 by Mark A. Pinsky, MD, PA
by Mark A. Pinsky, MD, PA
Dr. Pinsky is the founder and a plastic surgeon at Plastic Surgery Innovations, LLC in Palm Beach Gardens, Florida.
Funding: No funding was received for this study.
Disclosures: Dr. Pinsky is a minority stock holder in Alphaeon Corporation.
Abstract: Objective. The purpose of this study was to evaluate the efficacy of five topical test products intended to reduce the appearance of lines and wrinkles, increase skin moisturization, and reduce transepidermal water loss.
Design. Two studies—a controlled clinical trial for each individual test product and an experience trial involving more than 200 patients using a strategic combination of products—were done. Test products included a day facial cream, a night facial cream, an eye cream, a day lip cream, and a night lip cream.
Setting. The setting for the first study was AMA Laboratories (New York, New York)and 19 physician practices for the second.
Participant. For the first study, participants were recruited via advertisements, phone solicitation, or electronic media. Patients of the second study (N=222) were from 19 physician practices.
Measurement. For the first study, efficacies of the facial and eye creams were determined by measuring the surface evaluation of living skin, transepidermal water loss, and electroconductivity. Efficacy of the lip creams was evaluated by measuring surface evaluation of living skin and electroconductivity. These evaluation parameters were measured at the test sites prior to initial application of test product,15 minutes after the initial application, and at seven, 14, 28, and 56 days of test product use. For the second study, each subject completed an assessment of the topical product(s) effects after four weeks of use.
Result. With each of the five topical test products, an improvement in surface evaluation of living skin became apparent within 15 minutes of initial application of test products and continued for at least 56 days of regular use. Improvement of fine and coarse lines and wrinkles continued for at least 56 days of regular use. Electroconductivity data showed that each product dramatically increased the moisture content in the skin almost immediately after application and for at least 56 days. Improvement in transepidermal water loss was nearly immediate and continued over time with the day and night facial creams and eye cream. For the experience trial, a significant majority of subjects achieved one or more endpoints within 30 days or less.
Conclusion. The facial, eye, and lip creams are effective anti-aging products that reduce the appearance of both fine and coarse lines and wrinkles, dramatically increase the moisturization of the skin, and, in the case of the face and eye products, reduces transepidermal water loss. These changes last for at least 56 days without significant adverse effects.
Keywords: Electroconductivity, fine lines, hydration, lip cream, transepidermal water loss, wrinkles
J Clin Aesthet Dermatol. 2017;10(12):27–35
Introduction
A primary goal in the treatment of the skin is the accurate and efficient delivery of therapeutic agents. Liposomes have been extensively investigated for this purpose. A liposome is a vesicle with one or more double layers of phospholipid and cholesterol that might or might not enclose a large aqueous space. The main advantage of liposomes is that they can encapsulate and sometimes deliver both hydrophobic and hydrophilic drugs to a therapeutic target. This is possible because the lipid bilayer of the liposome can include anionic, cationic, or neutral phospholipids and can entrap hydrophobic compounds, while the large aqueous core can encapsulate hydrophilic drugs.1
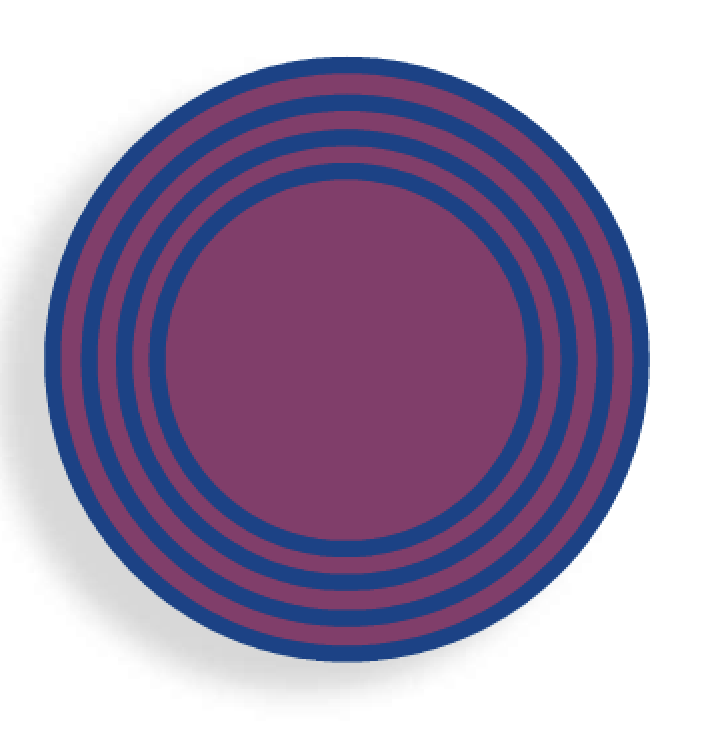
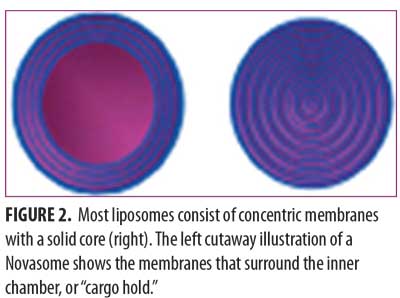
A Novasome™ is a unique type of liposome. Unlike most liposomes, the liposomes in Novasomes have 2 to 7 bilayer membranes surrounding a large central “cargo” area not present in most other liposomes (Figure 1). Most liposomes consist of concentric membranes with a solid core, much like a large onion. Figure 2 shows the membrane layer cutaway of a Novasome to reveal the innermost membrane that surrounds the inner chamber.
