 by Ricardo Augusto Sandoval Vasquez, MD; Kelly Park, MD, MSL; Katherine Braunlich, DO; Shino Bay Aguilera, DO
by Ricardo Augusto Sandoval Vasquez, MD; Kelly Park, MD, MSL; Katherine Braunlich, DO; Shino Bay Aguilera, DO
Dr. Vasquez is with the Department of Esthetic Medicine at Rosario University in Bogota, Colombia. Dr. Park is with the Section of Dermatology in the Medicine Service Line at Edward Hines Jr. VA Hospital in Hines, Illinois. Dr. Braunlich is with the Department of Dermatology at Largo Medical Center in Largo, Florida. Dr. Bay is with Shino Bay Cosmetic Dermatology & Laser Institute in Fort Lauderdale, Florida and the Department of Dermatology at Nova Southeastern University College of Osteopathic Medicine in Fort Lauderdale, Florida.
ABSTRACT: The injection of hyaluronic acid (HA), a naturally occurring biopolymer, is a common cosmetic procedure. Despite their efficacy and growing adaptation by the medical community, HA fillers occasionally give rise to adverse events. Adverse events from HA fillers range from temporary, such as edema and erythema, to more long-term effects, including granulomas or, in rare cases, sequelae from vascular occlusion. Here, we present a case of a 61-year-old Caucasian woman with prolonged infraorbital hollow edema after injection of HA filler for nasojugal groove correction. We review the anatomy of the nasojugal area and differing injection techniques as a possible explanation for the development of prolonged edema. Similarly, the rheological properties of the specific hyaluronic acid used during this procedure might be crucial in the development of this complication.
FUNDING: No funding was provided for this study.
DISCLOSURES: Dr. Bay is a trainer and speaker for Merz, Galderma, and Allergan. Drs. Vasquez, Park, and Braunlich have no conflicts of interest related to the content of this article.
KEYWORDS: Filler complications, fillers, hyaluronic acid, nasojugal, tear trough
J Clin Aesthet Dermatol. 2019;12(9):32–35
The face, particularly the periorbital region, reflects emotions, dispositions, and the aging process.1 As a result, this area garners special attention from the aesthetic point of view.2 According to the American Society of Plastic Surgery, aesthetic procedures around the eyes represent the fourth most common motivation for a patient to seek cosmetic intervention.3
There are a variety of nonsurgical and surgical modalities currently available for the modification of the periorbital region. Injection of hyaluronic acid (HA) filler is recognized as a safe and effective option for improving the appearance of the periorbital region, with excellent results for correction of the tear troughs and nasojugal grooves.4 The use of fillers for aesthetic purposes has increased dramatically over the years. In 2014, 1.9 million nonsurgical procedures were performed using filler injections.5 HA fillers play an integral part in the correction of age associated changes, particularly in the mid and lower face.6 Due to its biocompatibility, relative safety, stability, reversibility, and ease of administration, hyaluronic acid is now one of the most commonly utilized fillers for treating the aging face.6
However, despite the many favorable properties of hyaluronic acid, injection of HA filler is not without possible sequelae. To determine the best HA filler for a patient, a review of the different characteristics of hyaluronic acid is necessary. HA, also known as hyaluronan, is a glycosaminoglycan that consists of repeating non-sulfated disaccharide units of N-acetylglucosamine and glucuronic acid.6 In contrast to other glycosaminoglycans, HA has no defined shape, retains water, and integrates into the tissue where it is injected.7 Tissue integration occurs through electrostatic repulsion created by the negatively charged carboxyl groups of the molecule, favoring minimal tissue reactivity.8 The highly hydrophilic nature of HA is typically an advantageous property enabling HA fillers to withstand compressive forces. Conversely, the hydrophilic nature of HA also translates into a propensity for these fillers to occupy a large volume relative to their mass and their potential to cause chronic edema. That being said, the technique of HA injection largely determines the results and adverse reactions that occur.9 It is probable, however, that some adverse reactions are the result of individual anatomic variations or the inherent properties of the HA filler selected.
Adverse events from HA fillers range from temporary, such as edema and erythema, to more long-term effects, including granulomas or, in rare cases, sequelae from vascular occlusion.10 While edema ordinarily resolves several days post-injection, prolonged edema is occaisonally noted, particularly following HA filler injected into the infraorbital hollows.
