 by Zoe Diana Draelos, MD, and Isabelle Raymond, PhD
by Zoe Diana Draelos, MD, and Isabelle Raymond, PhD
Dr. Draelos is with Dermatology Consulting Services in High Point, North Carolina. Dr. Raymond is with Exeltis USA in Florham Park, New Jersey.
J Clin Aesthet Dermatol. 2018;11(5):30–32
Funding: This study was supported by a grant from Exeltis USA.
Disclosures: Dr. Draelos has no conflicts of interest relevant to the content of this article. Dr. Raymond is an employee of Exeltis USA.
Abstract: Objective. The goal of this study was to determine if a therapeutic cream containing PC-104 synthetic ceramides and other ingredients could help to ameliorate signs and symptoms of mild-to-moderate atopic dermatitis and other xerotic or pruritic dermatoses.
Design. In this single-site, four-week study, subjects (n=50) were instructed to apply the study product three times daily to all affected areas, including a target site for evaluation and photography at baseline, Week 2, and Week 4 visits.
Setting. This study took place in the private practice of the senior author.
Participants. Eligible subjects with mild-to-moderate sensitive skin were included.
Measurements. Evaluations included Investigator Global Assessment (IGA) scale score; subject satisfaction assessments; and noninvasive assessments of transepidermal water loss, increases in water content of the skin, and improvements in skin smoothness.
Results. Use of the ceramide cream resulted in a 100-percent improvement in IGA scores and a 67-percent improvement in overall subject skin assessment scores after four weeks of use in individuals with sensitive skin conditions. Improvements were statistically significant. Statistically significant improvements were also observed in transepidermal water loss, water content of the skin, and skin smoothness. Adverse events were not observed.
Conclusion. These data demonstrate that a proprietary combination of ceramide PC-104, palmitamide MEA, glycerrhetinic acid, and grape seed extract in a glycerin, dimethicone, and petrolatum vehicle was effective in reducing the signs and symptoms of mild-to-moderate atopic dermatitis and other types of pruritic dermatoses (e.g., senile itch, cosmetic intolerance syndrome) in children and adults.
Keywords: Ceramides, atopic dermatitis, sensitive skin, pediatric, adult
Introduction
Adequate moisturization and barrier repair are critically important for a number of dermatologic conditions. Various types of dermatoses and so-called “sensitive skin” are thought to be related to a dysfunctional skin barrier. This could be due to a number of conditions, such as atopic dermatitis, senile itch, eczema, allergic contact dermatitis, and cosmetic intolerance syndrome. A well-formulated cream for sensitive skin can enhance barrier repair, increase the water-holding capacity of the skin, and optimize healing.
Approximately 50 percent of intercellular lipids are ceramides, with cholesterol and free fatty acids representing the other constituents. In some individuals with sensitive skin, ceramides 1 and 3 are thought be deficient.1 It then follows that one approach to improving dermatitis and sensitive skin is the application of ceramides. Synthetic ceramides mimic natural ceramides but are contaminant-free and stable in formulation.
The synthetic ceramide combination PC-104 could augment the natural barrier function.2 However, the function of ceramides can be augmented with anti-inflammatory and antibacterial derivatives, such as glycerrhetinic acid, an extract of the licorice root, and proanthocyanidins, an extract of grape seeds.3 It is thought that the anti-inflammatory activity of these ingredients could be due to inhibited proliferative activity of T-cells via mitogen-activated protein kinase and nuclear factor kappa B signaling pathways. Other additives such as palmitoylethanolamide (PEA), a fatty acid amide, can bind to nuclear receptors, reducing the itch sensation.4 Thus, a cream composed of these ingredients addresses many of the physiologic issues associated with sensitive skin.
The objective of this study was to determine if a therapeutic cream containing PC-104 synthetic ceramides, glycerrhetinic acid, proanthocyanidins, and palmitoylethanolamide MEA could assist in the amelioration of the signs and symptoms of mild-to-moderate atopic dermatitis and other types of pruritic dermatoses (e.g., senile itch, cosmetic intolerance syndrome) in children and adults.
Methods
In this single-site, monadic study, investigators enrolled 50 healthy subjects (12 male and 38 female, 23 African-American and 27 Caucasian) who were 1 to 86 years of age (median: 38.0 years, interquartile range: 38.5 years) with mild-to-moderate atopic dermatitis or other xerotic or pruritic dermatoses. All subjects provided signed informed consent (Concordia Clinical Research, Beach Haven, New Jersey, USA). Subjects continued using their own self-selected cleansers, but were not allowed to apply any moisturizers on the affected areas except for the study product. Qualified subjects underwent baseline investigator assessment and subject self-assessment and received the study cream (NeoCera™; Exeltis USA, Florham Park, New Jersey, USA) to apply three times daily to the affected areas. In addition, a target site representing an area of sensitive skin was selected for evaluation of improvement characteristics in comparison with baseline. The target site was photographed at each visit by the investigator.