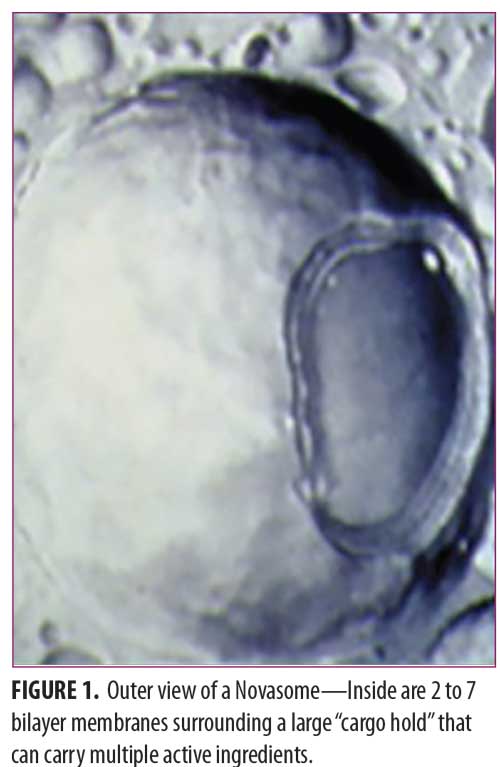
The liposomes in Novasomes have a hydrophobic tail group and a hydrophilic head group. Up to 80 to 85 percent of the Novasome core can be loaded with a medication from which the medication’s active ingredients can be released in a predictable manner. Encapsulated drugs travel through channels in the bilayer, making sustained, controlled release of active ingredients possible.2 Novasome microvesicles are stable, with a pH ranging from 2 to 13 and a temperature ranging from that of liquid nitrogen to higher than 100°C. They can therefore be used in a wide variety of applications.3
Since Novasomes can be made with a positive, negative, or neutral charge on the surface, they can adhere to specific sites on skin and hair shafts and thus can be used in cosmetic formulations.4 For example, if the charge of the Novasome is positive, then it can combine with skin that has a negative charge.2
DermaTriPlex™ is a globally patented technology platform comprising Novasome—a patented amphiphile and transdermal delivery platform designed for specific ingredients with performance characteristics that can be changed through the modification of the amphiphilic portion for a specific molecule; TruCollagen® (USV Private Ltd., Mumbai, India)—the largest segment of synthetically engineered natural collagen in the world (to the author’s knowledge); and and Teosyal Resilient Hyaluronic Acid® (RHA) The DermaTriPlex technology platform is supported by 20 synergistic, scientifically proven, anti-aging ingredients at concentrations known to be effective.
The purpose of this study was to evaluate the efficacy of five topical test products intended to reduce the appearance of lines and wrinkles, increase skin moisturization, and decrease transepidermal water loss. The test products (skIN3; Alphaeon Corp., Irvine, California) were a day facial cream, a night facial cream, an eye cream, a day lip cream, and a night lip cream, each using the DermaTriPlex technology platform.
Methods
methods
Two studies were performed: a 56-day controlled clinical trial for each individual test product and an experience trial involving more than 200 patients using a strategic combination of products.
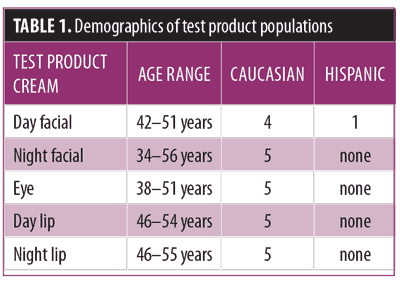
The 56-day study. In the first study, five separate groups of female subjects (n=5) were enrolled in each of the five studies. Subjects were recruited by advertisements in local periodicals, community bulletin boards, phone solicitation, and electronic media. Demographic data are shown in Table 1. Inclusion criteria were healthy women aged 35 to 60 years for the facial and eye creams, and Caucasian women aged 35 to 55 years for the lip creams. Subjects were asked to abstain from the use of any anti-aging and moisturizing products, including lotions, creams, gels, and nutritional supplements for at least eight weeks prior to the study. Each of the subjects exhibited fine lines and wrinkles at the test site and abstained from using any anti-aging products or moisturizing products on the lips or surrounding area (e.g., lotions, creams, lipsticks, chapsticks, gels, nutritional supplements) for at least 72 hours prior to the start of the study (for the lip creams).
Subjects were excluded if they were under a physician’s care or were taking any medication that might mask or interfere with the test results. Those who had a history of skin cancer, melanoma, lupus, psoriasis, connective tissue disease, diabetes, or any disease that would increase risk associated with study participation were excluded. Women who were pregnant, lactating, intending to become pregnant during the study period, or had given birth during the previous six months were excluded. Subjects were excluded if they had chronic skin allergies or a history of hypersensitivity to cosmetics in general. Further exclusion criteria included neurologic, dermatologic, or systemic disorders that would interfere with the results. Smokers, patients who had dermal filler injected into the lips or had fat grafting, silicone injections, or implants in the lips during the previous year were also excluded. Subjects who had botulinumtoxin type A around the lips during the previous six months, had laser resurfacing, chemical peels, or dermabrasion done to the lips were excluded. Finally, patients with lip disease, previous lip surgery, or systemic disease (e.g., diabetes, scurvy, or chelitis) were excluded.
All subjects provided signed, informed consent to participate in the study.
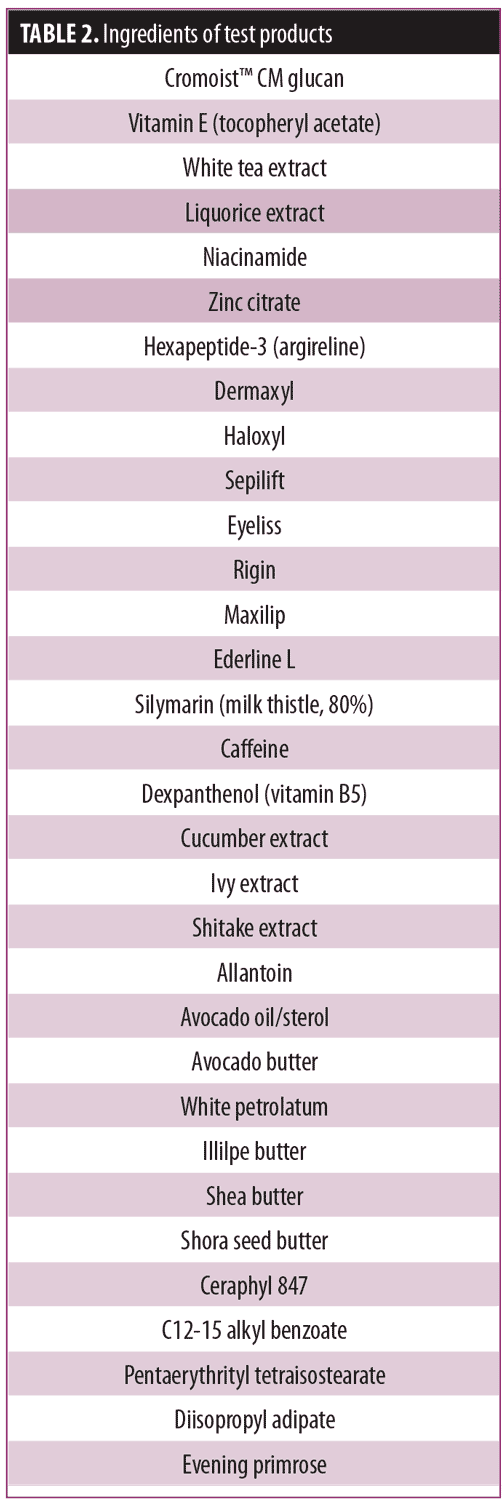
Test product. Each of the five test products contained the anti-aging ingredients of the technology platform (Table 2) at concentrations known to be efficacious. To be clear, not all of the ingredients used in the platform were present in each product, but some combinations of each were used. Table 2 represents the common tradenames; any single tradename product might have more than one active ingredient within its formulation and will have more than one International Nomenclature of Cosmetic Ingredients (INCI) name.
