 J Clin Aesthet Dermatol. 2019;12(5):E61–E68
J Clin Aesthet Dermatol. 2019;12(5):E61–E68
by Deirdre Edge, PhD; Maiken Mellergaard, PhD; Carsten Dam-Hansen, PhD; Dennis Dan Corell, MSc; Joanna Jaworska, PhD; Giovanni Scapagnini, PhD; and Michael Canova Engelbrecht Nielsen, PhD
Drs. Edge, Mellergaard, and Nielsen are with the Department of Research and Development at FB Dermatology, Ltd. in Ballerup, Denmark. Mellergaard is also with IVH, Immunology at the University of Copenhagen in Frederiksberg, Denmark. Dr. Dam-Hansen and Mr. Correll are with the Department of Photonics Engineering, Technical University of Denmark, Roskilde, Denmark. Dr. Jaworska is with Klox Technologies, Inc. in Laval, Québec, Canada. Dr. Scapagnini is with the Department of Medicine and Health Sciences “V. Tiberio” at the University of Molise in Campobasso, Italy.
FUNDING: No funding was received for this study.
DISCLOSURES: Drs. Edge and Nielsen are employees of FB Dermatology, Ltd./Kleresca®. The other authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background.We have previously reported clinical efficacy with a novel form of photobiomodulation—a biophotonic platform inducing fluorescent light energy (FLE)—in both disease-affected and healthy skin; however, the cellular mechanisms remain largely unknown.
Objective. This study investigated the cellular mechanism of action of FLE on key skin and immune cells.
Methods. We examined the effects of FLE on the clinical presentation of inflammation in a representative patient with acne vulgaris. The effect of FLE and an FLE-mimicking control lamp on collagen production from primary human dermal fibroblast (HDF) cells was assessed in the presence and absence of the proinflammatory cytokine, interferon gamma (IFN-?). Cytokine production was assessed from HDF and human epidermal keratinocytes (HEK) exposed to M1 macrophage-conditioned media following illumination with either a blue light-emitting diode (LED) or FLE. Finally, the effects of FLE on angiogenesis were assessed in human aortic endothelial (HAE) cells.
Results. FLE reduced inflammatory lesions and associated redness in the representative acne patient. Following the resolution of inflammation there was an overall enhancement of the skin’s texture. FLE enhanced collagen production from nonstressed HDF cells, decreased the inflammatory profile of HDF and HEK cells, and enhanced angiogenesis in HAE cells.
Conclusion. Our results suggest FLE is capable of enhancing collagen production, modulating cutaneous inflammation, and encouraging angiogenesis . While further research is required, our findings have important implications for approaches to treating inflammatory skin conditions and achieving better aesthetic outcomes.
KEYWORDS: Acne, aesthetic, angiogenesis, anti-inflammatory, biophotonics, chromophore, collagen, cytokines, fibroblasts, fluorescent light energy, FLE, inflammatory skin conditions, LED, macrophages, photobiomodulation, rejuvenation, therapeutic \
The physiological effects and therapeutic potential of photobiomodulation (PBM) have been investigated in several tissues and cell types using various low-level energy light sources, including low-level lasers, light-emitting diodes (LED), and broadband visible light lamps.1 LED therapy inducing PBM in dermatology has expanded in recent years, with promising results reported, including slowing the aging process of skin, improving inflammatory skin conditions, and healing lesions.2 While the complete cellular and molecular mechanisms of PBM are not fully understood, it is thought to affect cellular metabolism, increase adenosine triphosphate (ATP), and modulate reactive oxygen species (ROS).3,4 A change in ROS has been shown to affect transcription factors responsible for growth, inflammation, and cellular proliferation and oxygenation, eventually culminating in augmented tissue repair.3,4
The Kleresca® biophotonic platform (FB Dermatology, Dublin, Ireland), referred to as KBP in this article, is based on fluorescent light energy (FLE), which is produced by excited light-absorbing chromophores when illuminated with a multi-LED lamp, offers a new approach in dermatology. FLE has been shown in clinical trials to modulate both disease-affected and healthy skin, decreasing inflammation and enhancing the skin’s overall texture.5–12 Despite the reported clinical efficacy of FLE, the underlying cellular mechanism of action of this biophotonic platform has yet to be elucidated.
Inflammation is fundamental in many skin conditions.13 Macrophages play a vital role in the inflammatory response and tissue repair process.14 To determine whether FLE can influence the inflammatory responses of cutaneous cells, cytokine concentrations were assessed in supernatant samples of cultured human fibroblasts and keratinocytes exposed to macrophage-conditioned media (MCM). Additionally, as many light therapies aim to boost collagen production, this study examined the ability of FLE to enhance collagen production from human dermal fibroblasts. Finally, we explored a role for FLE in modulating angiogenesis, a final step in many healing processes.
