 by Richard Fried, MD, PhD; W. Phillip Werschler, MD; Judith Cenci, MD; Lauren Sternberg, MD; Priya Dhanaraj, MD; Dara Tolas, MA; and Suzanne Withrow, MA
by Richard Fried, MD, PhD; W. Phillip Werschler, MD; Judith Cenci, MD; Lauren Sternberg, MD; Priya Dhanaraj, MD; Dara Tolas, MA; and Suzanne Withrow, MA
All authors except Dr. Werschler are with Yardley Dermatology Associates in Yardley, Pennsylvania. Dr. Werschler is Assistant Clinical Professor of Dermatology at the University of Washington School of Medicine in Seattle, Washington.
Funding: Financial support was received from Galderma for this study.
Disclosures: Dr. Fried has served as a consultant, researcher, and/or speaker for Galderma, Allergan, Pfizer, Jannsen, Sun Pharmaceuticals, and Johnson & Johnson. All other authors have no conflicts of interest to disclose.
Abstract: Background. Facial volume loss can substantially impact self-perception of the affected individual and his to her social/professional interactions and opportunities. Persons who are perceived as more youthful and attractive are rated as nicer, more energetic, healthier, and more likely to be productive than those perceived to be older and unattractive. Objective. The goal of this study was to assess the changes in emotional and functional status of subjects treated with 2 to 4 monthly PLLA injections to the upper, mid, and lower face. Methods. Fifty subjects were recruited from two community dermatology centers in the United States (Bucks County, Pennsylvania, and Spokane, Washington). Two patient self-report measures were used—The Facial Volume Restoration Outcome Questionnaire and the Rosenberg Self Esteem Scale. Results. Approximately 30 percent of the subjects who completed pre- and post-injection instruments reported increased confidence, increased sense of control, more comfort with others, increased happiness when looking in the mirror, more happiness when their faces were touched, healthier eating, increased contentment, beliefs that they were seen as less stressed by others, increased happiness with their bodies, exercising more, and that their lives were better. Additionally, 43 percent felt more optimistic and 33 percent felt less anxious. The changes in self-esteem, which was self-reported on the Rosenberg scale, were very modest but also favorable. Investigator’s Global Assessment (IGA) of facial volume loss improved from 2.2 at baseline to 1.2 at six-month follow-up assessment. Conclusion. Treatment of facial volume loss with PLLA was associated with improvements in patient-perceived emotional and functional status at six months after initial injection.
Keywords: Poly-L-Lactic acid, fillers, volume loss, volume restoration
J Clin Aesthet Dermatol. 2018;11(7):40–43
Introduction
Facial volume loss can substantially impact self-perception of the affected individual and his or her social/professional interactions and opportunities. Persons who are perceived as more youthful and attractive are rated as nicer, more energetic, healthier, and more likely to be productive than those perceived to be older and unattractive.1–3
The observed facial changes that result from underlying volume loss associated with age and photodamage can produce a subjectively older and less attractive appearance. Loss of facial volume can produce a sad, sunken, deflated, dull, tired, and/or lackluster appearance, subjectively, which might suggest to others that the individual is older than he or she actually is. In addition, some individuals might consciously or subconsciously see these changes as harbingers of their own progressive deterioration and decline. Injection of approved fillers into the upper face, mid face, and lower face can create a more youthful, better rested, and kinder appearance. Randomized, controlled trials (RCTs) demonstrating the effectiveness of injectable fillers in improving emotional and functional status remain scarce.
A hallmark of hyaluronic acid and calcium hydroxylapatite filler products is their ability to produce notable volumetric changes immediately.4 The recent proliferation of technologically improved hyaluronic fillers has dramatically enhanced the armamentarium available to active clinicians.5 Claims of anatomic regional superiority and longer duration might be considered enticing reasons to use hyaluronic fillers.6 The early days of fillers characterized by “briefly there til completely gone” are being supplanted by “there now, persistently there, and stimulatory for a reasonable period of time.”7 The persistence of filler presence with enhancement of collagen production is a meaningful step forward in filler products, as these characteristics might slow the facial aging process. In contrast to the immediate deposition of hyaluronic acid and calcium hydroxylapatite fillers, another option exists for enhancement of collagen production and persistence of benefit. Poly-L lactic acid (PLLA) (Sculptra®, Galderma Laboratories, L.P., Ft. Worth, Texas) has been shown to effectively stimulate collagenases and fibroplasia, which can produce a gradual volumizing effect of the treated areas.8 While there is apparent immediate volume repletion with PLLA injections, it is mostly from the sterile water and lidocaine commonly used to prepare the product, which largely dissipates within days after injection. The subsequent enhancement in collagen production and facial volumization occurs gradually over the next 1 to 3 months following each injection. Optimal correction usually occurs 1 to 3 months after 3 to 4 monthly injections of PLLA. While the exact dilution of PLLA varies among injectors, 6- to 7cc of sterile water with 2cc of lidocaine was used in this study.