Procedure. Qualified subjects reported to the test facility and were examined by a trained technician. Faces were devoid of topical treatments. Subjects equilibrated to the ambient environment for 30 minutes before baseline measurements of surface evaluation of living skin (SELS), transepidermal water loss (TEWL), and electroconductivity (EC). SELS uses an optical system in a special camera that measures four parameters related with skin surface topography: roughness, scaling, smoothing, and wrinkling. This method provides analysis of living skin directly; indirect methods (e.g., profilometric) require replicas and pictorial representations of skin topography. However, when using SELS, the four skin parameters are mutually interdependent.5
Subjects were instructed to apply the day cream test product to the face and neck every morning. After the use of a facial cleanser, subjects patted their skin dry and applied enough of the test product to form a thin layer on the skin. The test product was then massaged into the skin. The night cream test product was applied to the face every evening, and the eye cream was applied above and below the eyes every morning and evening. The day lip cream was applied in and around the lips twice in the morning and twice in the afternoon, and the night lip cream was applied in and around the lips once at night.
The efficacy of each test product was objectively evaluated by measuring the evaluation parameters (i.e., SELS, TEWL, EC) at the test sites prior to the initial application of the test product and 15 minutes after the initial application. Evaluation parameters were then measured after seven, 14, 28, and 56 days of test product use.
SELS was measured by a Visioscan (Courage-Khazaka electronic GmbH, Cologne, Germany) which uses a specially designed camera and halogen lamps to obtain images that permit the user to characterize the topography of the skin surface.5 Of interest, in the present study, skin roughness (SEr; measured in relative units) was used as an indicator of the depth of fine and coarse wrinkles.
TEWL represents the level of water that constantly transpires through the skin. In this study, TEWL was used as an indicator of skin barrier protection.6 TEWL was measured with a computerized evaporimeter (cyberDERM, Inc., Media, Pennsylvania) in a quiet area, apart from the general traffic and activity of the test center. Readings were obtained by placing the probe lightly in contact with the skin.
EC was measured with a Nova Dermal Phase Meter (DPM 9003; Nova Technology Corp., Gloucester, Massachusetts). The DPM measures skin impedance, an indicator of the retained water content of the skin.7
Analysis of results. The data used in the statistical analysis reflect changes from baseline. Differences were tested for significance by a Student’s t-test using p<0.05 as the cutoff level. Topographical analyses of target areas before and after treatment were provided by AMA Laboratories, Inc. (New York, New York) using data from spatial and pixel coordinates to provide high-resolution images.
The experience trial. In an open-label study, patients from 19 physician practices were screened, and approximately 244 subjects were enrolled in the study. The objective of the study (a survey) was to evaluate subject satisfaction and perception of results after one month of daily use of the same five skin care products that contain Novasome, RHA, TruCollagen, and the supporting base formulation.
Qualified subjects were aged 40 to 60 years of age and were generally healthy with Fitzpatrick Skin Types I to IV. Subjects regularly used moisturizer and had mild-to-moderate facial lines. Subjects agreed to not use facial skin products other than those provided for the study, to avoid excessive sun exposure, minimize use of sunscreen, not use plumping lipsticks, and not undergo facial procedures during the study. Subjects were willing to comply with all instructions during the study. Subjects were excluded if they had skin disease, a photosensitivity condition, acne, adverse reactions to skin care products, recent injections of neurotoxin or dermal filler, or recent invasive facial procedures. Pregnancy, lactation, recent changes in oral contraceptives or hormone replacement therapy, or recent participation in a similar study were also grounds for exclusion. All subjects provided signed informed consent to participate in the study.
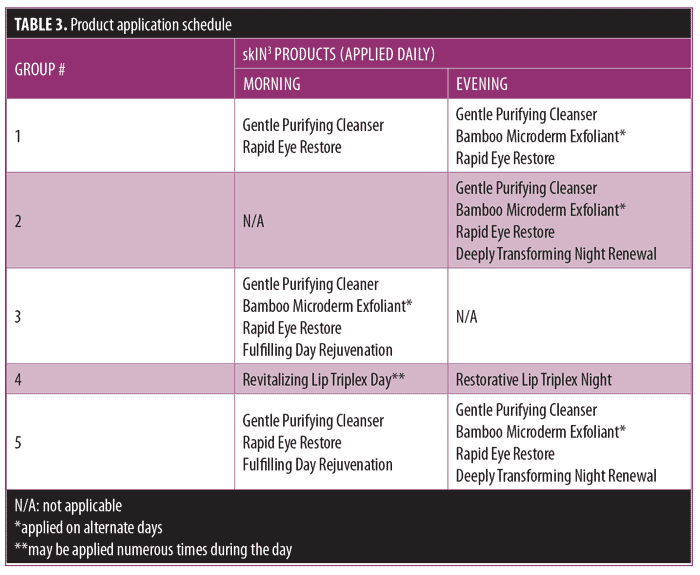
Subjects were divided into five groups in which they applied test products in the evening, morning, or both (Table 3). Group 1 comprised patients who had early signs of facial aging manifested by lines around the eyes. These subjects used the facial cleanser and eye product twice a day (morning and evening) and the bamboo microdermabrasion exfoliant 2 to 3 times per week. Group 2 comprised patients who normally performed their skin care regimen in the evening. These subjects used the facial cleanser twice a day, the bamboo microdermabrasion exfoliant 2 to 3 times per week, and the eye product and night facial product each evening. Group 3 comprised patients who normally performed their skin care regimen in the morning. These subjects used the facial cleanser twice a day, the bamboo microdermabrasion exfoliant 2 to 3 times per week, and the eye product and day facial product each morning. Group 4 was more of a niche group of patients, and comprised only those interested in lip products. Part of this group only used the day lip product applied multiple times throughout the day. The other part of the group used the night lip product once in the evening. Finally, Group 5 comprised those patients with dry skin or more mature skin who typically use skin care products twice a day. This group was instructed to use the facial cleanser twice a day, the bamboo microdermabrasion exfoliant 2 to 3 times per week, and the eye product in the morning and evening along with the revelant day facial and night facial product. Each subject completed an assessment of the topical product(s) effects after four weeks of use.
