 by Sujata Mehta-Ambalal, MD
by Sujata Mehta-Ambalal, MD
Dr. Mehta-Ambalal is a private practitioner and visiting dermatologist with Shalby Hospitals in Ahmedabad, Gujarat, India.
Funding: No funding was received for this study.
Disclosures: The author has no financial conflicts relating to the content of this article.
Abstract: Female acne is often associated with clinical signs of hyperandrogenism or metabolic syndrome. Various hormonal and biochemical factors as well as Vitamin D deficiency play a role in the etiopathogenesis of acne, and it is important to be able to detect the altered marker(s) indicative of certain abnormalities in order to diagnose and treat the cause. However, interpretation of these markers can be difficult, as there is ambiguity as to what is considered “normal” or “abnormal.” The aim of this study was to explore the associations that acne might have with certain clinical, hormonal, and biological factors among female patients with acne. Additionally, the available literature was reviewed in order to determine the prevalence of these associations, discussion of which is provided. The author’s investigations reveal a very high prevalence of abnormal metabolic and hormonal statuses among women with acne, indicating the need for dermatologists to maintain a high index of suspician for other disorders, especially metabolic disorders (and in particular, polycystic ovary syndrome), when treating female patients with acne.
Keywords: Female acne, clinical investigation, insulin, hormone, metabolic disorder, polycystic ovary syndrome, PCOS
J Clin Aesthet Dermatol. 2017;10(10):18–24
Acne is principally a disorder of adolescence. However, the prevalence of adult patients with acne is increasing. Acne tarda (adult acne) is defined as acne that develops (late-onset acne) or continues (persistent acne) after 25 years of age.1
Earlier puberty onset for female individuals triggers a higher incidence of acne vulgaris in the younger age ranges when compared to male individuals, regardless of a country’s economic level.2 In an Indian study, acne prevalence was highest among younger men and older women.3
Both types of adult acne are more common in women. Seventy-five percent of women with acne report a premenstrual flare.4 Hormonal factors, stress, increased use of cosmetics, and exposure to hot and humid conditions (e.g., while cooking) might play a role in increased prevalence of adult acne in women. In cases of persistent acne, female patients report lesions on most days and might experience a premenstrual flare. Late-onset acne can be further subdivided into chin acne (i.e., acne that occurs around the chin and perioral area) and sporadic acne. Chin acne is inflammatory and flares premenstrually, while sporadic acne occurs suddenly in adult life with no distinguishing features.5
The morphological characteristics of adult female acne are often distinct from adolescent acne (Figures 1 and 2). In adults, inflammatory lesions (particularly papules, pustules, and nodules) are generally more prominent on the lower chin, jawline, and neck, and comedones are closed (i.e., micro cysts). Adult acne is mainly mild-to-moderate in severity and might be refractory to treatment.6
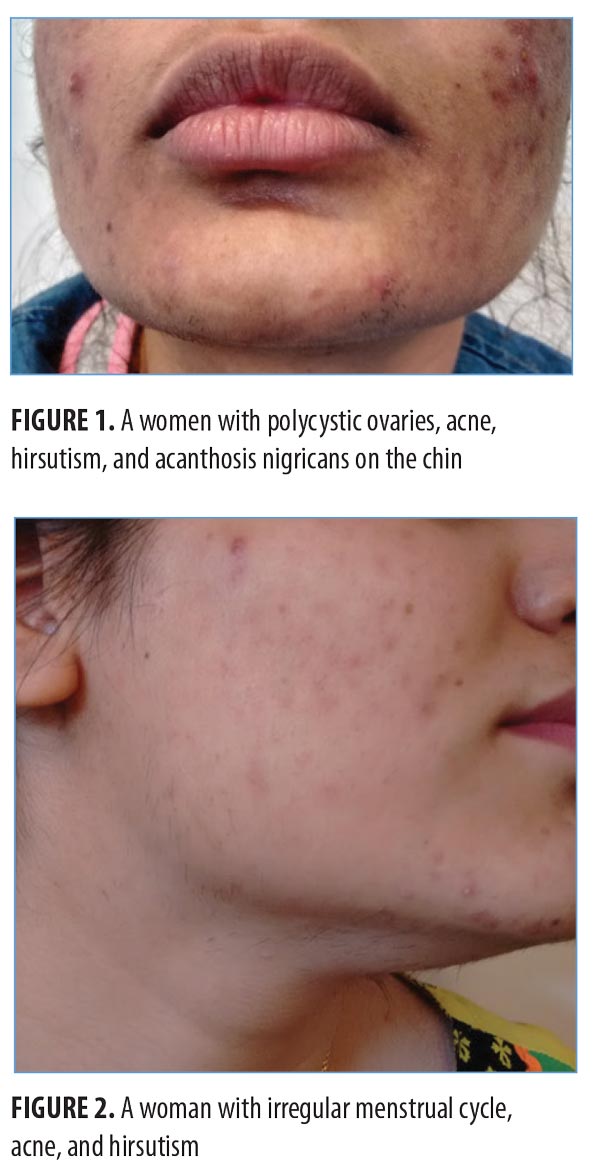
Use of cosmetics to hide lesions and post-acne pigmentation is higher in women, and the psychological effects of persistent adult acne might effect women at a higher rate than men,7 especially among professional or working women.
