 by Sabrina G. Fabi, MD; Lisa Zaleski-Larsen, DO; Joanna Bolton, MD; Rahul C. Mehta, PhD; and Elizabeth T. Makino, BS, CCRA, MBA
by Sabrina G. Fabi, MD; Lisa Zaleski-Larsen, DO; Joanna Bolton, MD; Rahul C. Mehta, PhD; and Elizabeth T. Makino, BS, CCRA, MBA
Dr. Fabi is with Cosmetic Laser Dermatology and is a volunteer Assistant Clinical Professor at the University of California, San Diego in San Diego, California. Drs. Zaleski-Larsen and Bolton are with Cosmetic Laser Dermatology in San Diego, California.
Dr. Mehta and Ms. Makino are with SkinMedica, Inc., an Allergan Company in Irvine, California.
Funding: Financial support for this study was provided by SkinMedica, Inc., an Allergan Company.
Disclosures: Dr. Fabi serves as an investigator, consultant, and speaker for Allergan, Galderma, and Merz. Drs. Zaleski-Larsen and Bolton have no financial conflicts relevant to the content of this article. Dr. Mehta and Ms. Makino are employees of SkinMedica, Inc., an Allergan Company.
Abstract: Background. Neuromodulator injection procedures are an effective treatment for moderate-to-severe facial wrinkles, but do not address the superficial fine lines and wrinkles caused by age-related loss of intrinsic hyaluronic acid levels in the epidermis.
Objective. In this study, the authors assess overall facial skin quality and patient satisfaction when combining topical treatment with a topical cosmetic serum (HA5) and applying to the entire face following a pre-elected neuromodulator injection treatment to the lateral canthal areas.
Methods and Materials. Twenty female subjects aged 36 to 63 years with moderate-to-severe under-the-eye fine lines and/or wrinkles enrolled in the study. HA5 was applied the entire face at baseline immediately post-injection and twice daily for eight weeks. Clinical assessments were conducted at baseline; 15 minutes post-procedure; and at Weeks 2, 4, and 8.
Results. Statistically significant improvements were observed immediately post-procedure and after eight weeks, along with high patient satisfaction.
Conclusion. The combination of topical serum and injectable procedure provided a rapid onset of improvements in fine lines/wrinkles appearance and skin texture and long-term overall improvements in areas not treated by the injection. These results support how this novel combination can provide physicians with a comprehensive approach to optimize patient outcomes.
Keywords: Neuromodulator injection, cosmeceutical, post-procedure, combination treatment, facial rejuvenation
J Clin Aesthet Dermatol. 2017;10(12):14–18
Introduction
The growth of the facial cosmetic industry reflects the increasing demand of patients seeking treatments to look younger. Steadily growing, the number of surgical and minimally invasive cosmetic procedures performed in the United States in 2015 totaled 15.9 million, a growth of two percent from the previous year, with minimally invasive procedures comprising the majority at 14.9 million.1 Facial wrinkling has been shown to strongly correlate with one’s perceived age2 and is one of the key features of diminished skin quality due to photodamage and intrinsic aging, along with dyspigmentation and uneven skin texture.3,4
From the patient perspective, the presence of facial lines or wrinkles is one of the top skin quality concerns that motivates patients to seek treatment. This demand is reflected by the growing number of neuromodulator injection procedures performed and supports why it is a key target for aesthetic treatments and procedures. Since it received United States Food and Drug Administration (FDA) approval for the treatment of glabellar lines in 2002, the neuromodulator onabotulinumtoxinA has hailed in a new era of minimally invasive cosmetic procedures. Growing over 700 percent since 2000, neuromodulator injections in general were the number one minimally invasive procedure performed in 2015.1
The efficacy of neuromodulator injection procedures for the treatment of moderate-to-severe facial lines and wrinkles has been well-established.5–7 The mechanism of action focuses on addressing the associated muscle activity causing the line or wrinkle formation. In addition to cosmetic in-office procedures, the topical skin care or “cosmeceutical” industry has also grown tremendously, with products targeting the improvement of skin quality. However, many of these cosmeceuticals lack the testing to support their efficacy. Recently, a novel topical serum, HA5 Rejuvenating Hydrator (SkinMedica, Inc., Irvine, California), was developed to restore diminished epidermal function and skin hydration associated with aging and improve the appearance of fine lines and wrinkles. When applied twice daily in subjects presenting with mild-to-severe periocular lines, HA5 provided both immediate and long-term significant improvements in wrinkle appearance and tactile roughness assessments as well as hydration instrumentation measurements.8 In-vitro testing with HA5 demonstrated enhanced expression of key markers for skin barrier function and associated increases in hyaluronic acid production.8
Combination therapies utilizing different treatment modalities have been shown to optimize clinical outcomes for skin rejuvenation and patient satisfaction.9–13 In this clinical study, we chose to combine HA5’s instant and long-term effects on fine lines and wrinkles, texture, and skin hydration with the established effects of a pre-elected neuromodulator injection to assess if the combination treatment enhances overall skin quality outcomes and achieves high patient satisfaction. Our aim was to assess the effects on overall facial skin quality and patient satisfaction when combining topical treatment with HA5 to the entire face following a pre-elected neuromodulator injection treatment to the lateral canthal areas.