Case Report
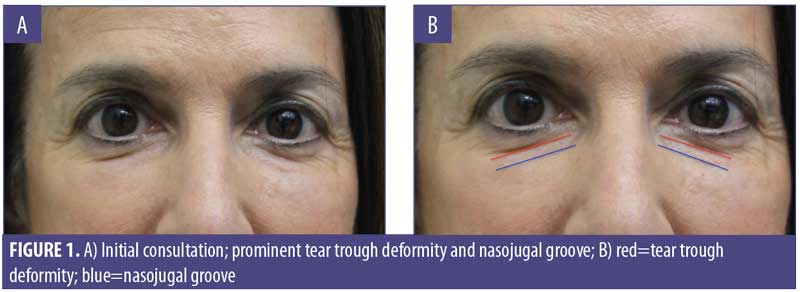
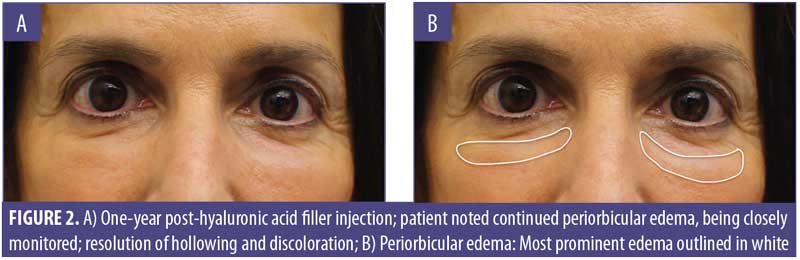
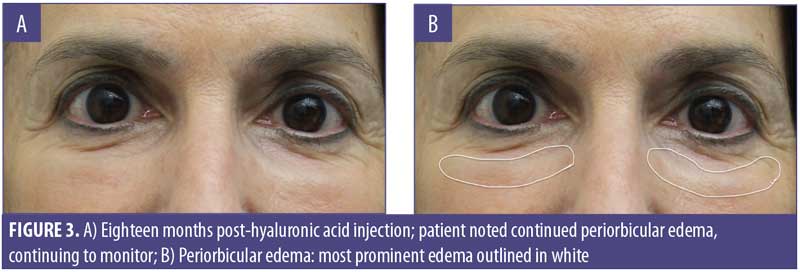
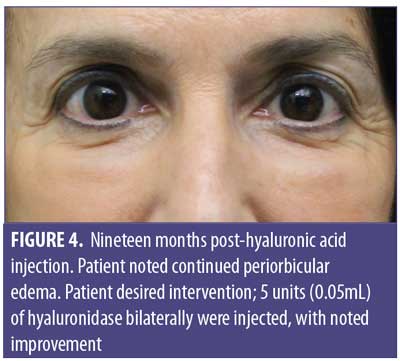
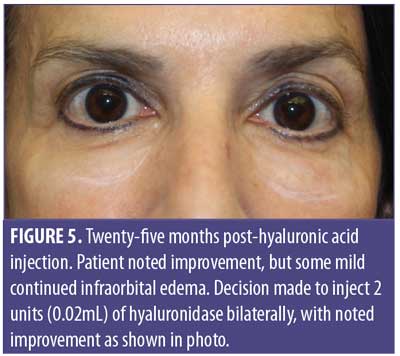
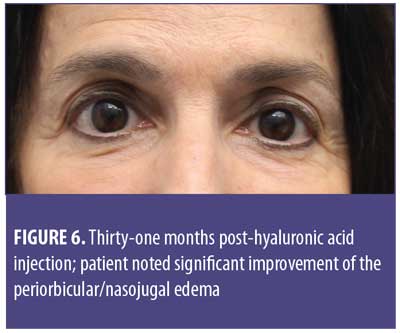
A 61-year-old Caucasian woman presented to our dermatology clinic for cosmetic consultation. The patient’s primary concerns included dark circles and etched lines in the infraorbital region (Figures 1A, 2B). Due to the prominence of her tear trough deformity and nasojugal groove, it was decided her cosmetic concerns could be improved with HA filler. One syringe (1mL) containing stabilized hyaluronic acid, 20mg/mL in phosphate buffered saline, pH 7 and 0.3% lidocaine (Restylane Silk®; Galderma, Lausanne, Switzerland) was injected to correct the patient’s cosmetic concerns. The patient tolerated the procedure well and there were no immediate complications. However, over the next few months, the patient noted development of infraorbital edema most prominent in the nasojugal region. At one-year follow up, the patient noted persistence of this edema (Figures 2A and 2B). The patient was informed this was not an isolated case and, while rare, had developed in other patients after HA filler was used to correct tear trough deformities or nasojugal grooves. The patient was asked if close monitoring would be acceptable, and the patient obliged. Eighteen months after initial HA injection, the patient returned with continued infraorbital edema. Again, the decision was made to monitor for resolution (Figures 3A and 3B). Nineteen months after initial HA injection, the patient noted persistent edema and requested intervention. Five units (0.05mL) of hyaluronidase (Vitrase®; Bausch + Lomb, Rochester, New York) was injected bilaterally in the area of greatest nasojugal edema, with noted improvement (Figure 4). Twenty-five months after initial HA injection, the patient returned with continued mild edema. The patient was injected with two units (0.02 mL) of hyaluronidase, bilaterally, in the area of greatest nasojugal edema, with noted improvement (Figure 5). Thirty-one months after the initial HA injection, the patient returned with significant improvement of her edema and declined further treatment (Figure 6). Ultimately, some subtle clinical findings persist despite the passage of time and appropriate intervention. At the time of this article’s publication, no further intervention has been performed, as the patient has expressed satisfaction with her outcome.
Discussion
Effective facial rejuvenation using fillers requires knowledge of facial anatomy, rheological properties of fillers, and injection technique. Variations among these three fundamental themes can elucidate the disparities and rare complications reported in the literature and in clinical practice. Adverse events in the periorbital region following HA filler injection include persistent edema, Tyndall effect, ecchymosis, contour irregularities, inflammation, granuloma formation, and, rarely, ischemic compromise.11 Our case highlights prolonged edema in the infraorbital region after HA injection for the correction of the nasojugal groove. This might have occurred as a result of several factors, including variation in our patient’s anatomy, the hydrophilic properties of our chosen HA filler, or injection technique.