Subjects agreed to a washout period of at least four days unless enrolled in Group 4. Subjects who had been consistently using face creams containing retinoids or hydroxyl acids were permitted to continue but did not start a new regimen of these products during the four-week study period.
Subjects completed the survey at the end of the study. Professional estheticians also examined the skin of the subjects for one or more improvements (e.g., overall improvement, hydration, softness, smoothness, radiance or brightness, and improvement in lines and wrinkles). The physicians who participated in this study had busy practices that required frequent use of highly trained estheticians with extensive experience in clinical assessments similar to those used in the study.
Results
The 56-day study. SELS, TEWL, and EC were measured at each time point as described above. Readings were also compared with those from baseline, and the percent differences between baseline and each time point were tabulated for each test product (Table 4).
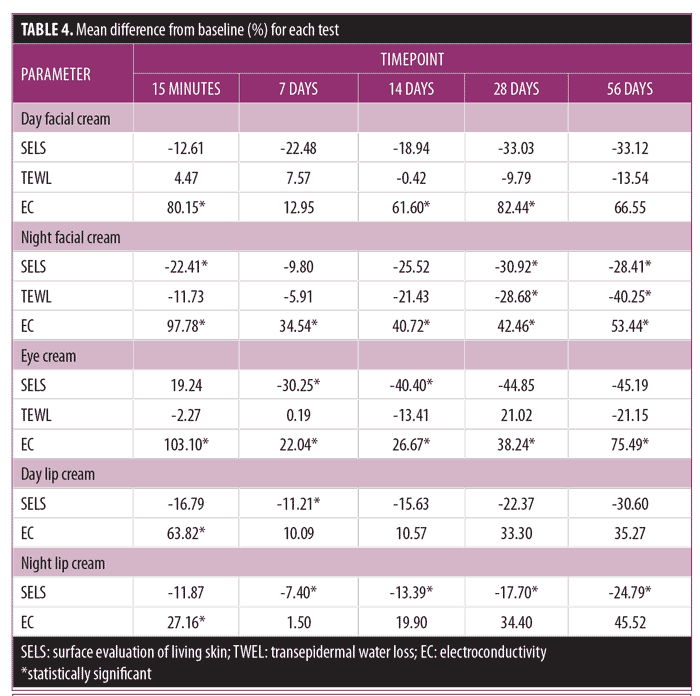
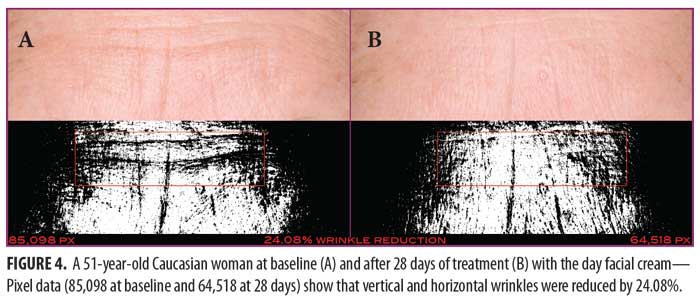
Day facial cream. As shown in Figure 3, SELS started to decline 15 minutes after product application and continued to decline during the remainder of the study period. Skin surface roughness, or depth of fine and coarse wrinkles, decreased immediately after product application and continued to improve for 56 days with regular use of the product. A decrease in SELS as compared with at baseline (Table 4) was also evident as early as 15 minutes (-12.61% difference) and reached a maximum at 56 days (-33.12%). The decrease was significant after 14 days (p=0.024) and after 28 days of use (p=0.012).
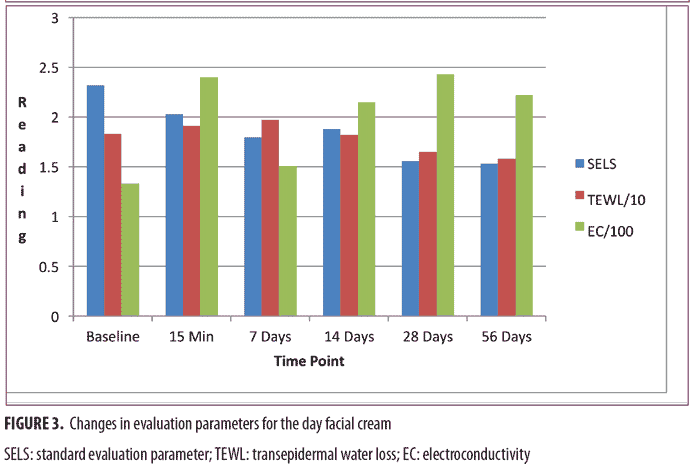
TEWL readings (Figure 3) began to decrease after 28 days of use and the decrease continued to progress until 56 days of using test products. A decrease in TEWL as compared with at baseline (Table 4) was evident at 28 days (-9.79%), and the trend continued through to 56 days (-13.54%). This signified that the test product reduced water loss after 28 days of use and the reduction progressively improved for 56 days.
EC, a measure of water content of skin, increased dramatically at 15 minutes (Figure 3) and remained above baseline for up to 56 days (Table 4). The increase was significant after 15 minutes (p = 0.009, 80.15%), 14 days (p = 0.048, 61.60%), and 28 days (p = 0.031, 82.44%). This showed that the test product helped to increase the moisture content in the skin almost immediately after application and continued to increase for 56 days with regular use of product.