Materials and Methods
The immediate and long-term clinical effects of HA5 when combined with a pre-elected neuromodulator injection procedure were evaluated in an open-label, single-center clinical study in subjects presenting with moderate-to-severe periocular (i.e., under-eye) wrinkles. Subjects were neuromodulator injection-experienced, defined as having received at least two neuromodulator injection procedures to the lateral canthal area within the last 18 months. Twenty healthy female subjects aged 18 years to 65 years old were eligible to enroll in the eight-week study. All subjects were required to present with mild-to-severe periocular lines or wrinkles, as determined by a grade of 4 to 9 on the investigator’s periocular fine lines and wrinkles scale based on the modified 10-point Griffith’s scale.14 Subjects were allowed continued use of their regular facial cosmetic products during the study, with regular use defined as products used for a minimum of three months prior to enrollment without any incidence of irritation.
Institutional review board approval from the Chesapeake Institutional Review Board and informed consent was obtained from subjects prior to commencement of any study procedures. This study was conducted according to ethical and regulatory principles for the protection of human subjects for research as outlined in 21 CFR 50, in accordance with the accepted standards for Good Clinical Practice (GCP) and the International Conference on Harmonization (ICH). Photoconsent from study subjects was also received. The study was conducted in San Diego, California, from September 2015 to February 2016.
At the baseline visit, clinical assessments and standardized digital photography (Canfield Vectra; Canfield Imaging Systems, Fairfield, New Jersey) of the subjects’ clean facial skin were conducted. Subjects then received a pre-elected neuromodulator injection in the left and right lateral canthal areas. After the procedure, HA5 was applied to the entire face, including the lateral canthal areas. Clinical assessments and standardized photography were conducted again to capture immediate effects 15 minutes post-procedure. Subjects were instructed to apply a thin layer of the HA5 formulation (e.g., a nickel-sized amount) to their entire face twice daily (morning and evening) after cleansing for eight weeks. At Weeks 2, 4, and 8, subjects returned to the study site for clinical assessments and photography, after washing their face with a cleanser and water. Subjects also completed a questionnaire regarding self-perceived skin quality from the treatment combination and were asked to compare their current experience to past injection procedure experiences.
Clinical efficacy of the HA5 formulation was assessed by the investigator at baseline (pre-neuromodulator injection and HA5 application); baseline again (post-neuromodulator injection and HA5 application); and at Weeks 2, 4, and 8 using the following grading scales: Facial Wrinkle Scale (FWS) with Photonumeric Guide (lateral canthal area); Fine Lines/Wrinkles in the periocular-under eye, periocular-lateral canthal area, forehead area, cheek areas, and perioral area; and Tactile Roughness (full face). The assessments are described in detail in Table 1. Safety and tolerability of the product was assessed through the capture of adverse events. Mean clinical grading scores (investigator assessments) at baseline (post-treatment) and at Weeks 2, 4, and 8 were compared with baseline scores (pre-treatment) using a Student’s paired t-test. Statistical differences were considered significant at the p<0.05 level. Analysis was conducted on the intent-to-treat population, comprising subjects who completed the baseline and the baseline post-treatment procedures and assessments.