We explored associations that acne might have with certain clinical, hormonal, and biological factors among a local cohort of female patients who presented to our clinic for acne treatment. We also reviewed the current literature on acne to determine the prevalence of these associations. Here, we describe and discuss the results of our study and literature review, and we interpret how these findings might be used to improve treatment and optimize patient outcomes in dermatology.
Methods
Thirty-six female subjects of reproductive age who had visited a private skin clinic in Western India between October 2016 and February 2017 for treatment of acne were recruited for this study, which followed the Declaration of Helsinki Ethical Principles of Medical Research. Patients gave informed consent to participate in the study, and patients whose photographs are used in this article provided written consent for the same.
Inclusion criteria was the presence of acne (irrespective of grade) with one or more of the following characteristics: hirsutism, acanthosis nigricans/acrochordons, patterned hair loss, weight gain, menstrual disturbances, and/or poor response to topical therapies or isotretinoin. Exclusion criteria were pregnancy and lactation, oral contraceptive use, any systemic illness including known hypothyroidism, current use of oral systemic medication, and/or infertility treatment. The typical age of the study participants ranged from 16 to 39 years. All patients belonged to the middle socioeconomic class. The clinical characteristics of the patients are described in Table 1.
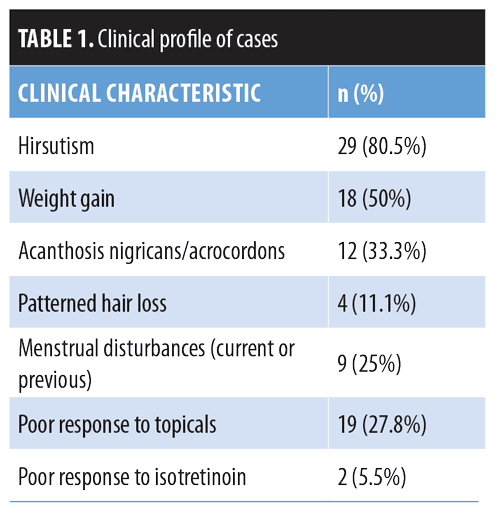
Because follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were being measured, the investigations were carried out within the first five days of onset of menses. An oral glucose tolerance test (OGTT) was performed in which blood sugar and insulin were measured after at least eight hours of fasting and two hours post-75g glucose. As some hormones have peak levels in the morning, FSH, LH, serum testosterone (total), anti-Müllerian hormone (AMH), thyroid stimulating hormone (TSH), serum prolactin, and serum vitamin D were measured from the morning fasting sample. Homeostatic Model of Assessment of Insulin Resistance (HOMA IR) score was calculated. Based on the method used by the laboratory and the references available, the following cut-off levels for “normal” were used: fasting blood sugar lower than 100mg/dL; two hours post-load blood sugar lower than 140mg/dL; fasting insulin lower than 17IU/mL; two hours post-glucose insulin lower than 41IU/mL; HOMA IR lower than 2.5; LH lower than FSH (as the test was done in follicular phase); Serum AMH lower than 6.8ng/mL; and vitamin D higher than 20ng/mL. Abdominopelvic ultrasound was done within 2 to 3 days of the blood tests and was checked for the presence of polycystic ovary syndrome (PCOS).
Results
Of the 33 study participants who consented to receive a pelvic ultrasound, 14 (45%) showed the presence of PCOS. Two patients were detected as having a single large (<5cm) cyst in their ovaries and were referred to a gynecologist for appropriate treatment.
