oxide, and prescription skin-lightening therapies have been indicated for the prevention and treatment of hyperpigmentation.17,18 Furthermore, zinc supplementation itself has been shown to interfere with uptake of copper,19 which is a necessary cofactor in tyrosinase and melanin synthesis.20
In this pilot study, we enrolled a group of patients with recalcitrant melasma, as indicated by the failure of prior therapies (75% of patients had received prior treatment), and conducted this study in a geographic area with a high average ultraviolet index (Jacksonville, Florida). We observed a significant decrease in MASI and MELASQOL scores from baseline through the follow-up time points. Physician and patient global assessment scores also showed broad agreement with regard to tolerability and clinical improvement. Although the spectrophotometric assessments did not show a significant difference between baseline and subsequent follow-up time points, this might be the result of the small anatomical area assessed with the device and differential rates of pigmentation changes of selected anatomical sites.
The present formulation of 2013-MCN-333 was well-tolerated by most patients. The most common adverse events were application-site erythema, scaling, and burning. In general, treatment-related adverse events were mild and decreased or resolved over the course of the study. The types of observed adverse events were consistent with the expected pharmacologic effects of topically applied skin-lightening agents. Incidental improvement in frequency and severity of acneiform lesions, as well as overall skin texture, were also reported by a high proportion of patients and represent additional benefits not seen with other treatments approved by the United States Food and Drug Administration (FDA) for melasma.10
Limitations. There are several limitations to this study. All patients were women, and, although this reflects the predominantly female population breakdown of melasma patients, the results might not necessarily be generalizable to men. It is also possible that the 2013-MCN-333 formulation is not optimal for routine use in all Fitzpatrick skin types and that the concentrations of active ingredients, as well as the vehicle, might need to be adjusted to balance efficacy with tolerability. Furthermore, although appropriate for a pilot study, the sample size was small. Additional investigations should incorporate a longer treatment duration and a randomized, double-blinded, and vehicle-controlled study design.
Conclusion
The results of this pilot study indicate that 2013-MCN-333 significantly improved moderate-to-severe melasma in our patients, as measured by MASI and MELASQOL scores. We also noted qualitative improvements in patient and physician global assessments of symptoms and complications. Response to treatment was noted as early as at Week 2 and continued through to the end of therapy in Week 20. Tolerability was good, and no serious adverse events were noted, which suggests a low overall risk to patients. Future studies are needed to fully evaluate the effectiveness of 2013-MCN-333 for the treatment of melasma.
References
- Brenner M, Hearing VJ. Modifying skin pigmentation—approaches through intrinsic biochemistry and exogenous agents. Drug Discov Today Dis Mech. 2008;5(2):e189–e199.
- Lee AY. An updated review of melasma pathogenesis. Dermatol Sin. 2014;32(4):233–239.
- Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melasma. J Dermatol Sci. 2007;46(2):111–116.
- Pathak MA, Riley FC, Fitzpatrick TB. Melanogenesis in human skin following exposure to long-wave ultraviolet and visible light. J Invest Dermatol. 1962;39:435–443.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199(9):601–605.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111(1):40–48.
- Gano SE, Garcia RL. Topical tretinoin, hydroquinone, and betamethasone valerate in the therapy of melasma. Cutis. 1979;23(2):239–241.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72(1):67–72.
- Kang WH, Chun SC, Lee S. Intermittent therapy for melasma in Asian patients with combined topical agents (retinoic acid, hydroquinone and hydrocortisone): clinical and histological studies. J Dermatol. 1998;25(9):587–596.
- United States Food and Drug Administration. Tri-Luma package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021112s009lbl.pdf. Accessed July 3, 2018.
- Dominguez AR, Balkrishnan R, Ellzey AR, Pandya AG. Melasma in Latina patients: cross-cultural adaptation and validation of a quality-of-life questionnaire in Spanish language. J Am Acad Dermatol. 2006;55(1):59–66.
- Prignano F, Ortonne JP, Buggiani G, Lotti T. Therapeutical approaches in melasma. Dermatol Clin. 2007;25(3):337–342, viii.
- Fitton A, Goa KL. Azelaic acid. A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs. 1991;41(5):780–798.
- Schallreuter KU, Wood JW. A possible mechanism of action for azelaic acid in the human epidermis. Arch Dermatol Res. 1990;282(3):168–171.
- Balina LM, Graupe K. The treatment of melasma. 20% azelaic acid versus 4% hydroquinone cream. Int J Dermatol. 1991;30(12):893–895.
- Ortonne JP. Retinoid therapy of pigmentary disorders. Dermatol Ther. 2006;19(5):280–288.
- Verallo-Rowell V. The role of sunscreens in the therapy of melasma. Clin Drug Invest. 1995;10(Suppl 2):49–56.
- Vazquez M, Sanchez JL. The efficacy of a broad-spectrum sunscreen in the treatment of melasma. Cutis. 1983;32(1):92, 95–96.
- Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7(4):1342–1365.
- Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem. 2009;284(2):717–721.