PLLA is a synthetic, biocompatible, biodegradable polymer. For its use in soft tissue augmentation, it is supplied as a lyophilized powder containing PLLA microparticles, the size and chemical attributes of which are tightly controlled.9 As a biocompatible material, PLLA generates a subclinical inflammatory tissue response that leads to encapsulation of the microparticles and stimulation of host collagen and fibroplasia production. Over time, as the PLLA degrades, the inflammatory response wanes and host collagen production increases. This response leads to the generation of new volume and structural support that occurs in a gradual, progressive manner and can last for years.8,10
While the efficacy, safety, and duration of PLLA injection benefits are well supported by the literature,11 its mechanism of action as a biostimulator that produces gradual tissue augmentation over several months is unique. One concern that clinicians might have when using PLLA is the possibility that its delayed onset of effects will result in treatment dissatisfaction among those patients who expect immediate, visible results. Other patients, however, might be more concerned that the immediately visible effects of a hyaluronic acid or calcium hydroxylapatite filler will be too obviously noticeable by others. The goal of this study was to assess the subjective changes in emotional and functional status of subjects treated with 2 to 4 monthly PLLA injections to the upper, mid, and lower face.
Methods
Fifty subjects were recruited from two community-based dermatology centers in the United States (Bucks County, Pennsylvania, and Spokane, Washington). All 50 subjects were eligible and enrolled according to protocol inclusion and exclusion criteria—49 women and one man. Subjects were required to be in good health without known contraindications to filler. Subjects were also required to be naive to fillers or not have had filler injections in the prior two years before enrollment. Fifty-eight percent of subjects were age 45 to 60 years, 33 percent were 60 to 75 years, and eight percent were 35 to 45 years. The protocol was approved by a central Institutional Review Board (IRB) and full informed consent was provided by all subjects.