Results
Twenty female subjects aged 36 to 63 years (mean age: 48 years) presenting with a grade of 6.4 (high moderate) for fine lines and wrinkles (periocular-under eye) were enrolled in the eight-week clinical study. Five subjects were lost to follow-up. No treatment-related adverse events were reported during the study. All subjects were neuromodulator injection-experienced, having received at least two neuromodulator injections in the lateral canthal areas in the last 18 months.
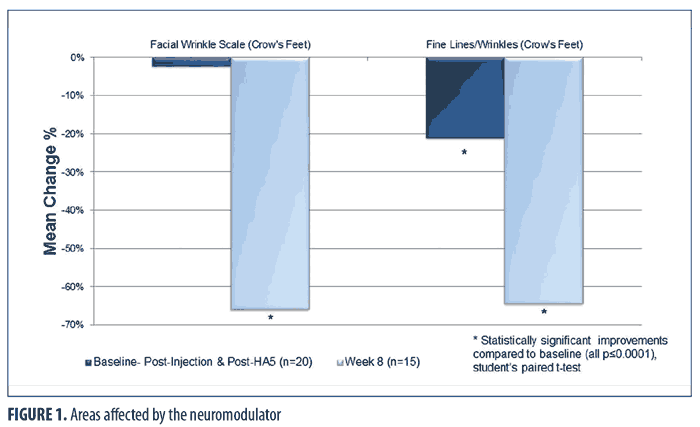
Figure 1 shows the immediate (baseline pre-treatment) and the long-term effects (at Week 8) from the combination treatment in the lateral canthal area, an area affected by the neuromodulator injection and HA5 application. Immediately after treatment at baseline, a statistically significant improvement in fine lines and wrinkles in the periocular-lateral canthal area was observed (all p?0.006; Student’s paired t-test; n=20). The FWS in this area did not show immediate significant improvement. By Week 8, fine lines and wrinkles and the FWS in the lateral canthal area showed significant improvements (all p?0.009; Student’s paired t-test; n=15).
For the facial areas not treated by the neuromodulator injection, Figure 2 shows the immediate (baseline pre-treatment) and the long-term effects (Week 8) after twice-daily use of HA5. Statistically significant improvements in the appearance of fine lines/wrinkles in the under-eye, forehead, and perioral facial areas were observed immediately after treatment at baseline (all p?0.009; Student’s paired t-test; n=20). Full-face tactile roughness grades also significantly improved immediately after HA5 treatment (all p?0.0006; Student’s paired t-test; n=20). At the interim visits of Weeks 2 and 4, all efficacy parameters in both the injection-treated and non-treated sites demonstrated statistically significant improvements (n=19 and n=16, respectively; all p?0.01; Student’s paired t-test). Long-term effects at Week 8 showed a statistically significant improvement of fine lines and wrinkles in all facial areas (i.e., under-eye, perioral, forehead, cheek) when compared with the same at baseline (all p?0.01; Student’s paired t-test; n=15). Figure 3 includes representative standardized digital photographs of a 62-year-old female subject at baseline, immediately after neuromodulator injection and HA5 application, and at Week 8, respectively.