Materials and Methods
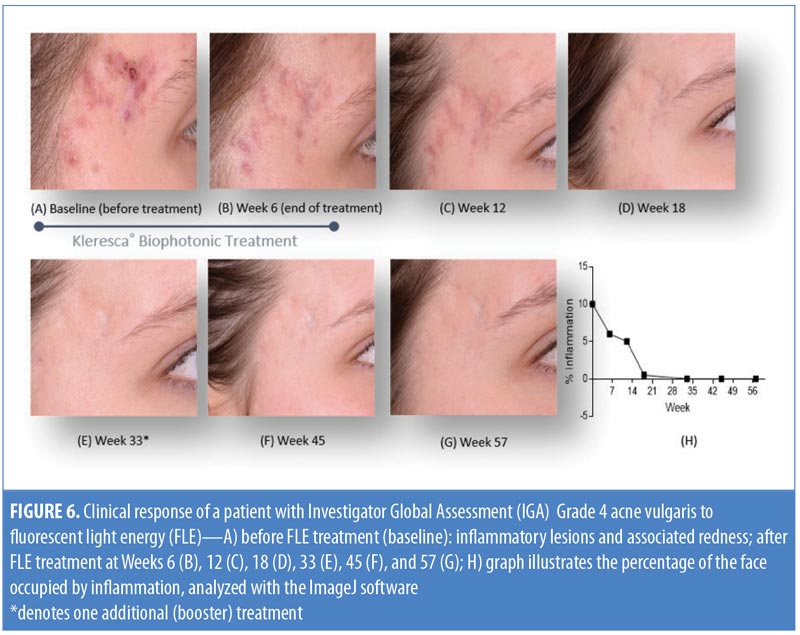
Clinical evaluation. A representative patient with acne vulgaris, (Grade 4, Investigator’s Global Assessment [IGA]) underwent treatment using the KBP (comprising a multi-LED lamp and proprietary chromophore-containing gel) twice a week for six weeks. For the treatment, a 2mm-thick layer of the chromophore-containing gel was applied to the clean face of the patient and immediately illuminated with the multi-LED lamp, applying approximately 30 to 40J/cm2 of blue light to the skin.7 This procedure was carried out with prior informed consent of the patient and was in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use’s Guidelines for Clinical Practice. Standardized photographs of the temple region of the patient’s face were taken before the treatment (baseline), at the end of the treatment period (Week 6), and at Weeks 12 and 18 from the start date. A single-session booster treatment was completed at Week 33, and photographs were taken at Weeks 45 and 57. The percentage of pixels occupied by inflammation (% inflammation) in each photograph of the facial skin was measured and expressed as a percentage of the total pixels per photograph (ImageJ; National Institutes of Health, Bethesda, Maryland).11
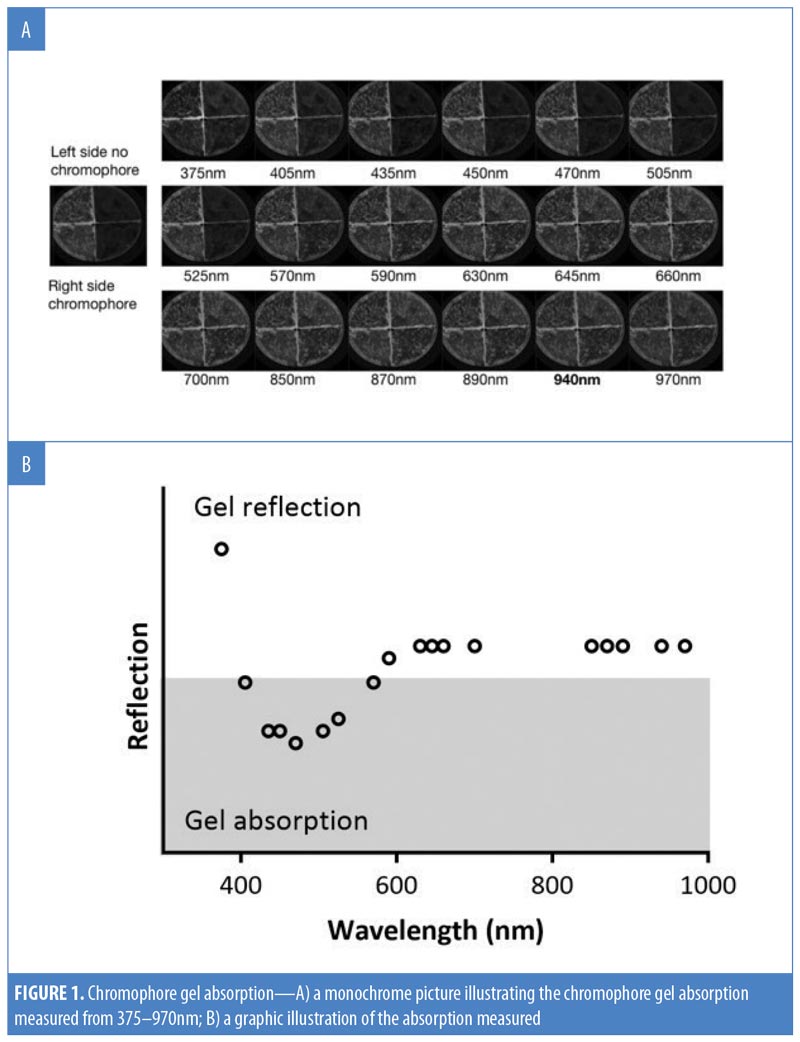
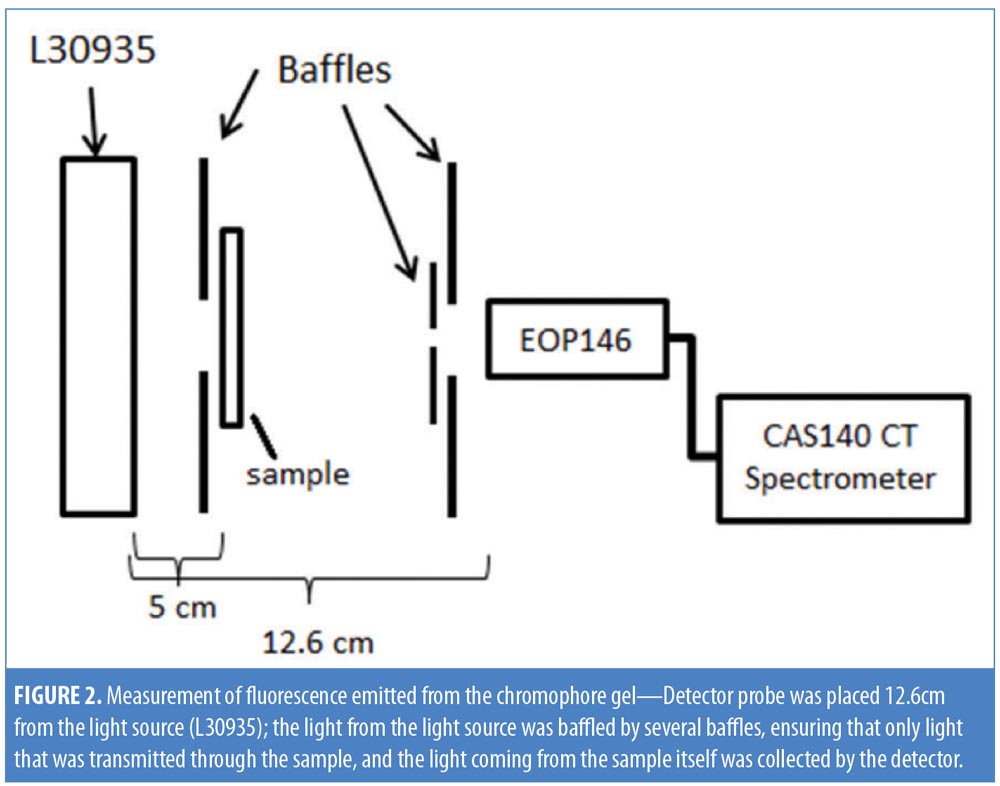
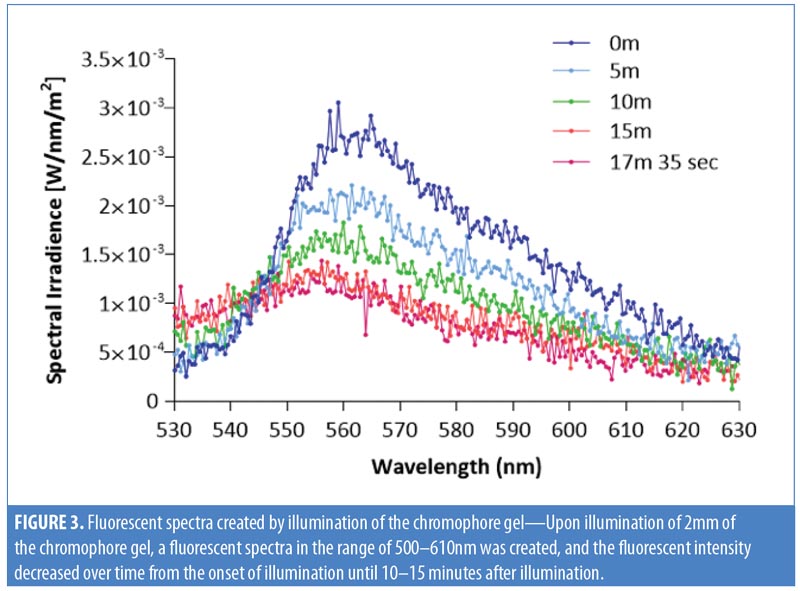