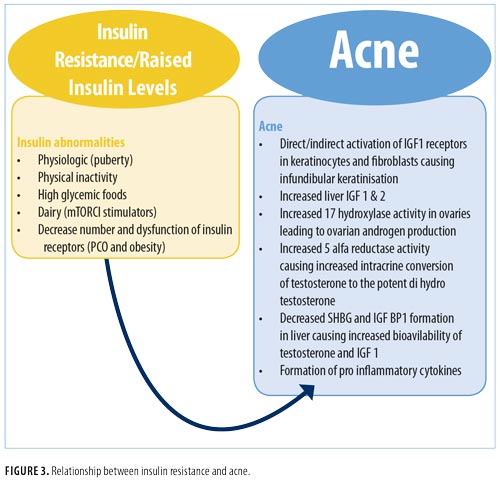
Prevalence rates of raised biochemical markers for insulin resistance and hormone dysfunction are shown in Table 2. Thirty-five out of the 36 patients (97.22%) showed at least one altered marker.
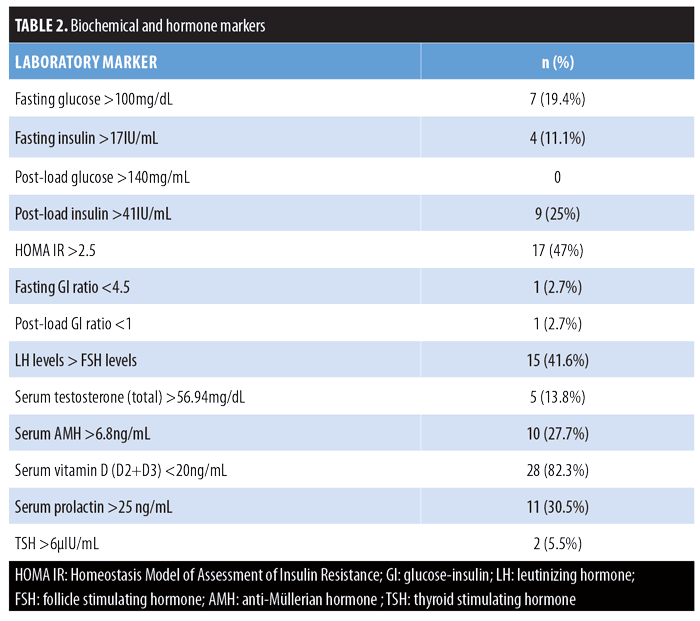
Twenty-one (58.3%) patients had at least one biochemical marker of insulin resistance. Mean fasting insulin in our study was 10.3 versus 7.7 from a healthy adult Indian cohort.45
Two patients were newly detected as having hypothyroidism. Prolactin levels were high, but were under 100ng/mL in 11 patients (30.5%).
Discussion
Serum testosterone. It has been shown that the development of acne can be a cutaneous symptom of hyperandrogenism, a medical condition characterized by excessive levels of male sex hormones (e.g., testosterone) in the female body.8 Other cutaneous clinical signs of hyperandrogenism include hirsutism, patterned hair loss, acanthosis nigricans, and acrochordons. Serum testosterone level estimation is a simple and cost-effective laboratory test used to determine the presence of hyperandrogenism. As serum testosterone has been associated with acne vulgaris, testosterone levels should be measured in patients presenting with acne vulgaris, especially in those cases that are treatment-resistant. In female cases of elevated testosterone levels, anti-androgen treatment should be considered.8
The total testosterone screening test, a marker of overall androgen production, has some advantages over free testosterone screening in that it is less expensive and might be considered easier to interpret. However, measuring free testosterone, which represents the biologically active testosterone, might be a more sensitive indicator of hormonal abnormality.