A robust understanding of facial anatomy is imperative for effective and safe treatment of the aging face. Many studies have previously described the nasojugal groove and tear trough deformity as the same entity. Lee et al12 effectively highlighted the clinical and anatomical differences of these two regions. This distinction can change how grooves and hollows in the infraorbital region are treated. The nasojugal groove occurs in the infraorbital region and corresponds with the inferior border of the orbicularis oculi muscle.12 The nasojugal groove is located slightly inferior and oblique to the tear trough deformity and is characterized by a natural depression that extends inferolaterally from the medial cheek to the mid-pupillary line.12,13
Facial aging brings about changes in the anatomical distribution of the orbicularis retaining ligament which is a fixation point for the orbicularis oculi, and resultant convexity of the central third of the face. Additional anatomical considerations of the aging face include herniation of the intraorbital fat, descent of infraorbital fat pads, atrophy of the subcutaneous tissue and skin, and malar bone resorption.12 Treatment should restore volume and address each of the aforementioned components as a unit.
Familiarity with the rheology of HA fillers is fundamental for facial rejuvenation procedures. Sound understanding of rheological properties helps prevent secondary events, such as edema. Differences in HA properties include HA concentration, elasticity, viscosity, cohesivity, and cross-linking.
The concentration of HA, often expressed in mg/mL, is proportional to the quantity of cross-linked (insoluble) HA and free (soluble) HA.14 Cross-linked HA gel is known to resist enzymatic degradation, extending the duration of a filler’s action. Free HA is readily metabolized and facilitates ease of extrusion.14 Consequently, providers must understand filler content in regards to cross-linked HA versus free HA. Additionally, gels can be equilibrated or nonequilibrated, a process used to hydrate the gel. Nonequilibrium gels tend to swell post-injection.14,15
HA is considered a viscoelastic gel. The viscosity relates to the ability of HA to deform during injection, while elasticity allows HA to regain its original form.16 Together, these components make HA an essentially ideal filler. Viscoelasticity is further subdivided into shear modulus (G*), energy required to deform; G prime or elastic modulus (G’), the energy fraction of G* restored after deformation; and viscous modulus (G”), the energy fraction of G* lost after deformation.17 HA fillers with a high G’ provide greater resistance to movement and thus, are more likely to retain their shape. HA fillers with a high G’ are ideal for deep injections with the goal of lift, but less ideal in areas of high muscle movement.