Chromophore gel measurement and mimicking lamp. Upon illumination, the K lamp delivers noncoherent blue light in continuous waves with a peak wavelength range of 440 to 460nm. The absorbance of the chromophore gel was measured using a multispectral imaging device (Videometer A/S, Hørsholm, Denmark) (Figure 1). The fluorescence emitted from the chromophore-containing gel was measured using a cosine-corrected optical probe (EOP-146; Instrument Systems, München, Germany) mounted 42mm above the output port of the setup. The optical probe was fiber-coupled to an array spectroradiometer (CAS140 CT; Instrument Systems, München, Germany), and the system was calibrated to measure the spectral irradiance from 356 to 830nm (Figure 2). Upon exposure to the blue LED light, the chromophore gel acts as a photoconverter, inducing the emission of FLE in a spectrum ranging from 500 to 610nm (Figure 3).
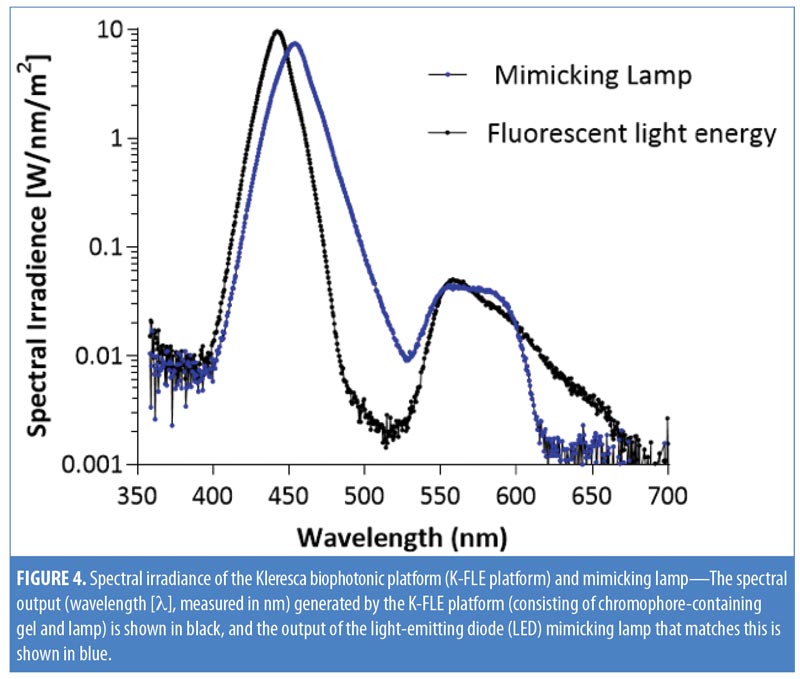
To recreate a control light source that mimicked the output from the KBP, we designed a mimicking lamp. The mimicking lamp had an identical spectral to the KBP, but instead of being generated by excited chromophore emissions combined with blue light, it was generated by continuous LED light. The blue light came from a single blue LED; the broader spectrum light, mimicking the chromophore emission spectrum, was spectrally filtered light from a cold white LED. Emission spectra were measured as above (Figure 4).