If total testosterone is elevated, the clinician should consider further testing for free testosterone, sex hormone binding globulin (SHBG), and other androgens. However, not all women with clinical signs of hyperandrogenism have elevated circulating androgen levels.5 Cutaneous signs of hyperandrogenism with a normal androgen level in the blood can be either due to increased responsiveness of the pilosebaceous unit to the normal androgen level (end-organ hypersensitivity) or increased activity of 5?-reductase, which converts testosterone to dihydrotestosterone, a sex steroid that is five times more potent than testosterone.9
In the skin, some follicles might be more acne-prone than others, and these follicles will have different levels of susceptibility to circulating hormones. Increased sensitivity of the sebaceous gland to androgens and the increased metabolization of local androgen hormones into potent androgen metabolites might offer alternative mechanisms in acne pathophysiology.10,11
Vitamin D. Vitamin D is a fat-soluble prohormone steroid with endocrine, paracrine, and autocrine functions.12 Seventy-nine percent of Indians are believed to be vitamin D-deficient,13 and in a study from South India, 76 percent of women were found to be vitamin D deficient.14
Vitamin D plays a physiologic role in reproduction, including ovarian follicular development and luteinization via altering AMH signaling, FSH sensitivity, and progesterone production in human granulosa cells.15 Low 25(OH)D levels are significantly correlated with insulin resistance in women with PCOS.16
There have been a few in-vitro studies that support the theory that vitamin D has a functional role in acne development.17–19 The identification of vitamin D receptors in human sebocytes and the modulation of lipid and cytokine production by vitamin D suggests a possible association between vitamin D and acne pathophysiology.17,18 Propionibacterium acnes (P. acnes) is a potent inducer of Th17, and 1,25(OH)2D inhibits P. acnes-induced Th17 differentiation; therefore, vitamin D could be considered an effective tool for modulating acne.19 Vitamin D(3) supplementation also improves postprandial insulin sensitivity.20 The level of 25(OH)D has been inversely associated with the severity of acne, and there is a significant negative correlation between 25(OH)D levels and the development of inflammatory lesions.21
Insulin. Acne is a common component of seborrhea-acne-hirsutism-androgenetic alopecia (SAHA) syndrome, PCOS, and hyperandrogenism, insulin resistance, and acanthosis nigricans (HAIR-AN) syndrome, all of which are linked to insulin resistance (Figure 3). Fasting insulin levels and HOMA are significantly higher among patients with acne compared with non-acne controls.22
Insulin resistance (IR) is a metabolic disorder in which target cells fail to respond to normal levels of circulating insulin, which results in compensatory hyperinsulinemia in order for the human body obtain an appropriate physiological response. It is particularly prevalent in individuals with diabetes mellitus (DM) Type 2, decompensated Type 1 DM, diabetic ketoacidosis, and obesity. In normal populations, IR has a prevalence rate of 20 to 25 percent.23
There are three types of IR: 1) Type A, caused by a reduced number and dysfunction of insulin receptors; 2) Type B, caused by the formation of antibodies against insulin receptors; and 3) Type C, which corresponds to a post-receptor defect. Patients with obesity and patients with PCOS tend to have Type A insulin resistance. Subsequent DM develops when insulin secretory capacity fails to reduce serum glucose.24
IR increases during puberty and appears to be related to fat accumulation.25 The insulin receptor belongs to the family of tyrosine kinase receptors, which includes the insulin-like growth factor (IGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet derived growth factor, colony stimulating factor I, and several cytokine receptors. A high concentration of insulin results in the direct and indirect activation of IGF-1 receptors in keratinocytes and fibroblasts, leading to their proliferation and formation of comedones. Other mediators can also contribute, including other tyrosine kinase receptors (e.g., epithelial growth factor receptor and fibroblast growth factor receptor).26,27 Hyperinsulinemia increases ovarian androgen, IGF-1, and IGF-2 production in the liver. Insulin and IGF-1 increase the activity of 17-hydroxylase in the ovaries, causing excessive production of androgens, especially 17-hydroxyprogesterone (17-OHP). Indirectly, insulin potentiates the action of LH in the ovaries.28,29 Another effect of hyperinsulinemia is a decrease in the hepatic production of SHBG (a sex-hormone carrier protein) and IGF-1 binding protein (IGFBP-I), contributing to greater action of free testosterone and IGF-1, respectively, in target cells. In-vitro studies have shown that insulin and IGF-1 might act with androgens to stimulate growth of hair follicles. Hyperinsulinemia can also increase the action of 5?-reductase, leading to increased conversion of testosterone into dihydrotestosterone.30,31
