 J Clin Aesthet Dermatol. 2019;12(3):12–14
J Clin Aesthet Dermatol. 2019;12(3):12–14
by Megan Wetzel, MD, MPH; John Strickley, BS; M. Tye Haeberle, MD; and Timothy S. Brown, MD
Drs. Wetzel and Haeberle and Mr. Brown are with the Division of Dermatology, Department of Medicine, University of Louisville School of Medicine in Louisville, Kentucky. Mr. Strickley is with the University of Louisville School of Medicine in Louisville, Kentucky.
FUNDING: No funding was received for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Objective. Dermal invasion is characteristic of more aggressive basal cell carcinoma (BCC) tumors. However, reporting the depth of invasion of BCC is not currently a standard or recommended practice. The purpose of this study was to document the depth of invasion of BCC.
Design. Original hematoxylin and eosin-stained slides of biopsied BCCs (N=500) were reviewed. The depth of invasion was measured for each case. Each case was also determined to be aggressive or nonaggressive based on the presence of additional histologic features.
Setting. Biopsy specimens from a large private practice were reviewed.
Measurements. Descriptive statistics were calculated for all cases. To compare the average depth of invasion of cases with aggressive features versus cases with nonaggressive features, two-tailed, independent t-tests were computed.
Results. The overall median depth of invasion was 0.68mm (range: 0.10–5.49). The median depth of invasion of aggressive cases (n=68) was 1.04mm, while the median depth of invasion of nonaggressive cases (n=432) was 0.62mm. The difference between median depth of invasion of aggressive and nonaggressive cases was found to be significant (p<0.0001). This significant difference (p<0.0001) was maintained even with transection, which was present in the majority of cases (n=347; 69.4%).
Conclusions. Dermal invasion depths of all subtypes of BCC have not been previously reported. The median dermal invasion of histologically aggressive BCC is greater than nonaggressive tumors. These findings might help clinicians in determining the depth of biopsy and choice of treatment.
KEYWORDS: Basal cell carcinoma, depth of invasion, tumor thickness, vertical growth
Basal cell carcinoma (BCC) is the most common cutaneous malignancy in the United States. Innumerable clinicopathological subtypes have been described, including nodular, superficial, morpheaform, and fibroepithelial.1–4 Clinical features sometimes allow for the distinction between these subtypes, but do not always permit clear delineation. Moreover, multiple subtypes can be found in a single lesion.4 Although management is often similar among the subtypes, the identification of certain histopathologic features is important for risk assessment. Findings of micronodular, infiltrating, sclerosing, and basosquamous differentiation are associated with more aggressive behavior such as higher rates of recurrence and invasion of the dermis.1–4
Treatment options for BCCs include standard surgical excision, Mohs micrographic surgery, curettage with or without electrodesiccation, cryosurgery, photodynamic therapy (PDT), radiation therapy, and medical therapy with topical agents or intralesional injections.5,6 Treatment options are dictated by tumor size, location, histologic subtype, patient demographics and adherence to treatment, medical comorbidities, and cosmetic outcome. The American Academy of Dermatology (AAD) recently released updated guidelines on the management of BCC.7
Topical agents, which have been available for over 10 years, fill a unique therapeutic niche. Fluorouracil (5-FU) and imiquimod creams are approved by the United States Food and Drug Administration (FDA) for the treatment of histologically confirmed, superficial BCC. More recently, treatment with electronic brachytherapy, a form of superficial radiation therapy, has provided patients with another topical intervention for superficial and nodular BCCs.8–10 The AAD recommends topical (e.g., imiquimod, 5-FU, methyl aminoaminolevulinate [MAL], or aminolevulinic acid PDT) or radiation therapy (e.g., superficial radiation therapy, brachytherapy, external electron beam, and other traditional radiotherapy) for low-risk tumors when surgery is not feasible or preferred.7 However, in practice, these agents can be used to treat other subtypes despite a lack of established efficacy and subpar clearance rates.6,11
Currently, it is not standard practice or recommended to report on the depth of invasion of BCCs as a discrete measurement. While deep dermal invasion is often associated with more aggressive subtypes, so long as a tumor maintains a connection with the epidermis and is confined to the upper layers of the dermis, it meets the diagnostic criteria for superficial BCC. This definition introduces variability, rendering superficial BCC a relative diagnosis.4
While a specific subtype can be associated with certain risk factors and management practices, it can be more efficient to use histologic features to guide management rather than subtype. This study aims to characterize a large sample of BCCs, of any subtype, by way of depth of invasion and histologic features.