Cell culture. Primary normal human epidermal keratinocytes (HEK) (ATCC® PCS-200-011; American Type Culture Collection, Manassas, Virginia) were grown in dermal cell basal medium supplemented with a keratinocyte serum-free growth kit (ATCC® PCS-200-400; American Type Culture Collection, Manassas, Virginia). Human dermal fibroblasts (HDF) (ATCC® PCS-201-012; American Type Culture Collection, Manassas, Virginia) were grown in fibroblast basal medium supplemented with a low-serum fibroblast growth kit (ATCC® PCS-201-41; American Type Culture Collection, Manassas, Viginia). All cells were passed at 80-percent confluency and keratinocytes in the third or fourth passage were used.
Measuring collagen production. HDF cells (7.5×104 cells/mL) were seeded in a two-well chamber slide, in-vitro fertilization (IVF) multi-dish (Nunc 167063; Thermo Fisher Scientific, Waltham, Massachusetts). To test the effects of FLE on collagen production, three sets of cells were prepared: 1) nonilluminated HDF control group; 2) HDF exposed to the mimicking lamp group; and 3) HDF exposed to FLE group. Illumination was performed according to manufacturer’s instructions. Briefly, the cells were positioned 5cm from the light sources and illuminated with 30 to 40J/cm2 (Figure 5). A physical glass barrier was placed between the cell monolayer and the illuminating systems to ensure that any biological response observed was induced by the mimicking lamp or the FLE and not through any physical or chemical interaction. Following 48 hours of incubation, cell-free supernatants were collected and assessed for total soluble collagen production using the Sircol™ Soluble Collagen Assay (Biocolor Ltd., Carrickfergus, United Kingdom), according to the manufacturer’s instructions. This was carried out on two separate occasions, and the results were pooled.
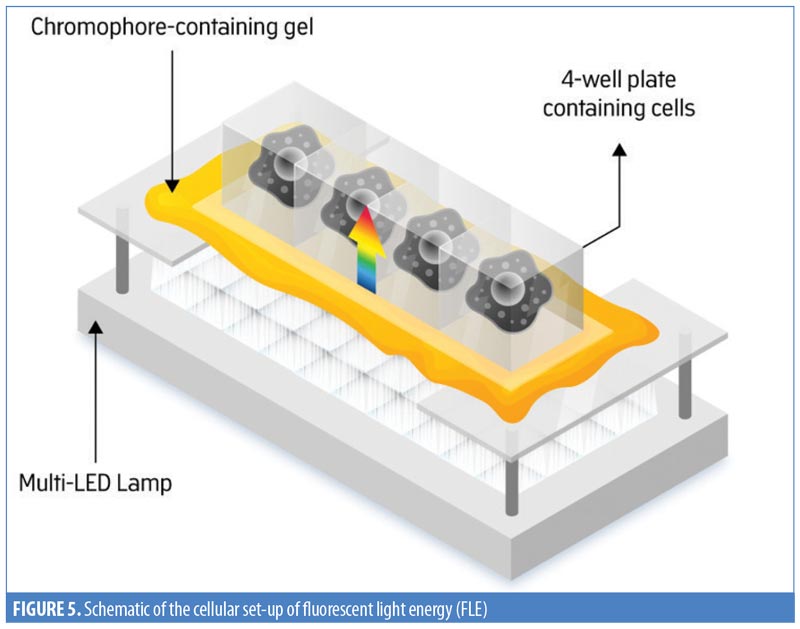
One subset of HDF slides (seeded at 7.5×104 cells/mL) was treated with 300U/mL of interferon gamma (IFN-?) human recombinant (R&D Systems, Minneapolis, Minnesota). These cells were exposed to either the mimicking lamp (control) or FLE. The collagen assay was subsequently carried out.
Macrophage conditioned media preparation. CD14+ monocytes were isolated from fresh, normal human peripheral blood mononuclear cells (PBMC), purchased from ZenBio (SER-PBMC-200; 200×106 cells/vial; Durham, North Carolina), using CD14 microbeads, human (30-050-201; Miltenyi Biotec, Bergisch Gladbach, Germany), and differentiated into macrophages according to the manufacturer’s protocols. Macrophage cells were then stimulated with lipopolysaccharide (LPS) (10ng/mL; L2630, Sigma-Aldrich, St. Louis, Missouri) and 50ng/mL of recombinant IFN-? for three days, polarizing them toward an M1 phenotype.15 MCM was subsequently collected and stored at -80°C until required to test the effect of inflammatory mediators on the activity of HDF and HEK cells.
Stimulation of fibroblast and keratinocyte cells with MCM. Prior to exposing the HEK and HDF cells to the collected MCM, HEK at 35,000 cells/mL per well were seeded in four-well culture glass slides (Corning® Biocoat™, Corning, New York, New York) and HDF at 75,000 cells/mL per well were seeded in two-well chamber slides for adherence overnight. Next, cells were stimulated with 600?L of MCM overnight at 37°C, before the media was removed and replaced by phosphate-buffered saline (PBS) prior to illumination. The cells were divided into three groups: 1) a nonilluminated control group; 2) a blue-LED lamp group; and 3) an FLE group. Subsequently, cells were cultured with fresh media, supernatants were collected after six and 24 hours, and cytokine release was assessed using enzyme-linked immunosorbent assay (ELISA) including human tumor necrosis factor alpha (TNF-?) (DuoSet TNF-? Human ELISA Kit; Invitrogen™, Waltham, Massachusetts) and human interleukin (IL)-6 (R&D Systems Inc., Minneapolis, Minnesota). Fold changes of cytokine concentrations were calculated and compared with nonilluminated controls, and data were averaged from three independent experiments.