A Western diet is characterized by high glycemic load and increased levels of milk/dairy protein, containing abundant amounts of branched-chain amino acids (leucine, isoleucine, and valine). These two dietary stimuli overstimulate a kinase termed mammalian target of rapamycin complex 1 (mTORC1). The activation of mTORC1 signaling is involved in both acne pathogenesis (altering sebaceous gland homeostasis with the promotion of cell growth and proliferation) and IR (stimulating the kinase S6K1, which negatively controls insulin signaling at the level of insulin receptor substrate-1 phosphorylation).32 Insulin resistance has been recognized as an inflammatory disease, and there is a close relation between inflammatory acne and insulin resistance.33,34
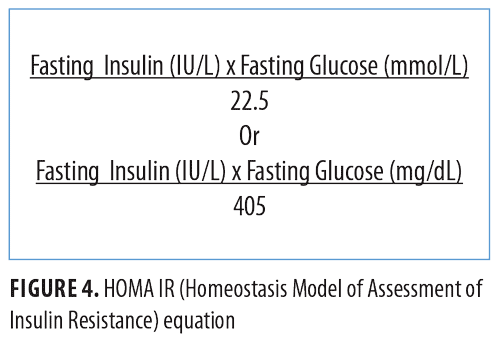
To date, there is no single test that can diagnose insulin resistance. A World Health Organization (WHO) consensus group concluded that the Insulin Sensitivity Index (SI) of the lowest 25 percent of a general population can be considered insulin-resistant. Insulin sensitivity shows ethnical- and gender-based variations.35–37 The hyperinsulinemic euglycemic clamp technique, considered the gold standard for diagnosing insulin resistance, is impractical in a clinical setting. HOMA IR might be a more practical alternative as its value is calculated using an equation (Figure 4) and is considered to replicate the clamp technique.38
A HOMA IR cut-off value of 2.5 has been identified to diagnose metabolic syndrome in urban Indian adolescents.39 An Indian study of adults identified a cut-off value of 2.41 to diagnose insulin resistance.40
The OGTT with insulin measurements at fasting and two hours after 75g glucose ingestion is a practical approach to diagnose insulin resistance. WHO defines the upper level of normal fasting blood glucose as 110mg/dL; the American Diabetes Association has defined it as 100mg/dL.41 An Indian consensus group has recommended that for the diagnosis of impaired fasting glucose, a cut-off value of 100mg/dL should be applied to Asian Indians.42 A fasting insulin level above 25mg/dL is considered abnormal.43 In a study of Caucasian women with obesity, a level of fasting insulin above 9µIU/mL was determined as a marker of pre-diabetes.44 A recent study from Western India (where our study was conducted) recommends a cut-off level of 17mg/dL for diagnosis of hyperinsulinemia.45 As insulin response pattern, post-glucose, varies considerably in patients with impaired fasting glucose and impaired glucose tolerance, fasting measurements are generally recommended in population studies. Measuring only fasting insulin levels is sometimes inadequate for a diagnosis of mild insulin resistance in women with acne.46 There are very few studies defining cut-offs for post-load insulin. The cut-off value of 41µIU/mL was used here in the present study and in another Indian study as well.47 Other non-Indian studies have used a cut-off value of 35µIU/mL.48,49
The glucose-insulin (GI) ratio at baseline, one, and two hours is lower in women with PCOS than in healthy controls.50 A fasting GI ratio of less than 4.547 and a two-hour GI ratio less than 1 is supportive of a diagnosis of insulin resistance in women with PCOS.51
It seems wise to perform an OGTT with HOMA IR calculation to avoid missing insulin resistance in patients with acne. Most of the data for cut-offs available to us are for patients with PCOS. Large-scale, cross-control studies in subjects who are acne prone compared to normal controls are needed to determine the cutoff levels of fasting and post-load insulin, HOMA IR, and GI ratios for diagnosis of insulin resistance in patients with acne.
AMH. Excessive production of AMH secreted by growing follicles is now considered a feature of PCOS. Acne is the most common cutaneous manifestation of PCOS,52 so dermatologists can play an important role in PCOS diagnosis. Girls with severe acne or acne resistant to oral and topical agents, including isotretinoin, have a 40-percent likelihood of developing PCOS.53 Twenty to 40 percent of female patients with acne suffer from PCOS.54
The performance of transvaginal ultrasound is not possible in women who are not yet sexually active. While FSH and LH testing is dependent on the menstrual cycle, the menstrual cycle has little influence on AMH. AMH testing is useful for diagnosing PCOS in patients with persistent acne.55 Serum AMH level is also correlated with the severity of PCOS, and thus a raised AMH should prompt the clinician to do an ultrasound for PCOS.