Clinical examples of patients at baseline and after 28 and 56 days of treatment with the day facial cream are shown in Figures 4 and 5.
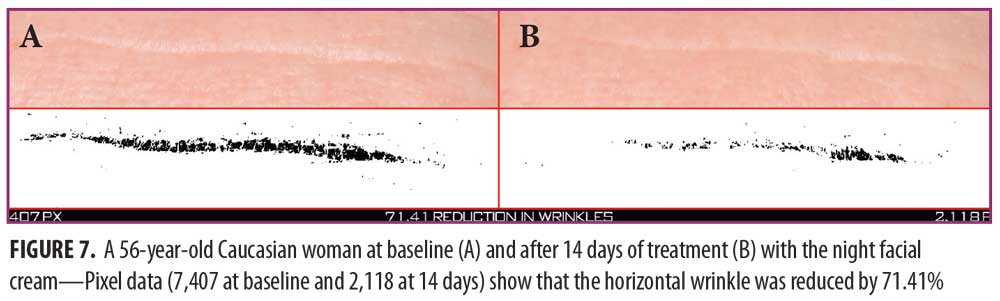
Night facial cream. As shown in Figure 6, SELS started to decline 15 minutes after product application and continued to decrease during the study period. A decrease in SELS as compared with baseline (Table 4) was also evident as early as 15 minutes (-22.41% difference), reached a maximum at 28 days (-30.92%), and remained well below baseline at 56 days (-28.41%). Differences from baseline were significant after 15 minutes (p=0.037), 28 days (p=0.041), and 56 days of use (p=0.016).
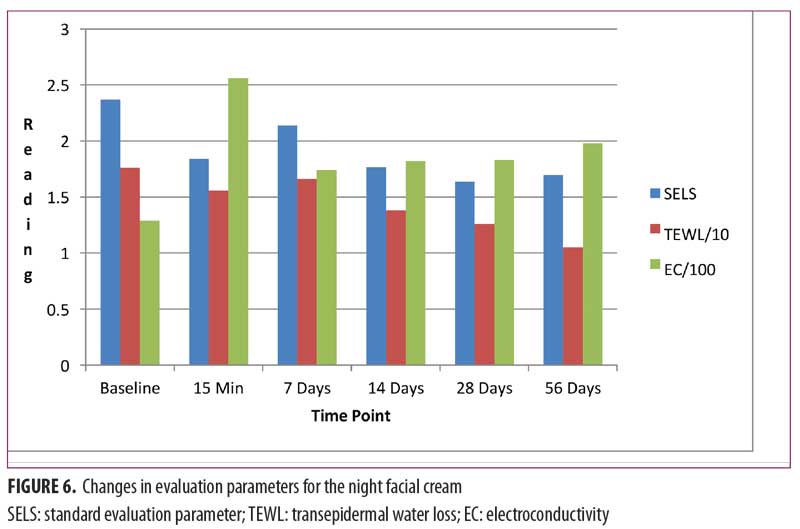
TEWL readings (Figure 6) began to decrease 15 minutes after the initial application and progressively declined for the remainder of the study. A decrease in TEWL as compared with baseline (Table 4) was evident after 15 minutes (-11.73%) and reached a maximum decrease at 56 days (-40.25%). Differences from baseline were significant after 28 days (p=0.029, -28.68%) and after 56 days of use (p=0.017).
EC increased dramatically after 15 minutes (Figure 6) and continued to increase above baseline for up to 56 days (53.44%) (Table 4). Differences from baseline were significant after 15 minutes (p=0.034, 97.78%), seven days (p=0.009, 34.54%), 14 days (p=0.006, 40.72%), 28 days (p=0.012, 41.46%), and 56 days (p=0.017) of use.
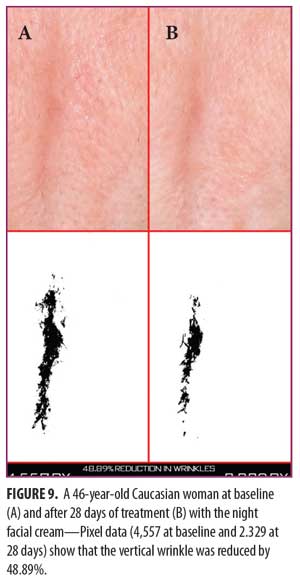
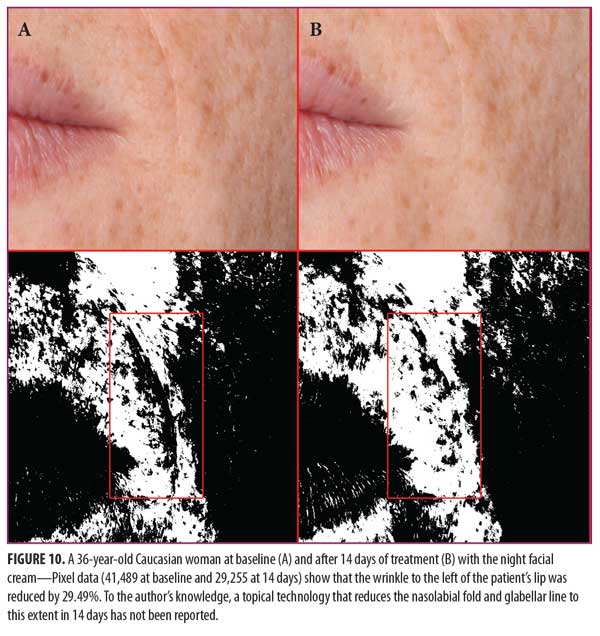
Clinical examples of patients at baseline and after 14 and 28 days of treatment with night facial cream use are shown in Figures 7–10.