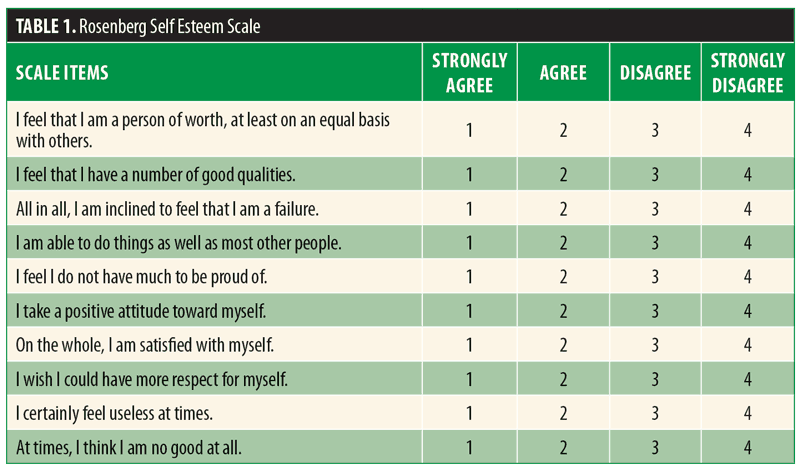
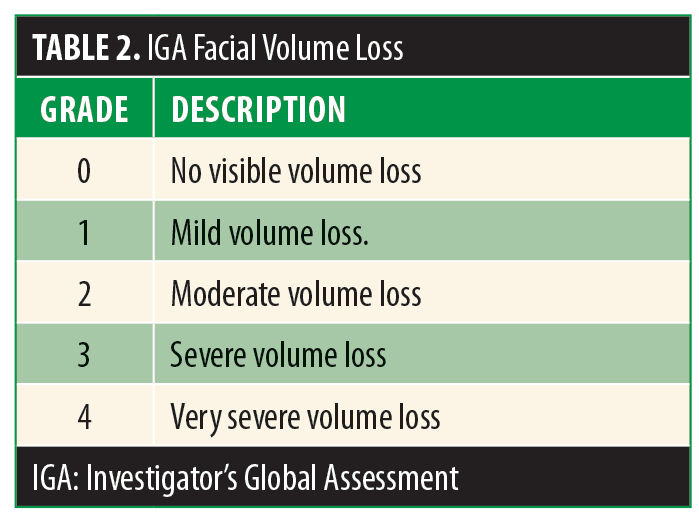
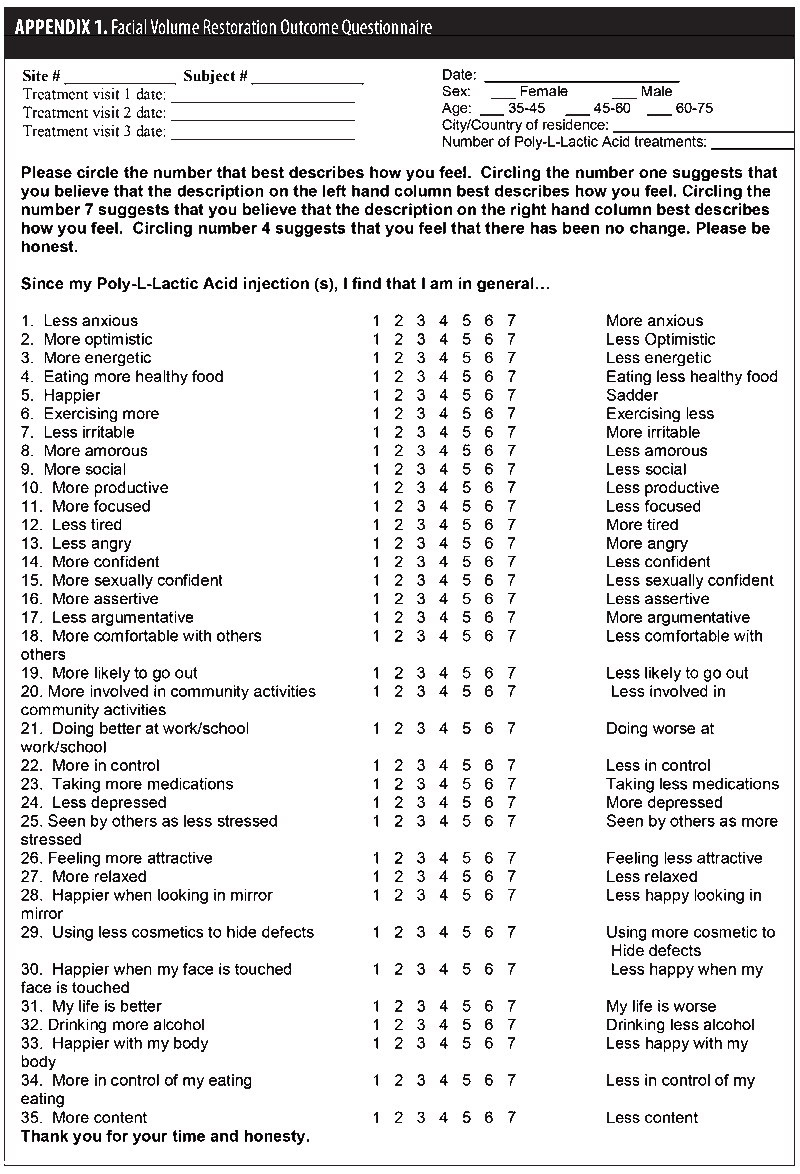
Two patient self-report measures were used: The Facial Volume Restoration Outcome Questionnaire (Appendix 1) and the Rosenberg Self Esteem Scale (Table 1). The Facial Volume Restoration Outcome Questionnaire is a 35-item patient self-report Likert scale-based instrument. Subjects self-report and self-rate selected areas of their emotional and functional status. The Rosenberg Self Esteem Scale is also a Likert scale-based instrument measuring overall self-esteem. Subject self-report measures were completed at baseline and six months after the subjects’ first injections. Facial volume loss was assessed on a 0 to 4 scale (Investigator Global Assessment [IGA])(Table 2) before and after treatment by either the principle investigator or a sub-investigator.
Subjects were injected monthly with PLLA over 3 to 4 consecutive months. Subjects received one full vial at each treatment visit. The PLLA was reconstituted with 7cc of sterile water and 2cc of lidocaine without epinephrine. The contents of the vial were injected in areas deemed appropriate by the certified injector and agreed upon by the study subject. Approximately 4 to 6 weeks later, a second vial was reconstituted as above and injected according to the above parameters. Finally, approximately 4 to 6 weeks later (12–16 weeks after the first injections), subjects were evaluated and a decision was jointly made with the injector regarding whether a third vial was desired and appropriate. All 50 subjects were injected at Week 1, 49 subjects were injected at the second visit, and 35 were injected at the third visit.