LH/FSH. In healthy women, LH/FSH ratio is approximately 1:1 during the follicular phase.57 LH hypersecretion is a characteristic hallmark of PCOS.58 High LH levels in the follicular phase indicate hypersecretion and probably an absence of LH surge, which causes anovulation in PCOS. Thus, in the present study we used the principle of LH/FSH ratio greater than 1 to indicate an abnormality in LH secretion. For PCOS, the ratio of greater than 2 is accepted as abnormal.
Prolactin. Women with hyperprolactinemia might present with signs of chronic hyperandrogenism, such as hirsutism and acne (possibly due to increased dehydroepiandrosterone sulfate secretion from the adrenals) and reduced SHBG, leading to high free testosterone levels. Normal serum prolactin levels range from 5 to 25ng/mL in female subjects, although physiological and diurnal variations occur.59 Hyperprolactinemia is usually defined as fasting levels of above
25ng/mL in women.60 Many common medications can cause hyperprolactinemia with prolactin levels 100ng/mL or less. Other common conditions that must be excluded when considering raised prolactin levels include samples taken from a non-fasting individual or from individuals who excessively exercise, have had chest wall surgery or trauma, have renal disease or cirrhosis, or have had a seizure within 1 to 2 hours of testing. Certain medications can also affect prolactin levels. All of these conditions can cause prolactin elevation over 50ng/mL.61
Study limitations. We found an almost universal prevalence of altered biochemical and hormone markers in our study. One reason for this might be the socioeconomic bias, as all patients in our study belonged to the middle class. Also, since only those patients who had some objective indication of insulin resistance or hyperandrogensim were included in our study, the prevalence rates do not reflect the real-world prevalence in female individuals with acne.
Conclusion
Rather than a transient pubertal cutaneous condition, acne is now recognized as a chronic systemic disease. There is a very high prevalence of altered metabolic and hormonal status in female individuals with acne. Although this study focused on women in India, there is no reason to believe that similar findings would not be seen in insulin-resistant men or in other countries.34
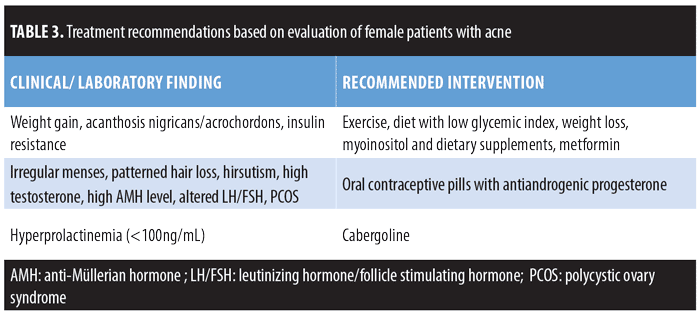
It has been suggested that PCOS should be renamed “female metabolic syndrome” or “syndrome XX.”62 Dermatologists frequently evaluate and manage the cutaneous manifestations of PCOS, and therefore can play a key role in PCOS diagnosis and management.63 Knowing the markers that indicate abnormalities can guide us to the correct acne treatment approach (Table 3). A patient with insulin resistance should be counseled about weight loss, exercise, and the consumption of a diet with low glycemic index. These patients can also be treated with metformin. Myoinositol supplements improve insulin sensitivity in PCOS patients.64 Oral contraceptives with antiandrogenic progesterone can be used in women with PCOS who are experiencing irregular menstrual cycles. Vitamin D supplementation can help treat acne directly or indirectly by improving insulin sensitivity and hormone dysfunction.20 Since the prevalence of vitamin D deficiency is ubiquitous, a study should be conducted to assess whether all acne patients should be given vitamin D supplements. Insulin resistance is also highly prevalent in patients with acne, so diet modification should be prescribed universally.
The skin reflects the internal health of the body. Dermatologists should keep a high index of suspicion for other disorders, including PCOS, in their patients with acne, and evaluate them appropriately. Dermatologists are in a unique position to introduce lifestyle modifications at an early age to their patients with acne in order to prevent or delay the onset of serious systemic disorders.
References
- Jansen T, Jansen OE, Plewig G. [Acne tarda. Acne in adults]. Der Hautarzt. 2013;64(4):241–51.
- Lynn DD, Umari T, Dunnick CA, Dellavalle RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25.
- Balaji A, Thappa DD. Profile of acne vulgaris— a hospital based study from south India. Indian J Dermatol Venereol Leprol. 2009;75(3):272–278.