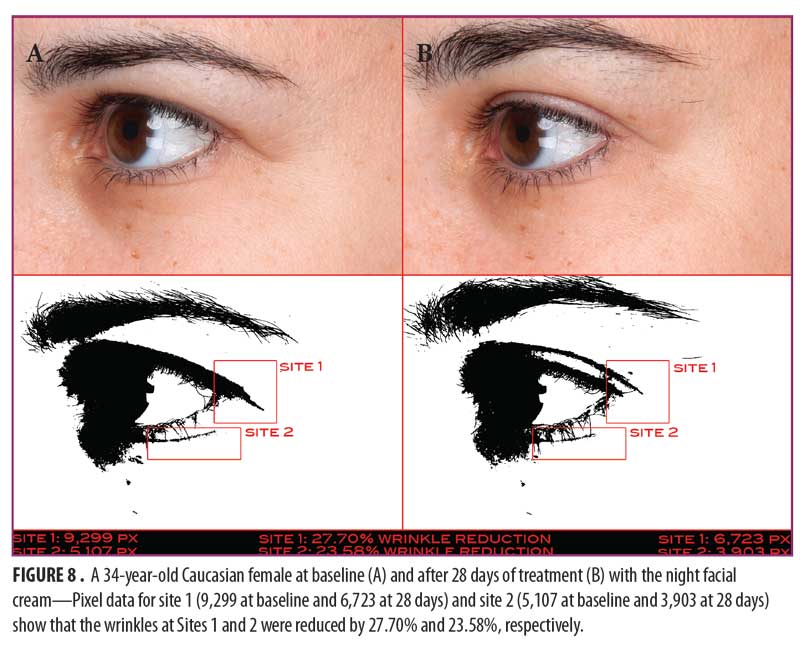
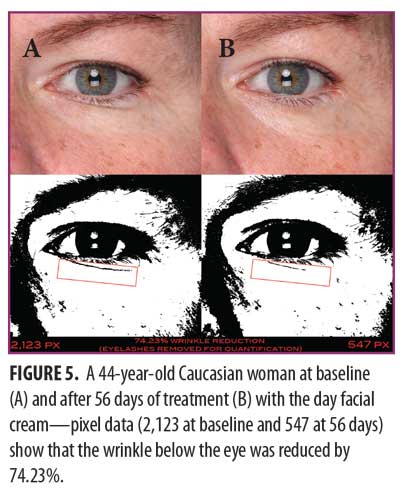
Eye cream. As shown in Figure 11, SELS started to decline 15 minutes after product application and continued to decline during the remainder of the study period. A decrease in SELS as compared with at baseline (Table 4) was also evident as early as 15 minutes (-19.24% difference) and reached a maximum at 56 days (-45.19%). Differences from baseline were significant after seven days (p=0.024, -30.25% and 14 days (p=0.027, -40.40%) of use.
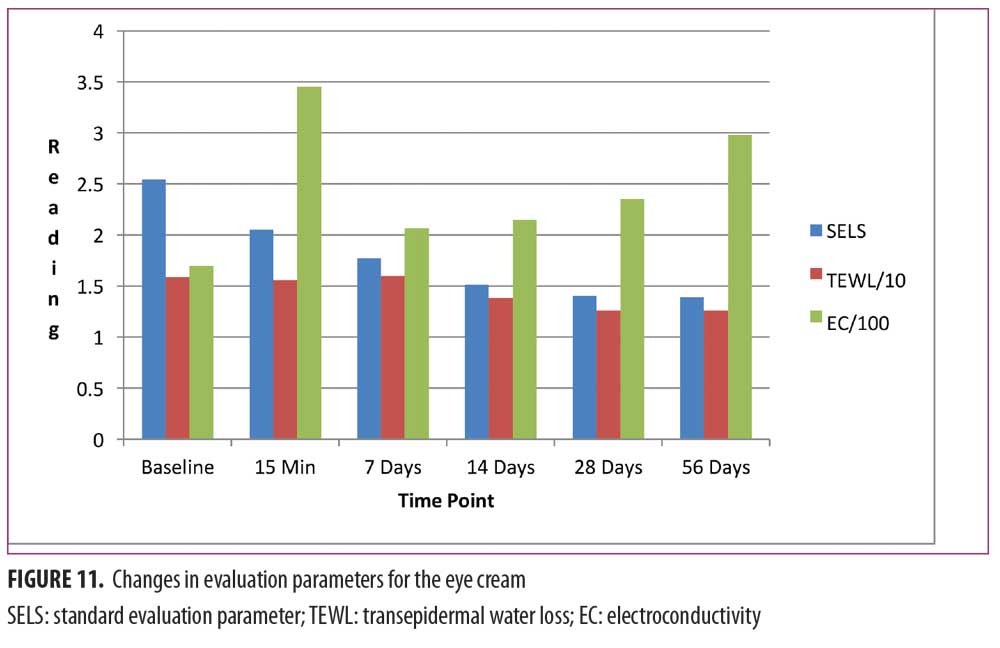
TEWL readings (Figure 11) began to decrease after 14 days of test product use and continued up to 56 days. A decrease in TEWL as compared with baseline (Table 4) was evident at 14 days (-13.41%) and continued until 56 days (-21.15%).
EC increased dramatically after 15 minutes (Figure 11), decreased at 14 days, and began an upward trend that reached a maximum above baseline at 56 days (75.49%; Table 4). Differences from baseline were significant after 15 minutes (p=0.001, 103.10%), seven days (p=0.002, 22.04%), 14 days (p=0.022, 26.67%), 28 days (p=0.045, 38.24%), and 56 days (p=0.009, 75.49%) of use.
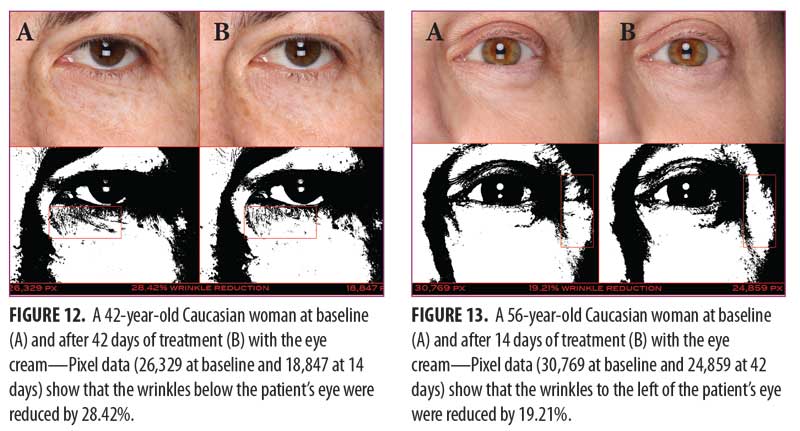
Clinical examples of patients at baseline and after 14 and 42 days of treatment with the eye cream are shown in Figures 12 and 13.