Methods
Five hundred original hematoxylin- and eosin-stained slides of BCCs diagnosed by skin biopsy from the years 2016 and 2017 were reviewed. Only histopathological slides already processed onto a glass microscope slide and previously diagnosed as BCC by a board-certified dermatopathologist were included. No new tissue specimens or tissue specimen processing were requested or included. All slides were then reviewed by the same board-certified dermatopathologist.
Depth of invasion was recorded for each tumor. This was measured as the distance from the granular layer or deepest portion of an ulcer to the deepest portion of the tumor. A tumor was labeled as aggressive based upon the following criteria: 1) dissection of tumor between collagen; 2) squamous metaplasia not underlying ulceration; 3) poor histologic tumor differentiation; and 4) perineural invasion. This study met the exemption criteria set forth by the local institutional review board.
Statistical analysis. Descriptive statistics were calculated for all cases. To compare average depth of invasion of cases with aggressive features versus cases with nonaggressive features, two-tailed, independent t-tests were performed. Statistically significant differences between groups were also examined using the Mann–Whitney U test due to the nonparametric nature of this population. Data were further stratified by level of transection (e.g., transected at the base, not transected at the base) followed by the performance of comparison using two-tailed, independent t-tests as well as Mann–Whitney I tests. Results with p<0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, California).
Results
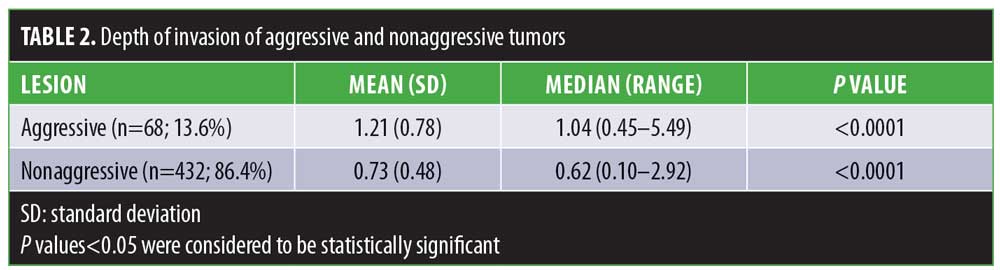
Of the 500 samples, the overall median depth of invasion was 0.68mm (mean: 0.79; range: 0.10–5.49; Table 1). Aggressive histology was identified in 68 cases (13.6%). The median depth of invasion of aggressive cases was 1.04mm (mean: 1.21; range: 0.45–5.49). Nonaggressive features were found in 432 cases (86.4%) with a median depth of invasion of 0.62mm (mean: 0.73; range: 0.10–2.9). The difference between the average depth of invasion in aggressive and nonaggressive cases was found to be significant (p<0.0001; Table 2).
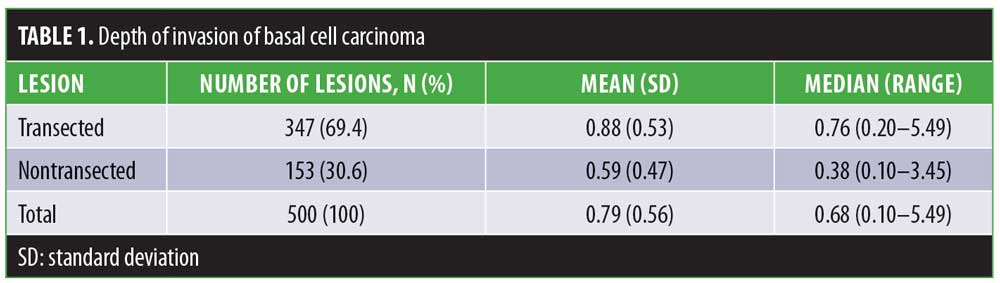
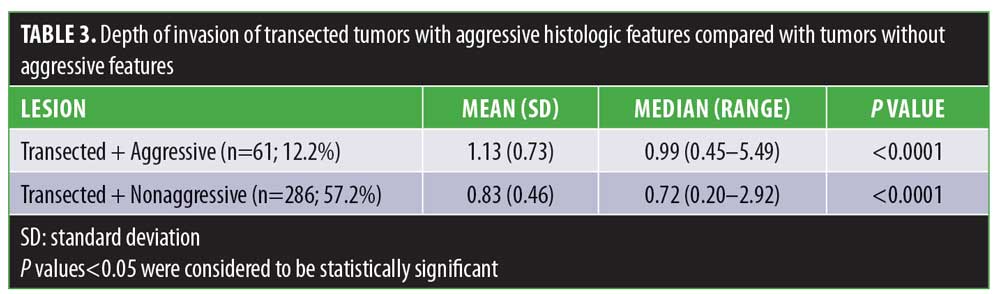
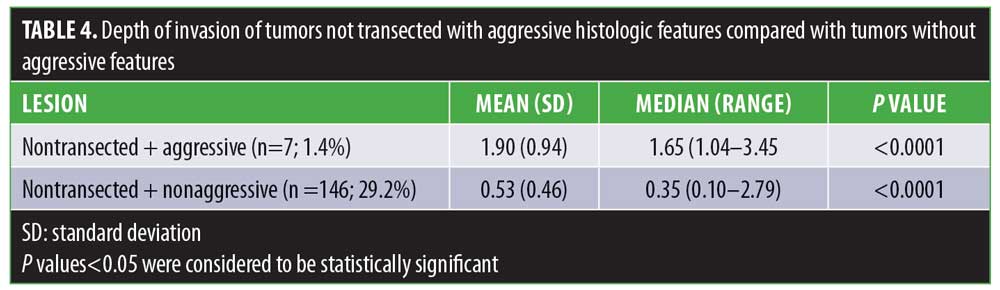
Overall, 347 cases (69.4%) were transected at the base with a median depth of invasion of 0.76mm. The median depth of invasion of cases not transected at the base (n=153; 30.6%) was 0.38mm. The difference in median depths of invasion was statistically significant (p<0.0001). The median depth of invasion of cases transected at the base with aggressive histology (n=61; 12.2%) was 0.99mm. The median depth of cases transected at the base without aggressive histology (n=286; 57.2%) was 0.72mm. The difference between the median depth of invasion between transected cases with and without aggressive histology as well as nontransected cases with and without aggressive histology were statistically significant (Tables 3 and 4; p<0.0001).