Results
Pre-and post-assessments were completed on 41 subjects. The remaining nine patients were lost to follow-up or withdrew and did not complete the post-filler assessments or return for investigator evaluation. None failed to return due to known adverse events.
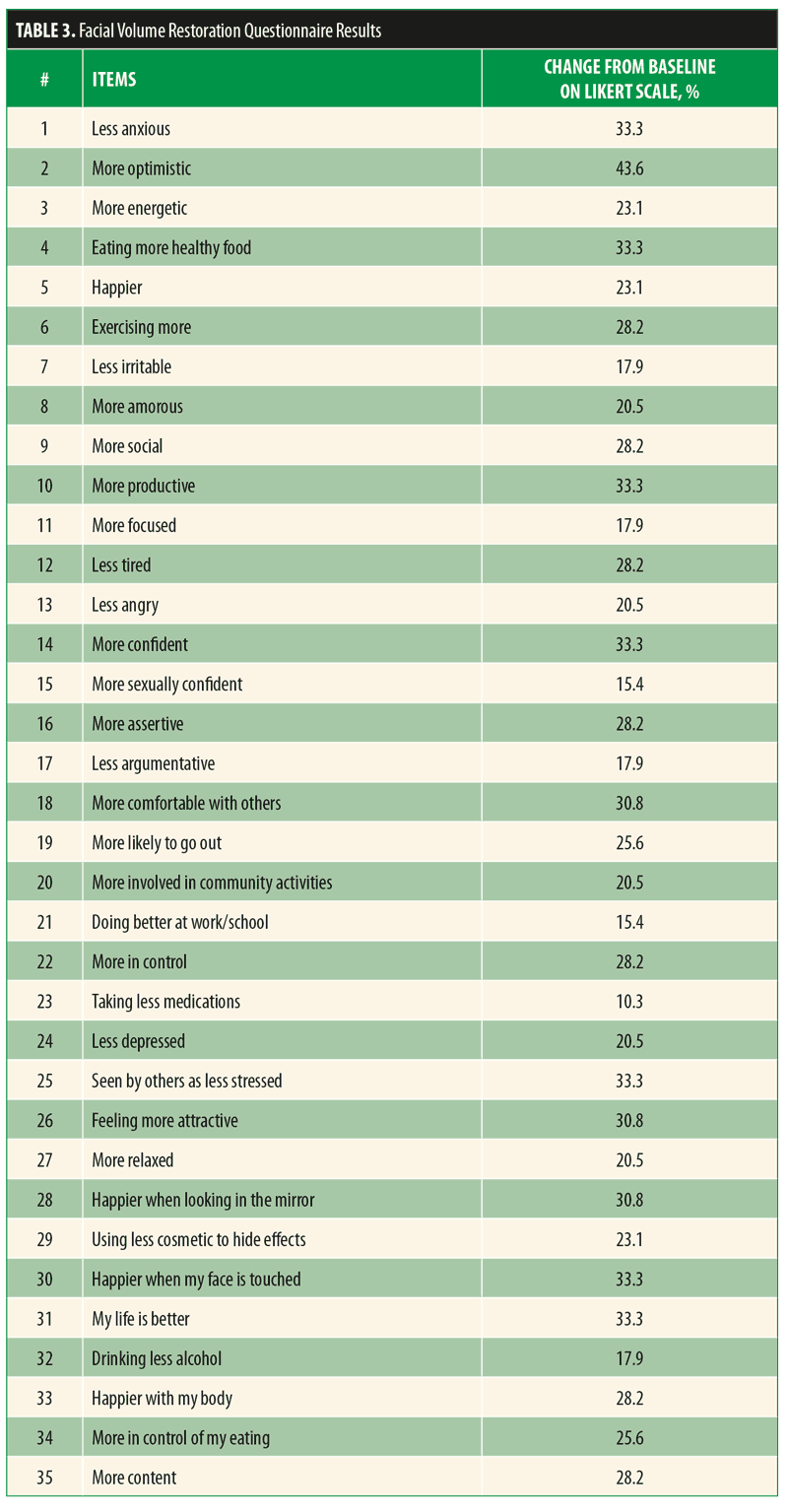
Approximately 30 percent of subjects who completed pre-and postinjection assessments reported increased confidence, increased sense of control, increased productivity, increased comfort with others, feeling more attractive, increased happiness when looking in the mirror, more happiness when their faces were touched, healthier eating, increased contentment, beliefs that they were seen as less stressed by others, being happier with their bodies, exercising more, and better lives. Additionally, 43 percent felt more optimistic, and 33 percent reported feeling less anxious (Table 3). The self-reported changes in self-esteem on the Rosenberg scale were modest but also favorable. IGA of facial volume loss improved from 2.2 at baseline to 1.2 at the six-month follow-up assessment (lower score suggesting less volume loss).