Day lip cream. As shown in Figure 14, SELS started to decline 15 minutes after product application and continued to decline during the remainder of the study period. The decrease as compared with at baseline was significant after seven days of use (p=0.046, -11.21%). A decrease in SELS as compared with at baseline (Table 4) was also evident as early as 15 minutes after (-16.79%) and reached a maximum at 56 days (-30.60%).
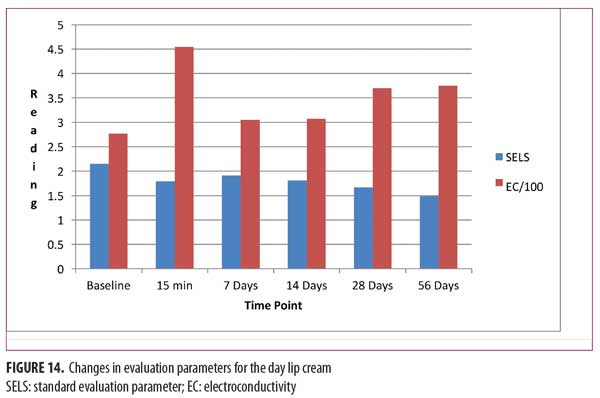
EC increased dramatically at 15 minutes (63.82%, Figure 14), decreased at seven days (10.09%), and began an upward trend until 56 days (35.27%; Table 4). The increase was significant after seven days of use (p=0.014).
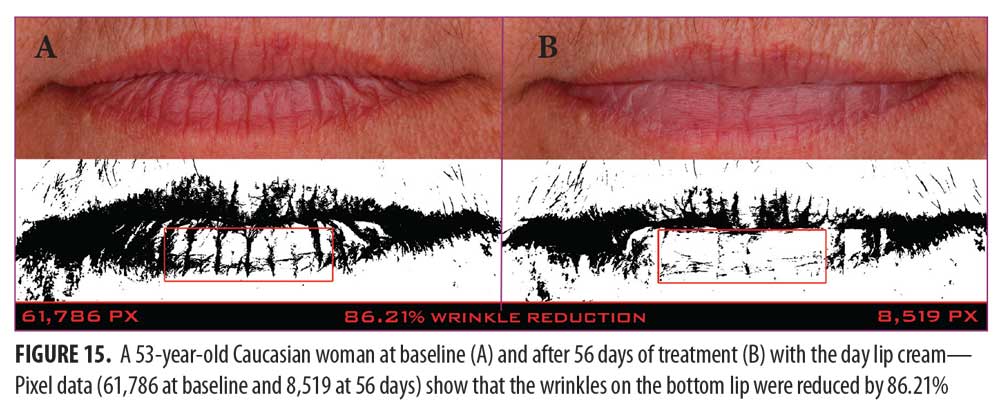
A clinical example of a patient at baseline and after 56 days of treatment with the day lip cream is shown in Figure 15.
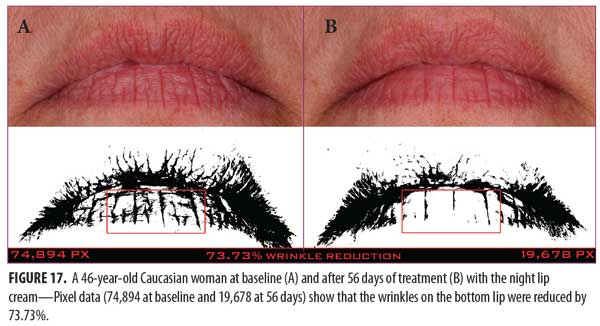
Night lip cream. As shown in Figure 16, SELS started to decline 15 minutes after product application and continued to decline during the remainder of the study period. A decrease in SELS as compared with at baseline (Table 4) was also evident as early as 15 minutes (-11.87% difference) and reached a maximum at 56 days (-24.79%). The difference from baseline was significant after seven days (p=0.040, -7.40%), 14 days (p=0.018, -13.39%), 28 days (p=0.019, -17.70%), and 56 days of use (p=0.011, -24.79%).
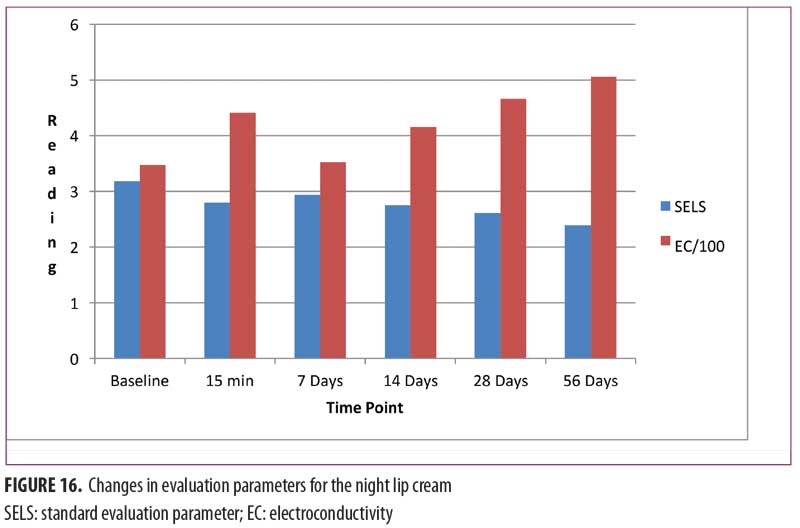
EC increased dramatically at 15 minutes (27.16%, Figure 16), decreased at seven days (1.50%), and began an upward trend that reached a maximum at 56 days (45.52%). The increase from baseline was significant at 15 minutes (p=0.003).
A clinical example of a patient at baseline and after 56 days of treatment with the night lip cream is shown in Figure 17.
The experience trial. Among the 244 enrolled subjects, 222 (91%) completed the survey at the end of the study. Serious adverse events were not observed. Most subjects (90%) reported that each of the five products absorbed well into their skin within three minutes of application and there were no objections to the scent of the products (they were fragrance-free).