Discussion
To our knowledge, this is the first large-scale evaluation of the depth of invasion of all subtypes of BCCs. Results of this study indicate BCCs of any subtype with aggressive histologic features has a statistically significant greater depth of invasion as compared with BCCs with nonaggressive histologic features. This difference was maintained even when the specimen was transected. The median depth of invasion of all BCCs, regardless of other histologic features and subtype, was 0.79mm (range: 0.10–5.49)
There is a paucity of literature examining the vertical depth of invasion of BCCs. Unlike with melanoma, the reporting of discrete measurement of tumor depth is not standard practice in BCC cases. The National Comprehensive Cancer Network recommends risk stratification based on histological subtype and perineural involvement.12 However, a formal grading and staging system, which would potentially require depth of invasion, has never been instituted. Current pathologic reporting practice is most likely reflective of the extremely low incidence of tumor metastasis, with the greatest risks being local tumor recurrence and treatment failure.
While treatment recommendations have been offered based upon horizontal size and subtype, analogous recommendations based on depth of invasion are absent in the current literature. However, with any topical therapy, adequate penetration of a drug to its target might be a concern with agents like imiquimod and 5-FU.6,11 Depth of invasion is also critical for determining the prescription dose of radiation therapy.10 Thus, one goal was to make a substantial contribution to an area of the field with limited data.
The relationship between depth of invasion and therapeutic response to topical agents in superficial BCC has been previously examined. McKay et al13 reported a mean tumor thickness of 0.3mm (range: 0.09–1.41). The authors found a tumor thickness greater than 0.40mm to be a predictor of treatment failure following topical imiquimod therapy (recurrence rate: 58%). Our study showed a relatively small portion of tumors (n=123; 24.6%) were less than 0.4mm in depth. Conversely, Roozeboom et al14 found no association between tumor thickness and treatment failure with imiquimod, fluorouracil, or MAL-PDT. In their study, the median tumor thickness reported was 0.35mm (range: 0.20–1.00).