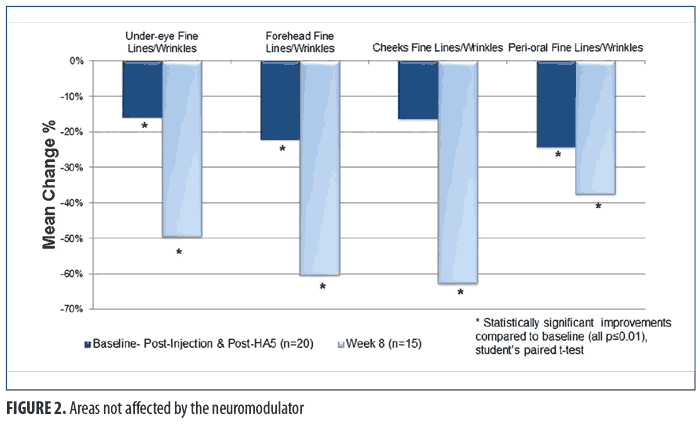
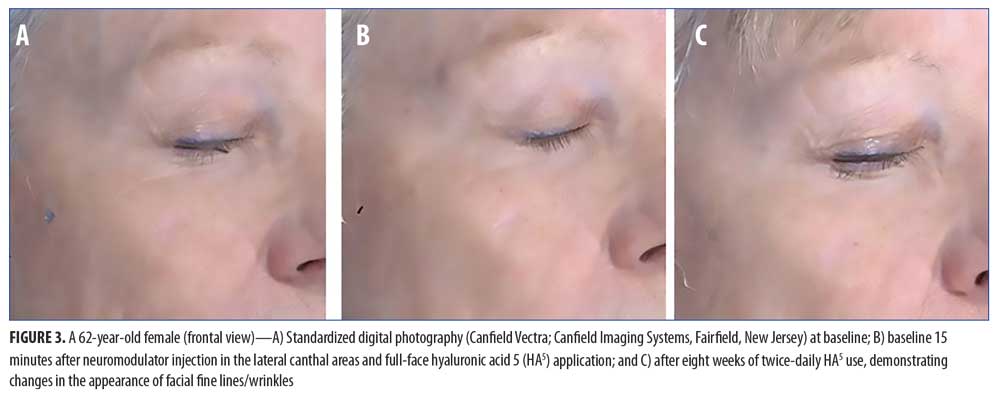
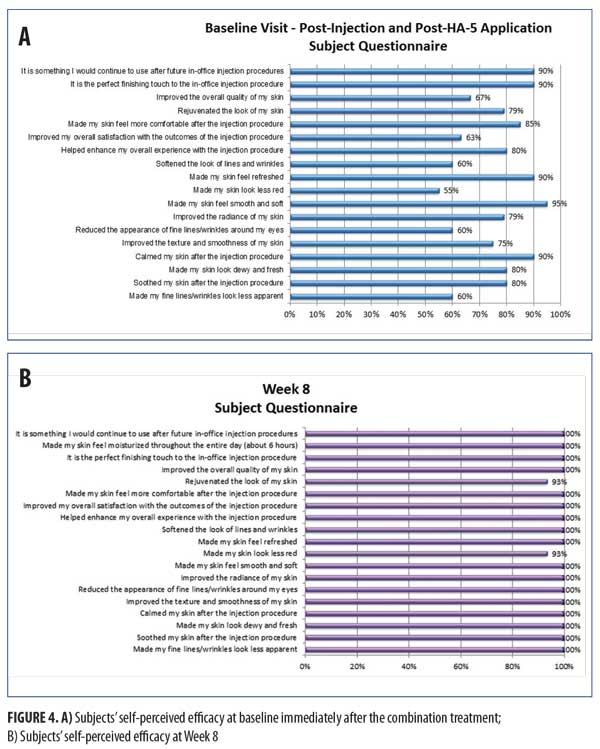
Figure 4 shows the subject’s self-perceived efficacy at baseline immediately after the combination treatment and long-term at the Week 8 visit. Subjects rated their experience with this combination as compared with their past neuromodulator injections. Parameters focusing on the immediate effects of HA5 application after the neuromodulator injection showed high ratings with 80 percent of subjects agreeing HA5 “soothed my skin after the injection procedure,” 85 percent of subjects agreeing HA5 “made my skin feel more comfortable after the injection procedure,” and 90 percent of subjects agreeing HA5 “is something I would continue to use after future in-office injection procedures.” Long-term effects at Week 8 were all consistently highly rated (all ?93%), with 100 percent of subjects agreeing the HA5 “improved my overall satisfaction with the outcomes of the injection procedure.”
Conclusion
Increases in the variety of treatment options available to address facial aging allow for physicians to tailor in-office procedures with topical therapies for optimal outcomes. In this study, we demonstrated that a combination of a topical cosmetic serum used after a pre-elected neuromodulator injection provided a rapid onset of improvements in fine lines/wrinkles and skin texture and long-term overall improvements in areas not treated by the neuromodulator injection.