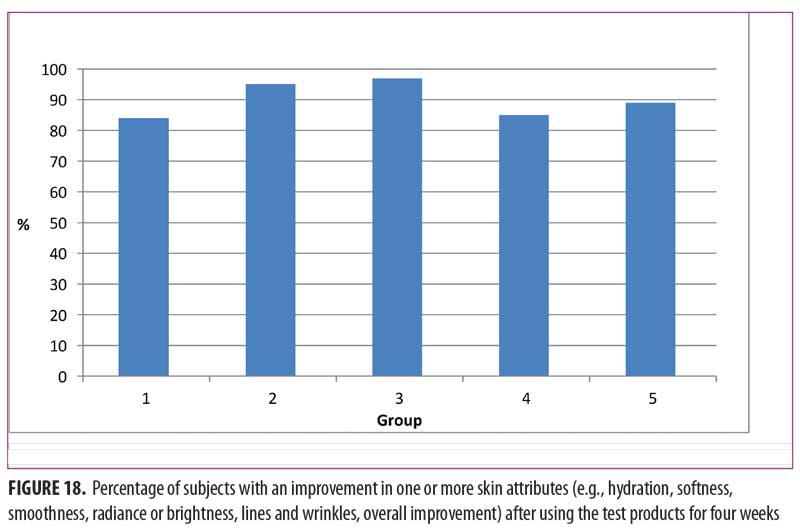
Subjects commented on improvement after one month of use for one or more of the following skin attributes: hydration, softness, smoothness, radiance or brightness, lines and wrinkles, and overall improvement. Among the five groups, the percentage of subjects citing improvement in one or more skin attributes after four weeks of use is shown in Figure 18. The highest was in Group 3 (97%), followed by Group 2 (95%), Group 5 (89%), Group 4 (85%), and Group 1 (84%). Willingness to recommend one or more of the products to a friend was greatest in Group 3 (97%), followed by Group 2 (95%), Group 1 (91%), Group 5 (90%), and Group 4 (62%). The low value (62%) for Group 4, in which both the day and night lip creams were used, might be attributed to the lesser clinical performance of the day lip cream in comparison with the superior performance of the night lip cream, as shown in Figures 15 and 16.
Among the medical estheticians, 87 percent observed improvement in one or more of the same attributes in their subjects.
Adverse effects. Adverse effects or unexpected reactions of any kind were not observed in any subject in the 56-day study or in the experience trial.
Discussion
The 56-day study. The results show that with each of the five topical test products, improvement in SELS becomes noticeable to the measuring device within 15 minutes of the initial application and that improvement continues for at least 56 days with regular use (Table 5). To the author’s knowledge, noticeable improvement in SELS within 15 minutes of application of the test product is unprecedented in cosmetic dermatology. Improvement was significant after 14 days and 28 days of use for the day facial cream; after 15 minutes, 28 days, and 56 days of use for the night facial cream; after seven days and 14 days of use for the eye cream; after seven days for the day lip cream; and after seven, 14, 28, and 56 days of use for the night lip cream. These results suggest that skin surface roughness, or the depth of fine and coarse lines and wrinkles, decreased immediately after product application and continued to decrease for at least 56 days with regular use of each test product.
Similar but even more favorable trends are apparent with EC. Improvements were significant after 15 minutes, 14 days, and 28 days of use for the day facial cream; after every timepoint for the night facial cream and eye cream; and after 15 minutes for both the day lip cream and night lip cream. These results show that each of the five test products helped to increase the moisture content in the skin almost immediately after application and that the favorable trends continued for at least 56 days with regular use.
These very early results and continuing improvement for at least 56 days have, to the author’s knowledge, not been reported for any topical product. Improvement in TEWL (measured only in those using the facial and eye creams) was nearly immediate with the use of the night facial cream, at 14 days with the eye cream, and at 28 days with the day facial cream. Improvement was significant at 28 and 56 days for the night facial cream.
The experience trial. The results (Figure 18) show that the selected products, which included facial, eye, and lip creams, performed well when applied daily according to the schedule in Table 3. The percentage of subjects who reported improvement in one or more of their skin attributes ranged from 84 to 97 percent. Subjects evaluated lines and wrinkles, softness, smoothness, radiance and brightness, and overall improvement.
Advantages of this study include high rate of survey completion (91%) and large number of reporting subjects (n=222). The five products provided a comprehensive anti-aging treatment for the face, eye, and lip areas, which age at different rates. Products were applied in the morning, evening, or both. It is important to note that 90 percent of subjects reported each of the five products absorbed well into their skin within three minutes of application. This observation, along with the continuous and progressive improvement in most parameters in the 56-day study, show that the early absorption of products into the skin is genuine rather than superficial, and that the beneficial effects of the products continue to increase despite regular cleaning of the face.
The likelihood of recommending one or more of these products in the future ranged from 57 percent (Group 4) to 91 percent (Group 1). The low percentage of Group 4 is, again, attributed to the reduced clinical performance of the day lip cream versus the night lip cream. Values in the remaining groups were 87 percent (Group 5), 84 percent (Group 3), and 80 percent (Group 2), respectively.
Conclusions
Although the 56-day trial included only five subjects for each test product, the improvements in endpoints from baseline to the end of the study were extremely high and achieved 95-percent confidence levels for all test products.
Results of this study support the effectiveness of the facial, eye, and lip creams used in this study as anti-aging products that reduce the appearance of both fine and coarse lines and wrinkles, increase the moisturization of the skin, and, in the case of the face and eye products, decrease transepidermal water loss. The test products showed early positive effects on the skin test sites, and these effects were seen for at least 56 days without significant adverse effects. We attribute these results to the integration of the dermal delivery technology, the cross linked hyaluronic acid, and synthetically engineered natural collagen utilized by the test products.
References
- Sercombe L, Veerati T, Moheimani F, et al. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286.
- Agarwal S, Kumari PVK. Advances in Novasome technology – a review. Int J Appl Pharm. 2013;5: 1–4.
- Pinsky MA, inventor. Materials and methods for delivering antioxidants into the skin. US Patent, filed 2010.
- Singh A, Malviya R, Sharma PK. Novasome – a breakthrough in pharmaceutical technology: a review article. Adv Biol Res. 2011; 5:184–189.
- Fischer TW, Wigger-Alberti W, Elsner P. Direct and non-direct measurement techniques for analysis of skin surface topography. Skin Pharmacol Appl Skin Physiol. 1999;12(1–2):1–11.
- Seitz JC, Spencer TS. The use of capacitive evaporimetry to measure the effects of topical ingredients on transepidermal water loss (TEWL) [Abstract]. Clin Res. 1982;30:608A.
- Leveque JL, de Rigal J. Impedance methods for studying skinmoisturization. J Soc Cosmet Chem. 1983;34(8):419–428.