Subjects completed a diary and made an entry for each product application, including comments, if necessary, to ensure adherence. In addition, subjects were given an application map, noting the target area on the arms or legs, to ensure that applications were made to the proper evaluation areas. Subjects underwent evaluation and photography at baseline, Week 2, and Week 4, with the examinations performed at least eight hours following the last application of the study cream. Evaluations consisted of an Investigator Global Assessment (IGA) scale score and subject self-assessment (SA), both with the scale of 0=none, 1=minimal, 2=mild, 3=moderate, and 4=severe for each skin parameter. IGA skin parameters included irritation, erythema, desquamation, roughness, dryness, and overall appearance, and the SA parameters included irritation, redness, peeling, roughness, and overall appearance. Target area noninvasive assessments were also made for transepidermal water loss (TEWL; Evaporimeter; Cortex Technologies, Hadsund, Denmark), corneometry (Pin Probe; Cortex Technologies, Hadsund, Denmark), and colorimetry (L*a*b scale; Minolta, Osaka, Japan) to ensure consistency in the evaluation site.
Since the data were either not normally distributed by the Shapiro–Wilk test or were not continuous, significant differences from baseline were evaluated by the nonparametric paired Wilcoxon signed-rank test. Since multiple nonindependent comparisons were made with baseline, Bonferroni correction was applied to the customary significance level of p=0.05. In this case, two comparisons (at 2 weeks and 4 weeks) were made with baseline, so the cutoff level was adjusted by dividing 0.05 by two to arrive at p=0.025. The safety population included all subjects exposed to the study product who provided any post-treatment safety information.
Results
All subjects completed the research project and adhered with the treatment protocol. No investigator- or subject-reported safety or lack of tolerance issues arose during the study. IGA data (Table 1) for the study cream revealed a 100-percent difference from baseline with respect to irritation, erythema, desquamation, roughness, and overall appearance after both two weeks and four weeks of application. The IGA score was based on an overall evaluation of all affected body areas. All differences were statistically significant compared to baseline (p<0.0001).
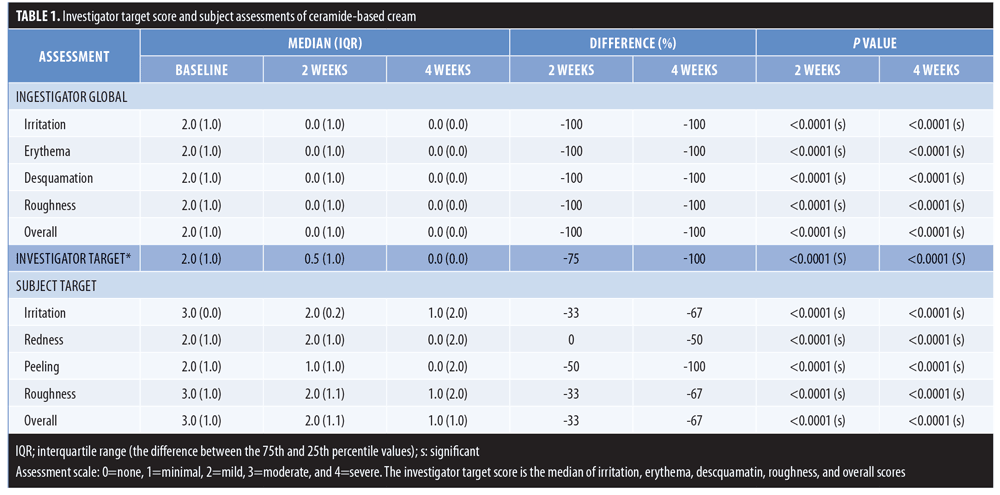
IGA data for the target site were composed of the median score of all five parameters at baseline, two weeks, and four weeks. Subjects entered the study with a median score of 2.0, which decreased to 0.5 at two weeks (75% decrease) and 0.0 at four weeks (100% decrease). Both changes were statistically significant (p<0.0001).
Subject target area assessments (Table 1) also indicated a statistically significant decrease in irritation, redness, peeling, roughness, and overall assessment (p<0.0001) at both Week 2 and Week 4. Median scores at Week 2 ranged from 0 to 50 percent compared to baseline, and continued to decrease to 50 to 100 percent at Week 4. The reductions were all statistically significant (p<0.001) at Week 2 and Week 4.
Three noninvasive assessments were also conducted to provide more objective data. A colorimeter was used to evaluate the color of the skin pre- and post-application of the ceramide cream. The L scale was used to assess light reflection from the skin surface, which is directly related to skin smoothness.5 The L scale reading decreased compared to baseline at four weeks and achieved significance (p<0.0001). The amounts of water in the skin and water leaving the skin were also evaluated. Corneometry was used to assess the water in the skin. Over the four weeks of the study, the water content increased steadily as the dry skin condition resolved. The baseline median corneometry reading of 50.00 increased to 70.00 by Week 2 and 80.00 by Week 4, both representing a significant (p<0.0001) increase in skin water content. Conversely, the TEWL reading decreased at Week 2 and Week 4 as the barrier improved and approached significance at four weeks (p=0.0363).
Discussion
Use of the ceramide cream in this study resulted in a 100-percent improvement in IGA scores and a 67-percent improvement in overall subject skin self-assessment scores after four weeks of use in individuals with atopic dermatitis or other sensitive skin conditions. Improvements were statistically significant.