Cohesivity is the internal adhesion force that binds cross-linked HA.16 The greater the cohesivity, the more likely the filler is to keep its shape. As a result, filler with low cohesivity will provide less lift than a gel with high cohesivity. This becomes important when considering injection depth. In general, cross-linking of HA strands increases product firmness. As a result, G* and G’ increase with increased cross-linking and cohesivity.16 Additionally, lower cohesiveness might lead to fragmentation of HA and increase the effect of gravitational pull on the product. Several products, such as Restylane Refyne® (Galderma, Lausanne, Switzerland), Juvederm Volbella® and Vollure® (Allergan, Dublin, Ireland), and Belotero Balance® (Merz Pharma GmbH & Co. KGaA, Frankfurt, Germany) have shown ideal cohesivity properties.17,18,19 Ultimately, the optimal filler is determined by careful consideration of the properties that influence performance and clinical experience. A thorough knowledge of HA gel properties is fundamental for proper filler selection, outcome, and meeting patient expectations.14
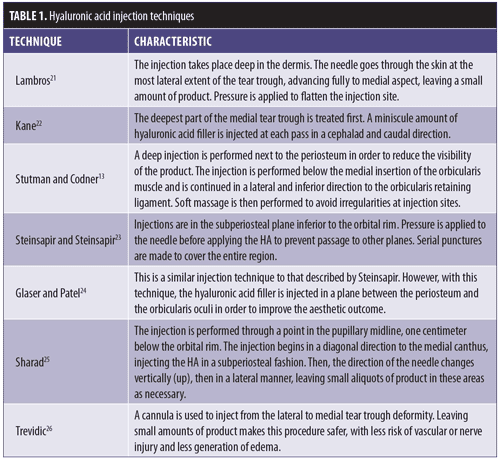
Generally speaking, augmentation of the infraorbital region with HA filler should follow specific placement technique. HA filler should be injected below the orbicularis retaining ligament, as a more superior injection might lead to migration of the HA and prolonged edema.13,20 There are multiple techniques for HA injection for the correction of the nasojugal groove (Table 1).
The injection technique used might favor the development of edema.27 Injection in the correct anatomical location is fundamental in all injection techniques. The development of injection devices, such as cannulas, might provide physicians the opportunity to feel resistance of the orbicularis retaining ligament and avoid a more superior injection.28 The optimal technique is one that requires the smallest amount of product.29
In the case presented here, the patient developed prolonged periorbicular edema despite the injector’s strong foundation in anatomy, clinical experience, and appropriate choice of HA filler for the anatomic region. Thus, it is our belief that this case of prolonged edema might be the result of the particulate, hydrophilic nature of the selected filler. The latter might result in increased gravitational pull, product in unwanted areas, and/or longstanding fluid attraction, resulting in prolonged edema. The specific cause remains unknown and thus, our interest in open discussion on this topic.
Conclusion
The use of HA fillers is increasingly common in aesthetic medicine. While infrequent, our case report serves to highlight persistent edema following injection of HA filler. There is no definitive cause for this event, but we theorize it might be a combination of differences in patient anatomy, technique, and type of HA technology utilized. In order to avoid this complication, we recommend products that are homogeneous, non-particulate, cohesive gels that have a low concentration of hyaluronic acid per mL. We emphasize that this is not an isolated case. While rare, this patient and others like her can develop chronic periorbicular edema despite appropriate filler and proper injection technique.
References
- Ramirez OM, Novo TA, Volpe CR. [The beautiful eye]. Cir Plást Iberolatinoam. 2007;33(2):79–90. Article in Spanish.
- Muñóz del Olmo JL, Serra Renom JM. [Non-invasive periorbital rejuvenation]. Cir Plást Iberolatinoam. 2008;34(1):11–18. Article in Spanish.
- Ross A. American Society of Plastic Surgeons. New statistics reflect the changing face of plastic surgery. 2016 Feb. https://www.plasticsurgery.org/news/press-releases/new-statistics-reflect-the-changing-face-of-plastic-surgery. 29 Oct 2018.
- Carruthers A, Cox SE, De Boulle K, Sommer B. Facial aesthetics: achieving the natural, relaxed look. J Cosmet Laser Ther. 2007;9:6–10.