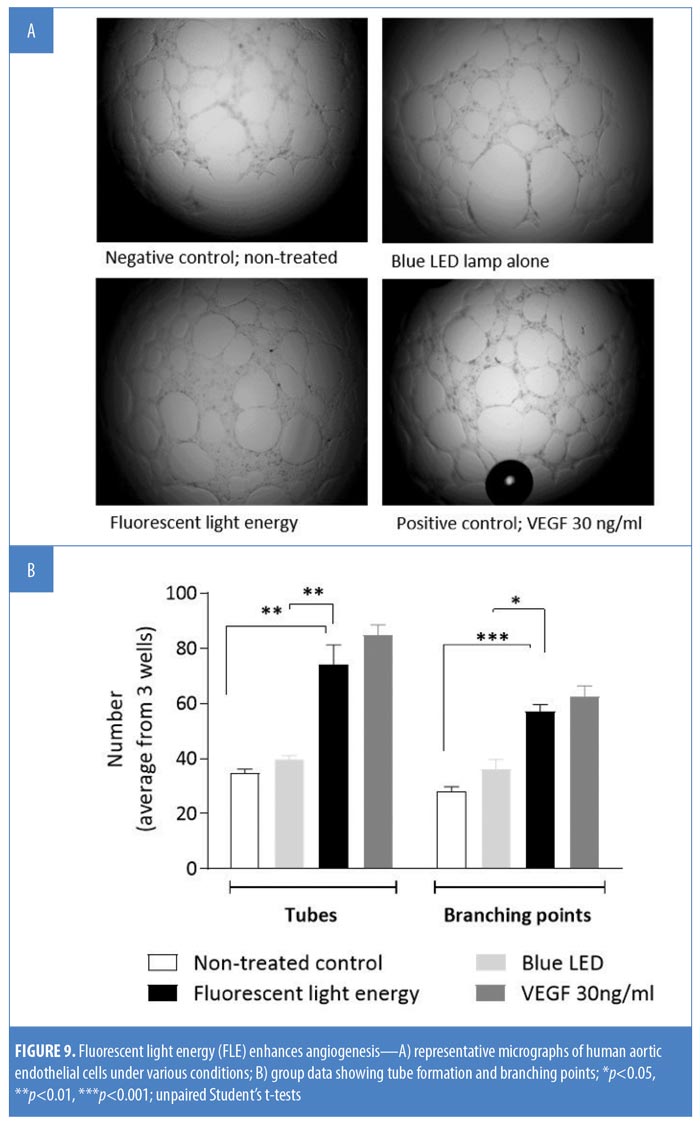
Angiogenesis and tube formation in human endothelial cells. HDF cells were illuminated with blue LED light only or with FLE. Seventy-two hours later, conditioned media (CM) was taken to assess new tube formation and the branching of human aortic endothelial cells (HAEC). HAEC were seeded (0.3×105/well) onto a Matrigel® layer (Corning, New York, New York) in a 96-well plate. Following cell adhesion, cell culture medium was replaced by 250?L of CM. HAEC were incubated in the CM for 18 hours at 37°C with 5% CO2; a negative control test and a positive control test using vascular endothelial growth factor (VEGF 30ng/mL), were run in parallel. Three images per well were taken and assessed for tube formation and branching points using an inverted microscope (Olympus IX50; Olympus, Tokyo, Japan). Group data were averaged from three images per well from three wells per condition.
Statistics. Data are expressed as means±standard deviations (SD) and analyzed using a one-way analysis of variance (ANOVA) or Student’s t-test where appropriate using the GraphPad Prism® Version 7 software (GraphPad Software, La Jolla, California. All samples were compared to controls, and experiments were performed up to three times in duplicate or triplicate. A p-value less than 0.05 was deemed significant in all cases.
Results
Clinical evaluation following FLE treatment. Figure 6 shows a woman with acne vulgaris, IGA Grade 4, which is representative of an inflammatory skin condition. A photo taken before treatment (Figure 6A) shows inflammatory lesions on the temple. There was a marked reduction in redness following six weeks of treatment with the KBP (Figure 6B). The redness and percentage of inflammation was resolved at the Week 18 time point (Figure 6D) and was maintained for the remainder of the study (Figures 6E–6H). As the inflammatory area was improving over time (Week 12 onward), there was a concurrent decrease in visible scarring (Figures 6E–6G). The treating practitioner chose to provide a single booster treatment at Week 33, after which the results were maintained to Week 52, demonstrating that the overall stress and inflammatory level was reduced significantly, thus suggesting that small manipulations over time might support a continuous improvement of a cyclic inflammatory skin condition.
Spectral irradiance of KBP and mimicking lamp. The spectral output (nm) generated by KBP ranged from 415 to 700nm, covering the blue, green, yellow, orange, and red wavelengths of the visible spectrum. The spectral output of the mimicking lamp matched this (Figure 4).