The HA5 serum used in this study contains a proprietary blend of Vitis vinifera flower stem cell extract, marine micro-organism polysaccharides, and a peptide complex to support the replenishment of endogenous levels of hyaluronic acid and epidermal homeostasis. Five forms of hyaluronic acid were also included in the formulation to provide immediate hydrating effects on the skin.
As expected in this study, the neuromodulator injection improved wrinkles in the area of treatment, as shown by the FWS. Improvement in the appearance of superficial fine lines and wrinkles in the injected area are not typically expected immediately after a neuromodulator procedure, yet rapid-onset improvement was observed in this study following HA5® application. Additionally, in facial areas not treated by the neuromodulator, HA5® application provided significant improvements in the appearance of fine lines and wrinkles and skin texture. The immediate and long-term effects observed in this study are consistent with a previous clinical study assessing the effects of HA5 on the appearance of fine lines and wrinkles and skin hydration.8
The causes of superficial fine lines or wrinkles are multifactorial and are not solely due to muscle contraction nor loss of underlying tissue, which injection procedures address. Age-related reductions in epidermal levels of hyaluronic acid also contribute to the formation of fine lines and wrinkles and uneven skin texture. Results from this study support how a combination of HA5 and neuromodulator injection can contribute to the development of a comprehensive approach to optimize patient outcomes. Better outcomes and perceived experience translated to great satisfaction in this study, and, if this treatment is used in practice, it might ultimately result in increased patient retention.
Acknowledgment
We would like to acknowledge Priscilla Tan, BA, employee of SkinMedica Inc., an Allergan Company, who conducted the data analysis for this study.
References
- American Society for Aesthetic Plastic Surgery. 2016. Available at: http://www.plasticsurgery.org/news/2016/new-statistics-reflect-the-changing-face-of-plastic-surgery.html. Accessed May 16, 2016.
- Liu F, Hamer MA, Deelen J, Lall JS, et al. The MC1R gene and youthful looks. Curr Biol. 2016;26(9):1213–1220.
- Uitto J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 Suppl):12–16.
- Gilchrest BA. Skin aging and photoaging: An overview. J Am Acad Dermatol. 1989;21(3 Pt 2):610–613.
- Carruthers JA, Bruce S, Cox SE, et. al. OnabotulinumtoxinA for treatment of moderate-to-severe crow’s feet lines: a review. Aesthet Surg J. 2016;36(5):591–597.
- Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840–849.
- Kerscher M, Rzany B, Prager W, et al. Efficacy and safety of incobotulinumtoxinA in the treatment of upper facial lines: results from a randomized, double-blind, placebo-controlled phase III study. Dermatol Surg. 2015;41(10):1149–1157.
- Narukar VA, Fabi SG, Bucay VW, et al. Rejuvenating hydrator: restoring epidermal hyaluronic acid homeostasis with instant benefits. J Drugs Dermatol. 2016;15(1 Suppl 2):s24–s37.
- Wisniewski JD, Ellis DL, Lupo MP. Facial rejuvenation: combining cosmeceuticals with cosmetic procedures. 2014;94(3):122–126.
- Bruce S. Complementary effects of topical antiaging treatments in conjunction with aesthetic procedures. J Drugs Dermatol. 2008;7(2 Suppl):s23–s27.
- Cuerda-Galindo E, Palomar-Gallego M, Linares-Garcivaldecasas R. Are combined same-day treatments the future for photorejuvenation? Review of the literature on combined treatments with lasers, intense pulsed light, radiofrequency, botulinum toxin, and fillers for rejuvenation. J Cosmet Laser Ther. 2015;17(1):49–54.
- Fabi, SG, Carruthers J. A single modality approach to rejuvenate the aging face and body: a thing of the past?. Derm Surg. 2016;42 Suppl 2:S73–S76.
- Tierney EP, Hanke CW. Recent advances in combination treatments for photoaging: review of the literature. Dermatol Surg. 2010;36(6):829–840.
- Griffiths CEM, Wang TE, Hamilton TA, et al. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992;128(3):347–351.