- Scheuer III JF, Sieber DA, Pezeshk RA, et al. Anatomy of the facial danger zones: maximizing safety during soft-tissue filler injections. Plast Reconstr Surg. 2017;139(1):50e–8e.
- Gold M. Use of hyaluronic acid fillers for the treatment of the aging face. Clin Interv Aging. 2007;2(3):369–76.
- Salwowska NM, Bebenek KA, Zadlo DA, Wcislo-Dziadecka DL. Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol. 2016 Dec;15(4):520–526.
- Collins MN, Birkinshaw C. Physical properties of crosslinked hyaluronic acid hydrogels. J Mater Sci Mater Med. 2008 Nov;19(11):3335–3343.
- Erazo PJ, de Carvalho AC, Alexander T, et al. Relleno facial con ácido hialurónico: técnica de pilares y malla de sustentación. Principios básicos para obtener una remodelación facial. Cir Plást.Iberolatinoam. 2009;35(3):185–194.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316.
- Hwang CJ. Periorbital injectables: understanding and avoiding complications. J Cutan Aesthet Surg. 2016;9(2):73–79.
- Lee JH, Hong G. Definitions of groove and hollowness of the infraorbital region and clinical treatment using soft-tissue filler. Arch Plast Surg. 2018 May;45(3):214.
- Stutman RL, Codner MA. Tear trough deformity: review of anatomy and treatment options. Aesthet Surg J. 2012 May;32(4):426–440.
- Kablik J, Monheit GD, Yu L, Chang G, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatologic Surg. 2009;35:302–12.
- Bogdan Allemann I, Baumann L. Hyaluronic acid gel (Juvederm) preparations in the treatment of facial wrinkles and folds. Clin Interv Aging. 2008;3(4):629–634.
- Michaud T. Rheology of hyaluronic acid and dynamic facial rejuvenation: topographical specificities. J Cosmet Dermatol. 2018 Oct;17(5):736–743.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and Drug Administration-approved fillers. Plast Reconstr Surg. 2015 Oct;136(4):678-686.
- Wu Y, Sun N, Xu Y, Liu H, et al. Clinical comparison between two hyaluronic acid-derived fillers in the treatment of nasolabial folds in Chinese subjects: BioHyalux versus Restylane. Arch Dermatol Res. 2016 April;308(3):145–151.
- Prasetyo AD, Prager W, Rubin MG, Moretti EA, et al. Hyaluronic acid fillers with cohesive polydensified matrix for soft-tissue augmentation and rejuvenation: a literature review. Clin Cosmet Investig Dermatol. 2016;9:257–280.
- Jordan DR, Stoica B. Filler migration: a number of mechanisms to consider. Ophthalmic Plast Reconstr Surg. 2015;31(4):257–262.
- Lambros VS. Hyaluronic acid injections for correction of the tear trough deformity. Plast Reconstr Surg. 2007;120:74S–80S.
- Kane MA. Treatment of tear trough deformity and lower lid bowing with injectable hyaluronic acid. Aesthetic Plast Surg. 2005;29(5):363–367.
- Steinsapir KD, Steinsapir SM. Deep-fill hyaluronic acid for the temporary treatment of the naso-jugal groove: a report of 303 consecutive treatments. Ophthal Plast Reconstr Surg. 2006;22(5):344–348.
- Glaser DA, Patel U. Enhancing the eyes: use of minimally invasive techniques for periorbital rejuvenation. J Drugs Dermatol. 2010;9:118–128.
- Sharad J. Dermal fillers for the treatment of tear trough deformity: a review of anatomy, treatment techniques, and their outcomes. J Cutan Aesthet Surg. 2012 Oct;5(4):229–238.
- Trevidic P. The use of blunt-tipped cannulas for tear trough correction. J Drugs Dermatol. 2012;11:38–40.
- Berguiga M, Galatoire O. Tear trough rejuvenation: a safety evaluation of the treatment by a semi-cross-linked hyaluronic acid filler. Orbit. 2017 Feb;36(1):22–26.
- Bertucci V, Lynde CB. Current concepts in the use of small-particle hyaluronic acid. Plast Reconstr Surg. 2015;136:132S–138S.
- Micheels P, Besse S, Sarazin D. Two crosslinking technologies for superficial reticular dermis injection: a comparative ultrasound and histologic study. J Clin Aesthet Dermatol. 2017 Jan;10(1): 29–36.