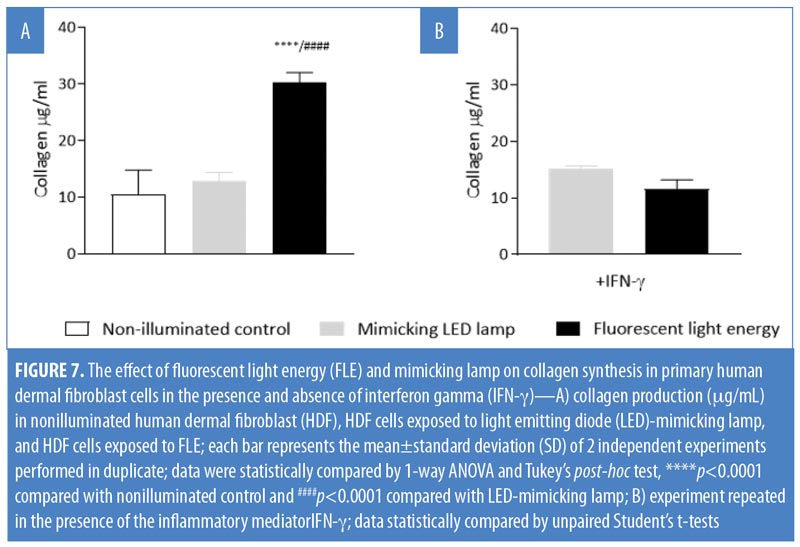
FLE increased collagen production. Exposure of HDF cells to the mimicking lamp did not affect collagen production in fibroblasts (p>0.05). Collagen production in fibroblasts treated with FLE was significantly increased compared with nonilluminated control cells and cells treated with the mimicking lamp (p<0.0001, Figure 7A). HDF cells pretreated with IFN-? did not significantly increase their collagen production when exposed to FLE versus cells exposed to the mimicking lamp alone (p>0.05; Figure 7B). In subsequent experiments, the blue LED (not the mimicking lamp) was used as an appropriate control, since there was no difference in the response between them. Additionally, it was more convenient to work with the blue LED due to its relatively small size and simple set-up.
FLE modulates the release of TNF-? and IL-6. Both HDF and HEK cells were exposed to MCM before being nonilluminated, treated with blue LED alone, or exposed to FLE. Both blue LED (p<0.05) and FLE treatment (p<0.0001) significantly reduced TNF-? release from HDF cells relative to control HDF cells (Figure 8A). FLE significantly reduced TNF-? release from HDF cells compared with blue LED treated HDF cells (p<0.01; Figure 8A). TNF-? release from HEK cells was not affected by blue LED treatment alone compared with nonilluminated control cells (p>0.05; Figure 8C). FLE-treatment decreased the production of TNF-? from HEK cells compared with nonilluminated control cells (p<0.01) and blue LED-treated cells (p<0.05; Figure 8C).
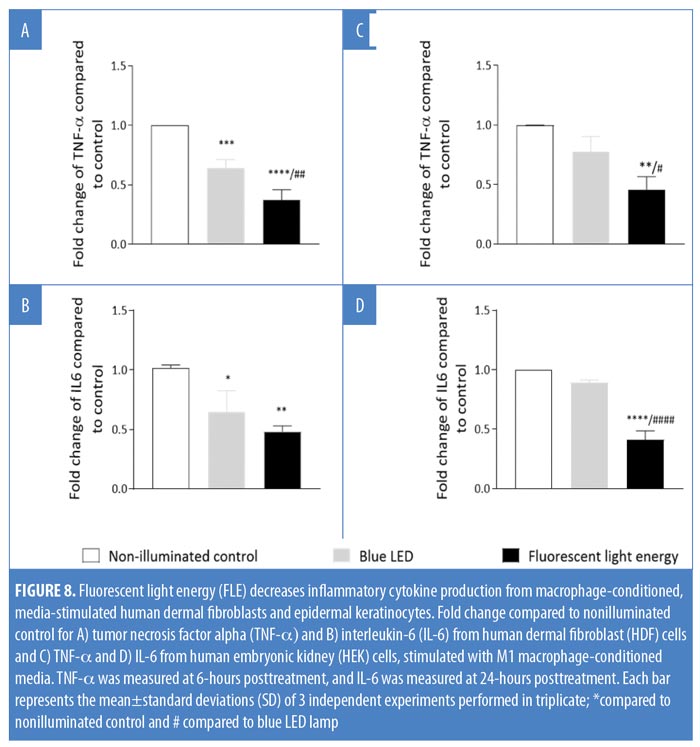
IL-6 release was significantly reduced from HDF cells following both blue LED light (p<0.02) and FLE treatments compared with nonilluminated control cells (p<0.01; Figure 8B). IL-6 release was not significantly different between blue LED or FLE-treated HDF cells (p>0.05, Figure 8B). IL-6 release from HEK cells was not affected by blue LED light treatment alone compared with nonilluminated control cells (p>0.05). However, FLE treatment decreased IL-6 release from HEK cells in comparison with nonilluminated control cells (p<0.0001) and blue LED-treated cells (p<0.0001, Figure 8D).
FLE enhances angiogenesis. Exposing HAEC to VEGF significantly increased both microvascular tube and branching formation compared with nontreated controls (p<0.05); blue LED alone did not affect either tube formation (p>0.05) or branching (p>0.05). Treatment of HAEC with CM significantly increased both branching and tube formation compared with nonilluminated control cells (p<0.01 and p<0.001) or blue LED alone (p<0.01 and p<0.05, Figure 9B).
Discussion
This study evaluated how FLE modulates cellular activity that underlyies the observed improvements in inflammatory dermatological conditions5,7,9,10 and rejuvenation of the skin using this treatment.6 We describe the mechanisms of action behind a technology used on a representative patient with acne vulgaris (IGA Grade 4) with visible redness and inflammatory lesions. A persistent resolution of inflammation and associated redness was observed after six weeks of exposure to FLE. There was also a visible improvement in the skin’s texture and a concomitant fading of existing scars. Previous clinical work with FLE has produced reports of significant decreases in inflammation and associated lesions in moderate and severe acne vulgaris;5,7 reductions in the appearance of fine lines and wrinkles as a stand-alone rejuvenation therapy,6 or normalization of the skin’s structure postlaser treatment for solar lentigines;11 and improvements in the signs and symptoms of rosacea.9,10 Together, these clinical findings highlight the broad application and utility of FLE.