- Cunliffe W, Gollnick H. Clinical features of acne Acne Diagnosis and Management. London: Martin Dunitz. 2000;49–67.
- Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne?. Indian J Dermatol Venereol Leprol. 2012;78(3):335–341.
- Dréno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27(9):1063–1070.
- Kellett SC, Gawkrodger DJ. The psychological and emotional effect of acne and the effect of treatment with isotretinoin. Br J Dermatol. 1999;140(2):273–282.
- Rahman M, Sikder AU, Rashid MM, et al. Association of serum testosterone with acne vulgaris in women. BSMMU J. 2012;5:1–5.
- Ghosh S, Chaudhuri S, Jain VK, Aggarwal K. Profiling and hormonal therapy for acne in women. Indian J Dermatol. 2014;59(2):107–115.
- Chrousos GP, Peck GL, Gross EG, et al. Adrenal function in women with idiopathic acne. J Invest Dermatol. 1982;78(6):468–471.
- Carmina E, Lobo RA. Evidence for increased androsterone metabolism in some normoandrogenic women with acne. J Clin Endocrinol Metab. 1993;76(5):1111–1114.
- Norman A, Bowman BA, Russel RE. Present knowledge of Nutrition. 8th ed. Washington, DC: ILSI Press; 2001.
- Beloyartseva M, Mithal A, Kaur P, et al. Widespread vitamin D deficiency among Indian health care professionals. Arch Osteoporos. 2012;7:187–192.
- Harinarayan CV, Sachan A, Reddy PA, et al. Vitamin D status and bone mineral density in women of reproductive and postmenopausal age groups: a cross-sectional study from South India. J Assoc Physicians India. 2011;59:695–701.
- Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102(2):460–468.e3.
- Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385.
- Kramer C, Seltmann H, Seifert M, et al. Characterization of the vitamin D endocrine system in human sebocytes in vitro. J Steroid Biochem Mol Biol. 2009;113(1-2):9–16.
- Lee WJ, Choi YH, Sohn MY, et al. Expression of inflammatory biomarkers from cultured sebocytes was influenced by treatment with vitamin D. Indian J Dermatol. 2013;58(4):327.
- Agak GW, Qin M, Nobe J. Propionibacterium acnes induces an IL-17 response in acne vulgaris that Is regulated by vitamin A and vitamin D. J Inves Dermatol. 2013;134(2):366–373.
- Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26(1):19–27.
- Lim SK, Ha JM, Lee YH, et al. Comparison of vitamin D levels in patients with and without acne: a case-control study combined with a randomized controlled trial. PLoS One. 2016;11(8):e0161162.
- Emiro?lu N, Cengiz FP, Kemeriz F. Insulin resistance in severe acne vulgaris. Postepy Dermatol Alergol. 2015;32(4):281–285.
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607.
- Hermanns-Le T, Scheen A, Pierard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5(3):199–203.
- Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32Supp3:24–34.
- Dunaif A, Xia J, Book CB, et al. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest. 1995;96(2):801–810.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14(9):2.
- Kidson W. Polycystic ovary syndrome: a new direction in treatment. Med J Aust. 1998;169(10):537–540.
- Goodarzi MO, Erickson S, Port SC, et al. ?-cell function: a key pathological determinant in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(1):310–315.
- Nermeen S.A. Fattah A Darwish Y.W. Is there a role for insulin resistance in nonobese patients with idiopathic hirsutism?. Br J Dermatol. 2009;160(5):1011–1015.
- Barbato MT, Criado PR, Silva AK, et al. Association of acanthosis nigricans and skin tags with insulin resistance. An Bras Dermatol. 2012;87(1):97–104.
- Napolitano M, Megna M, Monfrecola G. Insulin resistance and skin diseases. Scientific World Journal. 2015;2015:479354.
- Garg R, Tripathy D, Dandona P. Insulin resistance as a proinflammatory state: mechanisms, mediators, and therapeutic interventions. Curr Drug Targets. 2003;4(6):487–492.
- Del Prete M, Mauriello MC, Faggiano A, et al. Insulin resistance and acne: a new risk factor for men? Endocrine. 2012:42(3):555–560.
- Wijeyaratne CN, Seneviratne Rde A, Dahanayake S, et al. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): results of a large database from a specialist endocrine clinic. Hum Reprod. 2011;26(1):202–213.