Importantly, however, their data were collected from only superficial BCCs diagnosed by punch biopsy, unlike in our study, which examined all BCCs. A punch biopsy allows for the complete examination of the dermis and at least the superficial subcutaneous tissue. In our experience, shave biopsy is both preferential and more commonplace in clinical practice. However, while shave biopsy allows for the accurate diagnosis of BCC, all cutaneous layers might not be visualized. Coexistence of another histologic subtype present in deeper tissue might not always be identified, with missed identification of an aggressive component.4 Indeed, our data showed a majority of BCCs with nonaggressive features were transected at the base (n=286; 57.2%) with a median depth of invasion of 0.62mm (range: 0.10–2.92).
Monotherapy with imiquimod has been shown to be less efficacious in nodular BCC than superficial BCC, which was attributed to a possible deeper invasion of tumor.11 Intuitively, this explanation makes sense, but concrete data regarding depth of tumor and treatment response are needed. We found only a small portion of cases (n=85; 17.0%) were less than 0.40mm, not transected at the base, and had nonaggressive features. Our study supports current AAD recommendations as well as the labeled usage of imiquimod to be only used as monotherapy in superficial BCCs. The response to other topical interventions in regard to tumor depth has not been adequately studied. The current study can help further research in this area by determining the ideal depth of penetration of a potential topical therapy.
Limitations. We did not examine the association of specific histologic features with depth of invasion, which might also be useful data for characterizing BCC. Additionally, we did not control for anatomic location. However, as the majority of cases were transected, inherent differences in anatomic location might be less relevant. Additionally, since we did not specify histological subtype. Future studies could examine the vertical growth of each subtype.
Conclusion
Tumor thickness has been used both as a feature as well as a proxy of histologic subtype. While subtypes are associated with specific histologic features, they are not synonymous. This study further supports that BCCs with aggressive histology have deeper invasion than nonaggressive tumors. However, when quantified, even nonaggressive tumors can have deeper tumor extension than what has been previously documented. While nonsurgical interventions for the treatment of BCC are often efficacious, they should be applied judiciously as monotherapy, as deeper dermal invasion is the rule and not the exception.
References
- Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol. 2006;19(Suppl):S127–S147.
- Dixon AY, Lee SH, McGregor DH. Histologic features predictive of basal cell carcinoma recurrence: results of a multivariate analysis. J Cutan Pathol. 1993;20(2):137–142.
- Mosterd K, Arits AH, Thissen MR, Kelleners-Smeets NW. Histology-based treatment of basal cell carcinoma. Acta Derm Venereol. 2009;89(5): 454–458.
- Haws AL, Rojano R, Tahan SR, Phung TL. Accuracy of biopsy sampling for subtyping basal cell carcinoma. J Am Acad Dermatol. 2011;66(1): 106–111.
- Telfer NR, Colver BG, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159(1):35–48.
- Bath-Hextall FJ, Perkins W, Bong J, Williams HC. Interventions for basal cell carcinoma. Cochrane Database Syst Rev. 2007:1:CD003412.
- Bichakjian C, Armstrong A, Bordeaux JS, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78(3):540–559.
- Bhatnager A. Nonmelanoma skin cancer treated with electronic brachytherapy: results at 1 year. Brachytherapy. 2013;12(2):134–140.
- Paravati AJ, Hawkins PG, Martin AN, et al. Clinical and cosmetic outcomes in patients treated with high-dose-rate electronic brachytherapy for nonmelanoma skin cancer. Pract Radiat Oncol. 2015;5(6):e659–e664.
- Ballester-Sanchez R, Pons-Llanos O, Candela-Juan C, et al. Efficacy and safety of electronic brachytherapy for superficial and nodular basal cell carcinoma. J Contemp Brachytherapy. 2015;7(4)231–238.
- Schumack S, Robinson J, Kossard S, et al. Efficacy of topical 5% imiquimod cream for the treatment of nodular basal cell carcinoma. Arch Dermatol. 2002;138(9):1165–1171.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016 May;14(5):574–597.
- McKay KM, Sambrano BL, Fox PS, et al. Thickness of superficial basal cell carcinoma (sBCC) predicts imiquimod efficacy: a proposal for a thickness-based definition of sBCC. Br J Dermatol. 2013;169(3):549–554.
- Roozeboom MH, van Kleef L, Arits AHMM, et al. Tumor thickness and adnexal extension of superficial basal cell carcinoma (sBCC) as determinants of treatment failure for methylaminolevulinate (MAL)-photodynamic therapy (PDT), imiquimod, and 5-fluorouracil (FU). J Am Acad Dermatol. 2015;73(1):93–98.