Although not fully elucidated, various mechanisms underpinning the beneficial clinical outcomes observed with PBM have been reported.4,16–18 Of interest is the ability of PBM to modulate inflammation, alter cellular activation, modulate collagen synthesis, and enhance blood flow.19 We investigated a role for these key biomolecular mechanisms using FLE.
To reduce signs of aging in the skin, many modalities typically induce a controlled form of wounding in the skin’s epidermis to promote the induction of new collagen biosynthesis.17 Studies using LEDs have reported promising results in skin rejuvenation by enhancing collagen production without inducing prior damage.17 In our study, FLE enhanced collagen production from HDF cells compared with nonilluminated control fibroblasts. Moreover, exposing HDF cells to the LED-mimicking lamp, which matched the spectral output of the KBP, did not significantly alter collagen production. These results suggest the FLE irradiating from the gel induces this biomolecular alteration.
Multiple wavelengths of light in the visible spectrum can penetrate the skin and activate endogenous chromophores.20 Intuitively, one might think that combining wavelengths will lead to an enhanced effect. Indeed, this has been demonstrated by some investigators in the treatment of acne vulgaris.21 However, for collagen synthesis, there is still some debate in the literature on whether combining wavelengths will have a beneficial effect.22
Pulsing wavelengths of light, as opposed to continuous wavelengths, seem to be favourable for collagen production.23 The photoconversion of the gel leads to the production of a dynamic, hyperpulsed, multiwavelength of fluorescent energy through the phenomenon of Stokes shift.8 This pulsing of light appears to be the key to enhancing collagen production by the illuminated fibroblasts, as the mimicking lamp with the same spectral output failed to alter fibroblastic collagen production. Theories for enhanced collagen production with PBM suggest cytochrome c activation increases mitochondrial energy production and leads to the downstream activation of genes for collagen synthesis.16 While our results show a clear differential between FLE and a comparable LED light, the mechanism of enhanced collagen production remains to be elucidated.
Inflammation is an underlying feature of many dermatological indications13 and affects normal cellular function. Prior exposure of HDF to the proinflammatory cytokine IFN-? interfered with the ability of FLE-exposed fibroblasts to increase their collagen production. In our clinical case, there was an improvement in visible scarring following the resolution of redness and inflammatory lesions. This is likely due to an enhancement of collagen production after the attenuation of inflammation. These findings bolster the KBP’s potential for use in both therapeutic inflammatory indications and aesthetic purposes, including normalization of the skin before or after treatments that cause inadvertent inflammatory responses.
The cutaneous environment is complex. Fibroblasts and other mesenchymal cells can respond to both pattern- and damage-associated molecular pathogens (i.e., PAMPs and DAMPs), as well as inflammatory cytokines released by resident macrophages.24,25 Indeed, chronic skin inflammation is often triggered and maintained by the production of a variety of cytokines and chemokines, such as TNF-? and IL-6.26–28 We found that both fibroblasts and keratinocytes exposed to MCM from M1-like macrophages altered their cytokine production following exposure to FLE. HDF cells responded to both LED treatment and FLE with a reduced output of both TNF-? and IL-6. For TNF-?, FLE decreased TNF-? production further compared with treatment with blue LED. Conversely, keratinocytes only responded to treatment with FLE. Taken together, these results suggest that the FLE has an overall anti-inflammatory effect; however, further research is required.
Differential effects of PBM on cytokine production have been reported.18 Although PBM therapy is noted for its clinical anti-inflammatory effects, it has been reported to activate nuclear factor kappa-light-chain-enhancer of activated B-cells—the master regulator of inflammation in normal, quiescent cells.29 However, decreased inflammatory responses from various cell types pretreated with proinflammatory cytokines have also been reported.30–32 As the skin is in a proinflammatory state in many conditions, light treatment might be effective in reducing this response. Additionally, these findings support the theory that, depending on the microenvironment of the skin, PBM can have differential effects (i.e., therapeutic and aesthetic). Our findings support this concept.
Macrophages play an integral role in the immune response. Upon recruitment from monocytes, they can become a critical local source of various mediators, including matrix metalloproteinases, cytokines, and chemokines.24 They can have a dual reciprocal function and can be pro-(M1) or anti-inflammatory and reparative (M2), depending on the local environment.33
FLE might, theoretically alter the cutaneous macrophage phenotype, polarizing these cells towards an M2 phenotype. This transition is known to be a key step in the process of normal tissue repair.24 Indeed, some investigators have reported the capacity of PBM to alter macrophage phenotype,18 which would have many implications for cutaneous inflammation. Future work will focus on whether FLE can modulate immune metabolism.
A key and final phase of the healing process is neovascularization (i.e., angiogenesis) from existing blood vessels. PBM has been reported to increase angiogenesis in cutaneous wounds in vivo.34 In our study, FLE increased both branching points and tubes in HAECs, which is indicative of angiogenesis. The angiogenic response was relative to that of cells stimulated with VEGF, a potent angiogenic factor. The ability of FLE to induce angiogenesis correlates with the latent, persistent, clinical inflammatory resolution and collagen build-up observed in the presented case and several other studies.9,10,12 Interestingly, this was an indirect effect induced by the media taken from the HDF cells exposed to FLE. While it is not clear how the KBP induced an angiogenic response, it is clear that immune cells can release a variety of angiogenic factors.35 Specifically, M2 macrophages are a key source of VEGF and can enhance cellular proliferation.24 If FLE can polarize cutaneous macrophages towards the M2 phenotype, this might explain the angiogenesis observed. The ability of FLE to induce healthy vasculature and a normalized, destressed environment in the skin could be of benefit in treating rosacea by improving blood distribution through enhanced lateral blood flow and attenuating erythema and blushing.