Typically, these skin conditions are treated with topical corticosteroids. The study cream used herein was a unique formulation of several ingredients with different mechanisms of action relevant to the improvement of sensitive skin. Such a cream could be combined with prescription therapy, used alone in cases of more minor disease, or used solely during the maintenance phase following treatment. Subjects with mild-to-moderate atopic dermatitis or other dermatoses associated with sensitive skin conditions were enrolled in the present study to examine the abilities of the cream to function beyond simple cosmetic moisturization.
The formulation utilized dimethicone and petrolatum as occlusive moisturizers to create a temporary skin barrier in combination with glycerin as a humectant to attract and hold water in the stratum corneum and epidermis. The active ingredients included ceramide PC-104, palmitamide MEA, glycerrhetinic acid, and grape seed extract. It is worthwhile to examine how this combination of ingredients resulted in sensitive skin improvement. Ceramide PC-104, with the corresponding chemical name N-(3-hexadecyloxy-2-hydroxypropyl)-N-2-hydroxyethyl hexadecanamide, is a synthetic pseudoceramide developed to allow higher concentrations of ceramides to be used in skin care products free of contaminants, as many natural ceramides are derived from bovine sources.6
Topical pseudoceramides have been shown to decrease improved stratum corneum water-holding properties and facilitate barrier repair, which is important in sensitive skin conditions.7,8
The second need of a sensitive skin cream is to reduce inflammation reduction and accompanying itch. Palmitoylethanolamine (PEA), also known as palmitamide MEA, belongs to the family of N-acylethanolamines. Cells naturally produce these substances in order to down-regulate the inflammatory response via cannabinomimetic action on cannabinoid (CB) receptors.9 This observation has led to postulation that this family of molecules might possess analgesic, antioxidant, and anti-inflammatory skin benefits.10 CB 1 receptors are found in the brain and peripheral tissues, while CB 2 receptors are distributed throughout the immune system and in cutaneous nerve fibers.11 CB receptor agonists, such as PEA, reduced histamine-induced itch and vasodilation when applied topically prior to histamine.12
The third need of sensitive skin is inflammation reduction. The study formulation included grape seed extract, which contains the antioxidants proanthocyanidin and polyphenol, as well as a licorice extract containing glycerrhetinic acid for this purpose. Proanthocyanidin possesses antioxidant free radical neutralizing effects 20 times more potent than vitamin C and 50 times more potent than vitamin E.13
Conclusions
The combination of ceramide PC-104, palmitamide MEA, glycerrhetinic acid, and grape seed extract in a glycerin, dimethicone, petrolatum vehicle resulted in the concurrence between the investigator, subject, and noninvasive assessments for successful improvement of the signs and symptoms of mild-to-moderate atopic dermatitis and other xerotic or pruritic dermatoses in both pediatric and adult populations.
References
- Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78(1):27–30.
- Vielhaber G, Lange S, Ley J, Koch O. N-palmitoyl-4-hydroxy-L-proline palmityl ester: a pseudoceramide that provides efficient skin barrier repair and protection. Int J Cosmet Sci. 2006;28(1):70.
- Hemmati AA, Foroozan M, Houshmand G, et al. The topical effect of grape seed extract 2% cream on surgery wound healing. Glob J Health Sci. 2014;7(3):52–58.
- Veraldi S, De Micheli P, Schianchi R, Lunardon L. Treatment of pruritus in mild-to-moderate atopic dermatitis with a topical non-steroidal agent. J Drugs Dermatol. 2009;8(6):537–539.
- Takiwaki H, Serup J. Measurement of color parameters of psoriatic plaques by narrow-band reflectance spectrophotometry and tristimulus colorimetry. Skin Pharmacol. 1994;7(3):145–150.
- Mizushima H, Fukasawa J, Suzuki T. Phase behavior of artificial stratum corneum lipids containing a synthetic pseudo-ceramide: a study of the function of cholesterol. J Lipid Res. 1996;37(2):361–367.
- Uchida Y, Holleran WM, Elias PM. On the effects of topical synthetic pseudoceramides: comparison of possible keratinocyte toxicities provoked by the pseudoceramides, PC104 and BIO391, and natural ceramides. J Dermatol Sci. 2008;51(1):37–43.
- Park BD, Youm JK, Jeong SK, et al. The characterization of molecular organization of multilamellar emulsions containing pseudoceramide and type iii synthetic ceramide. J Invest Dermatol. 2003;121(4):794–801.
- Noli C, Della Valle MF, Miolo A, et al. Efficacy of ultra-micronized palmitoylethanolamide in canine atopic dermatitis: an open-label multi-centre study. Vet Dermatol. 2015;26(6):432–440, e101.
- Eberlein B, Eicke C, Reinhardt HW, Ring J. Adjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J Eur Acad Dermatol Venereol. 2008;22(1):73–82.
- Facci L, Toso RD, Romanello S, et al. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci USA. 1995; 92(8):3376–3380.
- Ständer S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38(3):177–188.
- Nassiri-Asl, M., Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother Res. 2009;23(9):1197–1204.