Discussion
Injection of PLLA has been shown to be a safe and effective method for facial volume and contour restoration.11,12 A potential drawback of PLLA injection is the delay in persistent, clinically evident volume repletion. The improvements are gradual as enhanced endogenous collagen production and fibroplasia gradually replenish volume-depleted areas.10 In the present study, we assessed patient satisfaction with the delayed onset effects of PLLA on facial volume using two patient self-reported assessements. The data suggest that patients perceived favorable emotional and functional improvements at six months after their first PLLA injection.
A benefit of PLLA’s gradual onset of effects is the potentially less obvious observation from others that a patient has undergone a cosmetic procedure. Clinicians might include this point in their discussions with patients regarding volume repletion treatment options.
Dissemination of the data from this study together with existing data supports the theory that volume restoration can slow the aging process. Thus, the beneficial effects of injectable PLLA might extend beyond those of a cosmetic procedure, which might serve as a compelling recruitment tool for its use among maturing individuals who struggle with facial volume loss associated with aging, medications, stress, and illness.
Limitations. Limitations of the study include the self-reported nature of the data collected from study participants, thus any favorable changes reported by the patients might not be directly attributable to the filler treatment. Additionally, lack of quantification of Likert scale gradations limits our findings. Finally, the absence of a comparable group of subjects treated with a hyaluronic acid or calcium hydroxylapatite product does not allow for assessment of equivalence, superiority, or inferiority of immediate volumizing products. Additional research addressing these limitations is needed to support and clarify our data on the emotional and functional benefits of facial volume restoration using PLLA.
Conclusion
Treatment of facial volume loss with PLLA was associated with improvements in patient-perceived emotional and functional status at six months after initial injection. These data suggest that the gradual volumization produced by PLLA injections might result in better long-term results and higher patient satisfaction with treatment outcomes compared to the immediate, but shorter-lived, volumetric changes associated with hyaluronic acid and calcium hydroxylapatite filler products.
References
- Fried RG, Werschler WP. The botulinum toxin experience: results of a patient self-report questionnaire. J Clin Aesthet Dermatol. 2009;2(11):37–40.
- Fried RG. Optimal facial rejuvenation: looking for help from above. The Dermatologist. 2014;22(12):18–19.
- Fried RF. Filler cheeks, A bright idea? The “eyes” have it. J Clin Aesthet Dermatol. 2011; 4(5): 44–47.
- Werschler WP. Treating the aging face: a multidisciplinary approach with calcium hydroxylapatite and other fillers, part 1. Cosmetic Dermatology. 2007;20(11):
739–742. - Arsiwala SZ. Current trends in facial rejuvenation with fillers J Cutan Aesthet Surg. 2015;8(3):125–126.
- Li D, Wang X, et.al. A randomized, controlled, multicenter study of Juvederm Voluma for enhancement of malar volume in Chinese subjects. Plast Reconstr Surg. 2017;139(6): 1250e–1259e.
- Wilson AJ, Taglienti AJ, et. al.Current applications of facial volumization with fillers. Plast Reconstr Surg. 2016;137:872e–882e.
- Mercer SE, Kleinerman R, Goldenberg G, Emanuel PO. Histopathologic identification of dermal filler agents. J Drugs Dermatol. 2010;9(9):1072–1078.
- Scultra® injectable poly-L-lactive acid package insert. Revised July 2016. Galderma Laboratories, L.P., Ft. Worth, Texas. http://www.galdermausa.com/IFU/Sculptra_Aesthetic_IFU.pdf?_ga=2.41415394.1110726524.1531344681-1690417467.1531344681. Accessed 11 July 2018.
- Werschler WP, Smith Scott DO. Mechanism of action of poly-L-lactic acid: a stimulatory dermal filler. J Drugs Dermatol. 2007;6 Suppl(1):18–20.
- Fitzgerald R, Vleggaar D. Facial volume restoration of the aging face with poly-L-lactic acid. Dermatol Ther. 2011;24(1):2–27.
- Vleggaar D, Fitzgerald R, Lorenc ZP. Satisfying patient expectations with poly-L-lactic acid soft tissue augmentation. J Drugs Dermatol. 2014;13(4 Suppl):s40–43.