Conclusion
While further research is required to elucidate the KBP’s complete mechanism of action, in our study, we report its capacity to enhance fibroblastic collagen production, attenuate the inflammatory signature of a variety of cutaneous cells, and enhance angiogenesis—all contributing to normalizing and destressing the skin. This platform was superior to an equivalent mimicking nonfluorescent light and to conventional blue LEDs. These findings are relevant to a variety of inflammatory skin indications and can offer adjunct support to more invasive dermatological approaches.
Acknowledgments
The authors would like to thank the study participant who took part in this representative clinical case study. We would also like to thank Dr. Emmanuelle Devemy and Dr. Fethia Benyebdri from Klox Technologies, Inc. for their help with data generation and management and Ms. Anne Downes from FB Dermatology Ltd./Kleresca®, who copyedited and proofread the manuscript.
References
- Jagdeo J, Austin E, Mamalis A, et al. Light-emitting diodes in dermatology: a systematic review of randomized controlled trials. Lasers Surg Med. January 2018. [Epub ahead of print].
- Ablon G. Phototherapy with light emitting diodes: treating a broad range of medical and aesthetic conditions in dermatology. J Clin Aesthet Dermatol. 2018;11(2):21–27.
- Chung H, Dai T, Sharma S, et al. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2): 516–533.
- Hamblin MR. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol. 2018;94(2):199–212.
- Antoniou C, Dessinioti C, Sotiriadis D, et al. A multicenter, randomized, split-face clinical trial evaluating the efficacy and safety of chromophore gel-assisted blue light phototherapy for the treatment of acne. Int J Dermatol. 2016;55(12):1321–1328.
- Nikolis A, Bernstein S, Kinney B, et al. A randomized, placebo-controlled, single-blinded, split-faced clinical trial evaluating the efficacy and safety of KLOX-001 gel formulation with KLOX light-emitting diode light on facial rejuvenation. Clin Cosmet Investig Dermatol. 2016;9:115–125.
- Nikolis A, Fauverghe S, Scapagnini G, et al. An extension of a multicenter, randomized, split-face clinical trial evaluating the efficacy and safety of chromophore gel-assisted blue light phototherapy for the treatment of acne. Int J Dermatol. 2018;57(1):94–103.
- Jalili A. Chromophore gel-assisted phototherapy. J für Ästhetische Chir. 2018:1–5.
- Braun SA, Gerber PA. A photoconverter gel-assisted blue light therapy for the treatment of rosacea. Int J Dermatol. 2017;56(12):1489–1490.
- Sannino M, Lodi G, Dethlefsen MW, et al. Fluorescent light energy: treating rosacea subtypes 1, 2, and 3. Clin Case Reports. 2018;6(12):
2385–2390. - Scarcella G, Dethlefsen M, Nielsen M. Treatment of solar lentigines using a combination of picosecond laser and biophotonic treatment. Clin Case Reports. 2018;6(9):1–3.
- Mahendran A, Wong XL, Kao S, Sebaratnam DF. Treatment of erlotinib induced acneiform eruption with chromophore gel-assisted phototherapy. Photodermatol Photoimmunol Photomed. 2018 Dec 16. [Epub ahead of print].
- Houh YK, Kim KE, Park HJ, Cho D. Roles of erythroid differentiation regulator 1 (Erdr1) on inflammatory skin diseases. Int J Mol Sci. 2016;17(12):1–10.
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723.
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6(March):1–13.
- de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light Therapy. IEEE J Sel Top Quantum Electron. 2016;22(3): 348–364.
- Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52.
- Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361.
- Chung JH, Seo JY, Choi HR, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001;117(5):1218–1224.
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8(6): 36–44.
- Lee SY, You CE, Park MY. Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg Med. 2007;39(2):180–188.
- Barolet D. Photobiomodulation in dermatology: harnessing light from visible to near infrared. Med Res Arch. 2018;6(1).
- Barolet D, Roberge CJ, Auger FA, et al. Regulation of skin collagen metabolism in vitro using a pulsed 660nm LED light source: clinical correlation with a single-blinded study. J Invest Dermatol. 2009;129(12):2751–2759.
- Mescher AL. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration. 2017;4(2):39–53.
- Nowarski R, Jackson R, Flavell RA. The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell. 2017;168(3):362–375.
- Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-? (TNF-?) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004;279(31):
32633–32642. - Hernández MV, Meineri M, Sanmartí R. Skin lesions and treatment with tumor necrosis factor alpha antagonists. Reum Clin. 2013;9(1):53–61.
- Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2(4):288–294.
- Chen ACH, Arany PR, Huang YY, et al. Low-level laser therapy activates NF-?B via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6(7):e22453. doi:10.1371/journal.pone.0022453
- Yamaura M, Yao M, Yaroslavsky I, et al. Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg Med. 2009;41(4):282–290.
- Hwang MH, Shin JH, Kim KS, et al. Low level light therapy modulates inflammatory mediators secreted by human annulus fibrosus cells during intervertebral disc degeneration in vitro. Photochem Photobiol. 2015;91(2):403–410.
- Chen ACH, Huang YY, Sharma SK, Hamblin MR. Effects of 810-nm laser on murine bone-marrow-derived dendritic cells. Photomed Laser Surg. 2011;29(6):383–389.
- Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20.
- de Sousa APC, Paraguassú GM, Silveira NTT, et al. Laser and LED phototherapies on angiogenesis. Lasers Med Sci. 2013;28(3):981–987.
- Granata F, Frattini A, Loffredo S, et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol. 2010;184(9):5232–5241.