- Dasgupta A, Khan A, Banerjee U, et al. Predictors of insulin resistance and metabolic complications in polycystic ovarian syndrome in an Eastern Indian population. Indian J Clin Biochem. 2013;28(2):169–176.
- Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gender Medicine. 2009;6(Suppl 1):60–75.
- Keskin M. Homeostatic model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing Insulin resistance among obese children and adolescents. Paediatrics. 2004;115(4):500–503.
- Singh Y, Garg M, Tandon N, Marwaha RK. A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban Indian adolescents. J Clin Res Pediatr Endocrinol. 2013;5(4):245–251.
- Momin AA, Bankar MP, Bhoite GM. Determination of HOMA IR cut off value, and efficiency of lipids and lipoprotein ratios as discriminator of insulin resistance in type 2 diabetes mellitus patients. IOSR J Pharm Biol Sci. 2014;4(6):9–14.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Available at: http://care.diabetesjournals.org/content/39/Supplement_1/S13. Accessed on February 6, 2016.
- Misra A, Chowbey P, Makkar BM, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–170.
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia: Elsevier Saunders; 2011.
- Johnson JL, Duick DS, Chui MA, Aldasouqi SA. Identifying prediabetes using fasting insulin levels. Endocrine Practice. 2010;16(1):47–52.
- Shah D, Puthran S. Diagnosis of hyperinsulinaemia in a normoglycaemic healthy Indian population—developing ethnic reference ranges. J Assoc Physicians India. 2014;62(5):394–399.
- Aizawa H, Niimura M. Mild insulin resistance during oral glucose tolerance test (OGTT) in women with acne. J Dermatol. 1996 Aug;23(8):526–529.
- Saxena P, Prakash A, Nigam A. Efficacy of 2-hour post glucose insulin levels in predicting insulin resistance in polycystic ovarian syndrome with infertility. J Hum Reprod Sci. 2011;4(1):20–22.
- Ou HT, Chen PC, Wu MH. Effect of metformin by employing 2-hour postload insulin for measuring insulin resistance in Taiwanese women with polycystic ovary syndrome. J Formos Med Assoc. 2017;116(2):80–89.
- Stovall DW, Bailey AP, Pastore LM. Assessment of insulin resistance and impaired glucose tolerance in lean women with polycystic ovary syndrome. J Womens Health (Larchmt). 2011;20(1):37–e43.
- Kulshreshtha B, Ganie MA, Praveen EP, et al. Insulin response to oral glucose in healthy, lean young women and patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008 Nov;24(11):637–643.
- Medical University of South Carolina. Assessing Insulin Sensitivity. Available at: http://academicdepartments.musc.edu/family_medicine/rcmar/insulin_sens.htm. Accessed on: February 27, 2017.
- Gowri B V, Chandravathi P L, Sindhu P S, Naidu K S. Correlation of skin changes with hormonal changes in polycystic ovarian syndrome: A cross-sectional study clinical study. Indian J Dermatol. 2015;60(4):419.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to best practices in the evaluation and treatment of polycystic ovary syndrome. – part 1. Endocr Pract. 2015;21(12):1415–1426.
- Azziz R1, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488.
- Sardana K, Singh C, Narang I, et al. The role of antimullerian hormone in the hormonal workup of women with persistent acne. J Cosmet Dermatol. 2016;15(4):343–349.
- Dumont A, Robin G, Catteau-Jonard S, et al. Role of anti-Müllerian Hormone in pathophysiology, diagnosis and treatment of polycystic ovary syndrome: a review. Reprod Biol Endocrinol. 2015;13:137.
- Strauss JF, Barbieri RL. Yen & Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia: Elsevier Health Sciences; 2013.
- King J. Polycystic ovary syndrome. J Midwifery Womens Health. 2006;51(6):415–422.
- Melmed S, Jameson JL. Disorders of the anterior pituitary and hypothalamus. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw Hill; 2008:2076–2097.
- Halbreich U, Kinon BJ, Gilmore JA, Kahn LS. Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinol. 2003;28 Suppl 1:53–67.
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci. 2013;6(3):168–175.
- Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003;14(8):365–370.
- Houseman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71(5):847.e1–847.
- Artini PG, Di Berardino, Papini F. Endocrine and clinical effects of myo-inositol administration in polycystic ovary syndrome. a randomized study. Gynecol Endocrinol. 2013;29(4):375–379.

